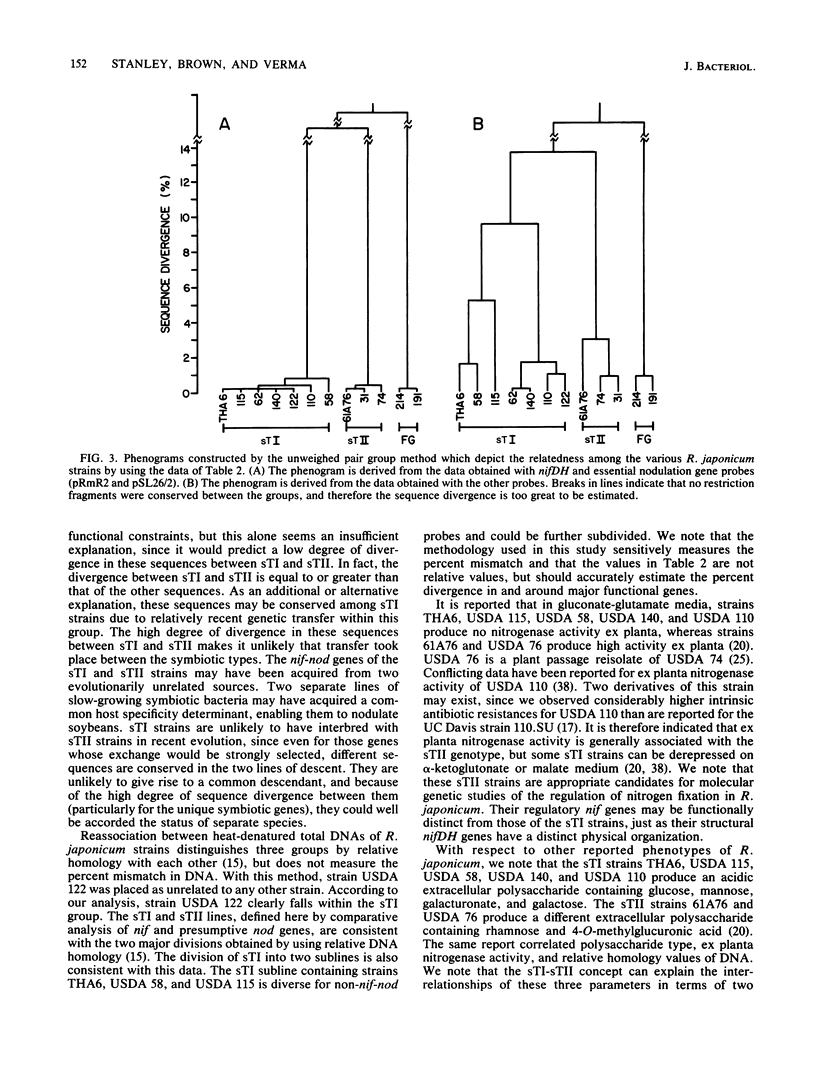
Abstract
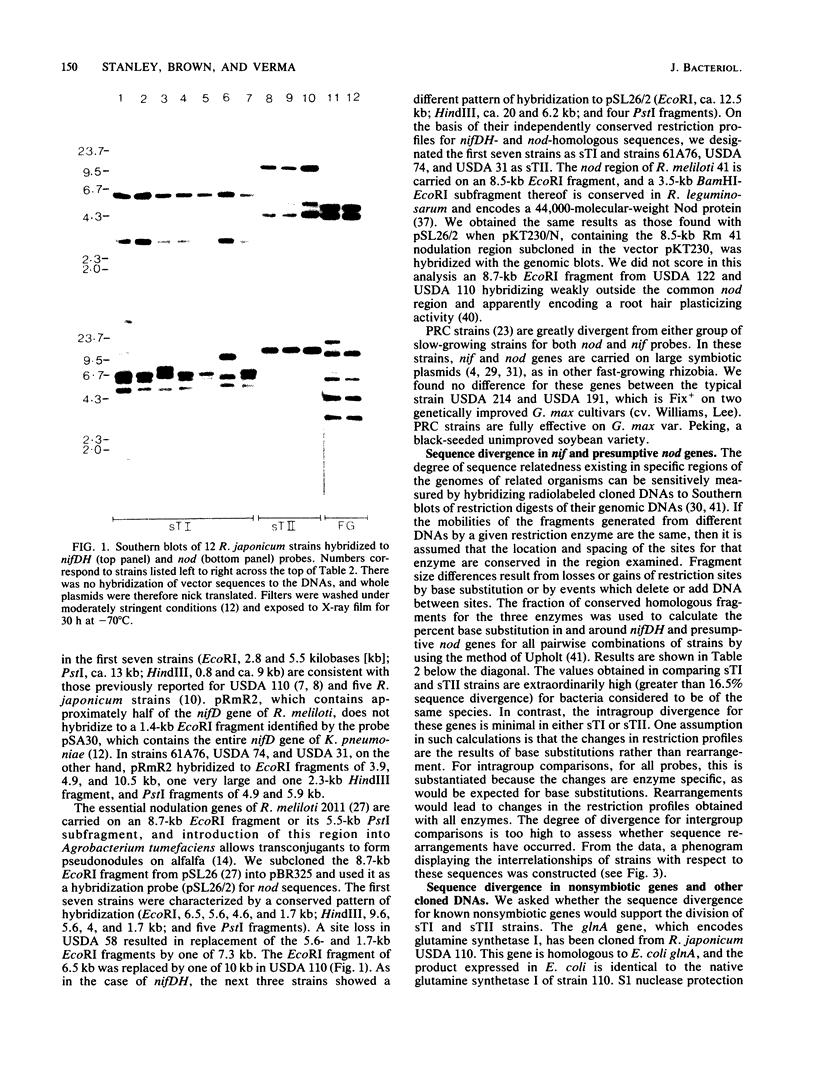
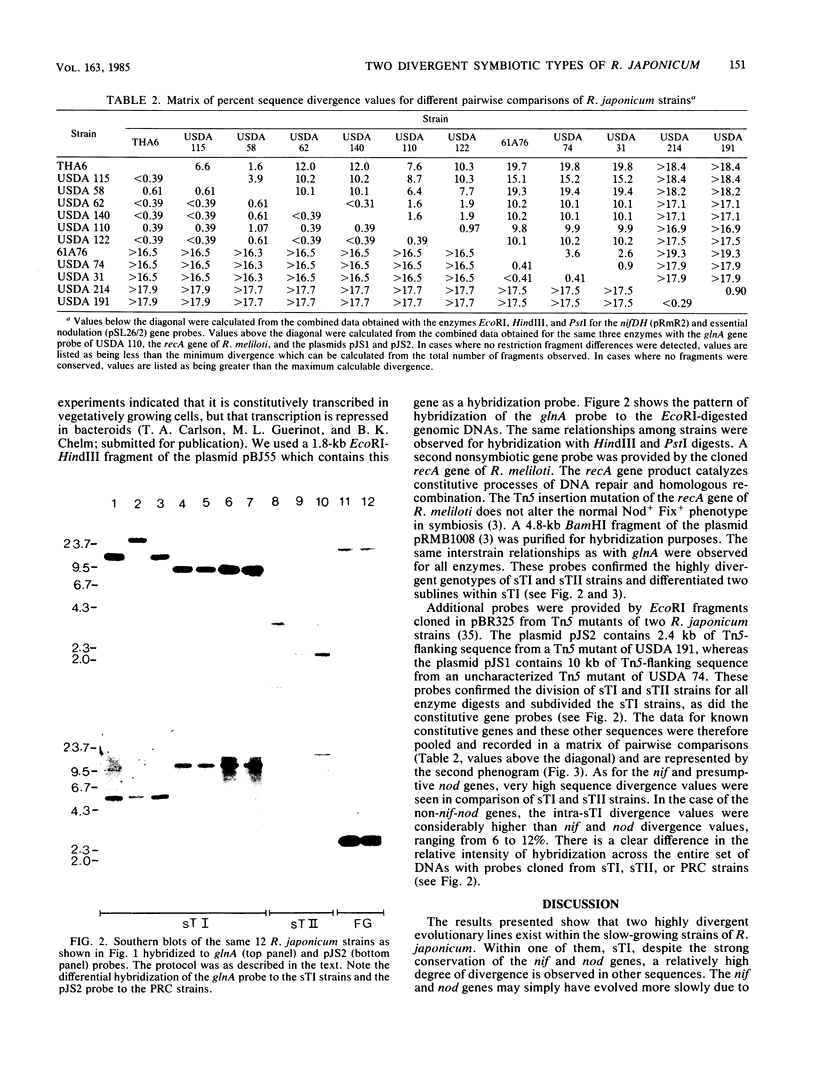
We examined the interrelationships of the genomes of 10 slow-growing strains of Rhizobium japonicum to provide a foundation for molecular genetic studies of these agriculturally important endosymbiotic bacteria of commercial soybeans. The degree of base substitution in and around known symbiotic genes (nif and presumptive nod), constitutively expressed genes (glnA and recA), and two other cloned sequences was estimated from restriction site variation by using cloned DNAs as hybridization probes to genomic Southern blots. Two highly divergent patterns of conservation of nifDH genes and nod-homologous sequences were found. On this basis, we classified the strains as the symbiotic genotypes sTI or sTII. Existing maps of the nif genes of R. japonicum apply only to strains of the sTI genotype. This division was further characterized by four other probes which also distinguished two sublines within sTI. Phenograms were constructed depicting interrelationships according to DNA sequence divergence. sTI and sTII are two highly divergent evolutionary lines consistent with the status of individual species. Neither is related to fast-growing Rhizobium strains (PRC strains) nodulating soybeans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. H., McClung C. R., Chelm B. K. Physical organization of the Bradyrhizobium japonicum nitrogenase gene region. J Bacteriol. 1984 Sep;159(3):857–862. doi: 10.1128/jb.159.3.857-862.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Helinski D. R. Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):311–316. doi: 10.1128/jb.155.1.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton W. J., Heycke N., Z A H. M., Pankhurst C. E. Plasmid-linked nif and "nod" genes in fast-growing rhizobia that nodulate Glycine max, Psophocarpus tetragonolobus, and Vigna unguiculata. Proc Natl Acad Sci U S A. 1984 May;81(10):3093–3097. doi: 10.1073/pnas.81.10.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Doctor F., Modi V. V. Genetic mapping of leucine and isoleucine-valine loci in Rhizobium japonicum. J Bacteriol. 1976 May;126(2):997–998. doi: 10.1128/jb.126.2.997-998.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann M., Hennecke H. Rhizobium japonicum nitrogenase Fe protein gene (nifH). J Bacteriol. 1984 Jun;158(3):1005–1011. doi: 10.1128/jb.158.3.1005-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. G., Eaglesham A. R., Szalay A. A. Conservation of DNA regions adjacent to nifKDH homologous sequences in diverse slow-growing Rhizobium strains. J Mol Appl Genet. 1983;2(3):225–236. [PubMed] [Google Scholar]
- Haugland R., Verma D. P. Interspecific plasmid and genomic DNA sequence homologies and localization of nif genes in effective and ineffective strains of Rhizobium japonicum. J Mol Appl Genet. 1981;1(3):205–217. [PubMed] [Google Scholar]
- Hirsch A. M., Wilson K. J., Jones J. D., Bang M., Walker V. V., Ausubel F. M. Rhizobium meliloti nodulation genes allow Agrobacterium tumefaciens and Escherichia coli to form pseudonodules on alfalfa. J Bacteriol. 1984 Jun;158(3):1133–1143. doi: 10.1128/jb.158.3.1133-1143.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hom S. S., Uratsu S. L., Hoang F. Transposon Tn5-induced mutagenesis of Rhizobium japonicum yielding a wide variety of mutants. J Bacteriol. 1984 Jul;159(1):335–340. doi: 10.1128/jb.159.1.335-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T. A., Agarwal A. K., Keister D. L. Extracellular polysaccharide composition, ex planta nitrogenase activity, and DNA homology in Rhizobium japonicum. J Bacteriol. 1984 Jun;158(3):1168–1171. doi: 10.1128/jb.158.3.1168-1171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza K., Fuhrmann M., Hahn M., Regensburger B., Hennecke H. In Rhizobium japonicum the nitrogenase genes nifH and nifDK are separated. J Bacteriol. 1983 Aug;155(2):915–918. doi: 10.1128/jb.155.2.915-918.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- Keyser H. H., van Berkum P., Weber D. F. A Comparative Study of the Physiology of Symbioses Formed by Rhizobium japonicum with Glycine max, Vigna unguiculata, and Macroptilium atropurpurem. Plant Physiol. 1982 Dec;70(6):1626–1630. doi: 10.1104/pp.70.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall L. D. Transfer of R factors to and between genetically marked sublines of Rhizobium japonicum. Appl Environ Microbiol. 1979 May;37(5):862–866. doi: 10.1128/aem.37.5.862-866.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson R. V., Russell P. R., Atherly A. G. Nitrogen fixation (nif) genes and large plasmids of Rhizobium japonicum. J Bacteriol. 1982 Nov;152(2):928–931. doi: 10.1128/jb.152.2.928-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Tajima F. DNA polymorphism detectable by restriction endonucleases. Genetics. 1981 Jan;97(1):145–163. doi: 10.1093/genetics/97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W. P., Schmidt E. L. Plasmid transfer within and between serologically distinct strains of Rhizobium japonicum, using antibiotic resistance mutants and auxotrophs. J Bacteriol. 1981 Feb;145(2):1025–1030. doi: 10.1128/jb.145.2.1025-1030.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. E., Ausubel F. M., Cannon F. C. Physical map of chromosomal nitrogen fixation (nif) genes of Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2866–2870. doi: 10.1073/pnas.76.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Schmidt J., John M., Kondorosi E., Kondorosi A., Wieneke U., Schröder G., Schröder J., Schell J. Mapping of the protein-coding regions of Rhizobium meliloti common nodulation genes. EMBO J. 1984 Aug;3(8):1705–1711. doi: 10.1002/j.1460-2075.1984.tb02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. B., Hennecke H., Lim S. T. The biosynthesis of nitrogenase MoFe protein polypeptides in free-living cultures of Rhizobium japonicum. Biochim Biophys Acta. 1979 Dec 17;565(2):365–378. doi: 10.1016/0005-2787(79)90212-0. [DOI] [PubMed] [Google Scholar]
- Sutton B. C., Stanley J., Zelechowska M. G., Verma D. P. Isolation and expression of Rhizobium japonicum cloned DNA encoding an early soybean nodulation function. J Bacteriol. 1984 Jun;158(3):920–927. doi: 10.1128/jb.158.3.920-927.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]