Abstract
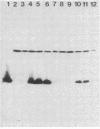
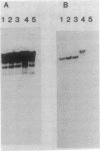
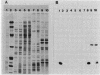
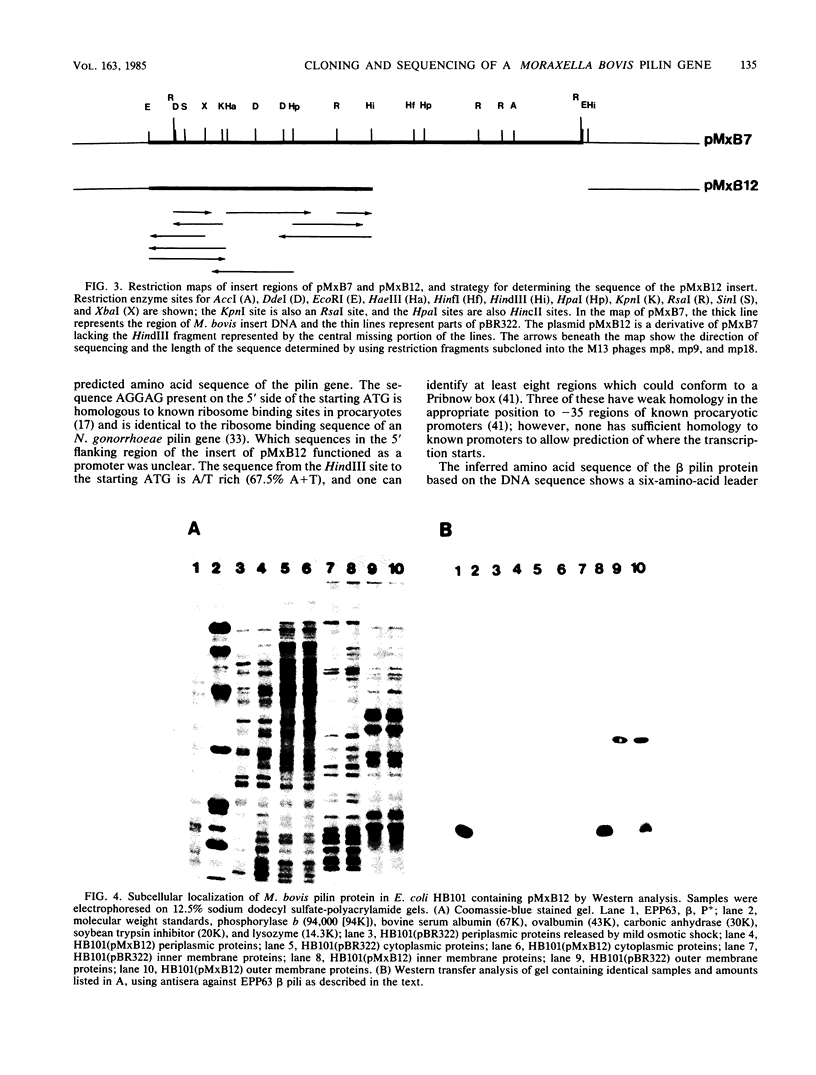
Moraxella bovis pili have been shown to play a major role in both infectivity and protective immunity of bovine infectious keratoconjunctivitis. Sonicated M. bovis DNA from the piliated strain EPP63 was inserted into the vector lambda gt11 with EcoRI linkers. Recombinant phage were screened with an oligonucleotide probe based on the amino-terminal portion of the DNA sequence of a Neisseria gonorrhoeae pilin gene. Two candidate phages produced a protein that comigrated with EPP63 beta pilin in sodium dodecyl sulfate-polyacrylamide gels and bound anti-pilus antisera. The 1.9-kilobase insert from one of these, lambda gt11M182, was subcloned in both orientations into pBR322, forming the plasmids pMxB7 and pMxB9, both of which produced beta pilin, as did pMxB12, a HindIII deletion derivative of pMxB7. In HB101(pMxB12), the M. bovis pilin protein was shown to be primarily localized in the inner membrane. The entire 939-base-pair insert of pMxB12 was sequenced, revealing a ribosome binding site just upstream of the coding region and an AT-rich region further upstream containing some potential RNA polymerase recognition sites. The translation of the sequence predicts a six-amino-acid leader sequence preceding the phenylalanine that begins the mature protein. Codon usage analysis of the M. bovis beta pilin gene revealed greater use of the CUA codon for leucine than usual for a well-expressed Escherichia coli gene. Comparisons of the M. bovis EPP63 beta pilin protein sequence with other pilin gene sequences are presented.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bovre K., Froholm L. O. Competence in genetic transformation related to colony type and fimbriation in three species of Moraxella. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):649–659. [PubMed] [Google Scholar]
- Bovre K., Froholm L. O. Variation of colony morphology reflecting fimbriation in Moraxella bovis and two reference strains of M. nonliquefaciens. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):629–640. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Falkow S. Protein antigens from Staphylococcus aureus strains associated with toxic-shock syndrome. Science. 1981 Feb 20;211(4484):842–844. doi: 10.1126/science.7466361. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Emery D. L., Stewart D. J., Clark B. L. Isolation of the gene encoding pilin of Bacteroides nodosus (strain 198), the causal organism of ovine footrot. FEBS Lett. 1984 Jul 23;173(1):103–107. doi: 10.1016/0014-5793(84)81026-1. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A. Nucleotide sequence of the gene encoding pilin of Bacteroides nodosus, the causal organism of ovine footrot. J Bacteriol. 1984 Dec;160(3):1184–1187. doi: 10.1128/jb.160.3.1184-1187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froholm L. O., Sletten K. Purification and N-terminal sequence of a fimbrial protein from Moraxella nonliquefaciens. FEBS Lett. 1977 Jan 15;73(1):29–32. doi: 10.1016/0014-5793(77)80008-2. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- HENSON J. B., GRUMBLES L. C. Infectious bovine keratoconjunctivitis. I. Etiology. Am J Vet Res. 1960 Sep;21:761–766. [PubMed] [Google Scholar]
- Henrichsen J., Froholm L. O., Bovre K. Studies on bacterial surface translocation. 2. Correlation of twitching motility and fimbriation in colony variants of Moraxella nonliquefaciens, M. bovis, and M. kingii. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(3):445–452. [PubMed] [Google Scholar]
- Henrichsen J. Twitching motility. Annu Rev Microbiol. 1983;37:81–93. doi: 10.1146/annurev.mi.37.100183.000501. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Hughes D. E., Pugh G. W., Jr A five-year study of infectious bovine keratoconjunctivitis in a beef herd. J Am Vet Med Assoc. 1970 Aug 15;157(4):443–451. [PubMed] [Google Scholar]
- Hughes D. E., Pugh G. W., Jr, McDonald T. J. Ultraviolet radiation and Moraxella bovis in the etiology of bovine infectious keratoconjunctivitis. Am J Vet Res. 1965 Nov;26(115):1331–1338. [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKern N. M., O'Donnell I. J., Inglis A. S., Stewart D. J., Clark B. L. Amino acid sequence of pilin from Bacteroides nodosus (strain 198), the causative organism of ovine footrot. FEBS Lett. 1983 Nov 28;164(1):149–153. doi: 10.1016/0014-5793(83)80039-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Billyard E., Haas R., Storzbach S., So M. Pilus genes of Neisseria gonorrheae: chromosomal organization and DNA sequence. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pedersen K. B., Froholm L. O., Bovre K. Fimbriation and colony type of Moraxella bovis in relation to conjunctival colonization and development of keratoconjunctivitis in cattle. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):911–918. doi: 10.1111/j.0365-5563.1973.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Pugh G. W., Jr, Hughes D. E., Booth G. D. Experimentally induced infections bovine keratoconjunctivitis: effectiveness of a pilus vaccine against exposure to homologous strains of Moraxella bovis. Am J Vet Res. 1977 Oct;38(10):1519–1522. [PubMed] [Google Scholar]
- Pugh G. W., Jr, Hughes D. E. Experimental bovine infectious keratoconjunctivitis caused by sunlamp irradiation and Moraxella bovis infection: correlation of hamolytic ability and pathogenicity. Am J Vet Res. 1968 Apr;29(4):835–839. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Fernandez R., Schoolnik G. K. Strain-specific and common epitopes of gonococcal pili. J Exp Med. 1984 Jul 1;160(1):208–221. doi: 10.1084/jem.160.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu T. S., White F. H. Production and characterization of Moraxella bovis hemolysin. Am J Vet Res. 1977 Jun;38(6):883–885. [PubMed] [Google Scholar]
- Sandhu T. S., White F. H., Simpson C. F. Association of pili with rough colony type of Moraxella bovis. Am J Vet Res. 1974 Mar;35(3):437–439. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry P. A., Pearlstone J. R., Smillie L. B., Paranchych W. Amino acid sequence of pilin isolated from pseudomonas aeruginosa PAK. FEBS Lett. 1983 Jan 24;151(2):253–256. doi: 10.1016/0014-5793(83)80080-5. [DOI] [PubMed] [Google Scholar]
- Schoolnik G. K., Fernandez R., Tai J. Y., Rothbard J., Gotschlich E. C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984 May 1;159(5):1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm J. W., Moore D. D., Blattner F. R., Howe M. M. Correlation of the genetic and physical maps in the central region of the bacteriophage Mu genome. Virology. 1980 Aug;105(1):185–195. doi: 10.1016/0042-6822(80)90166-x. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]