Abstract
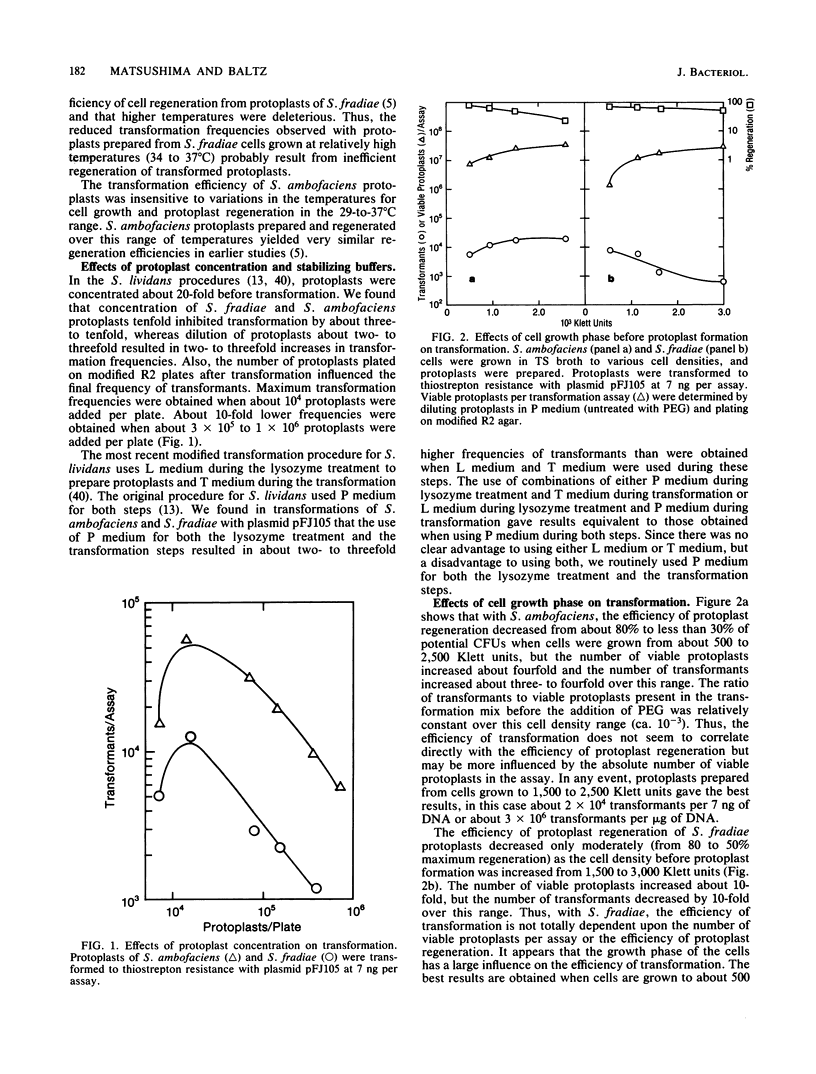
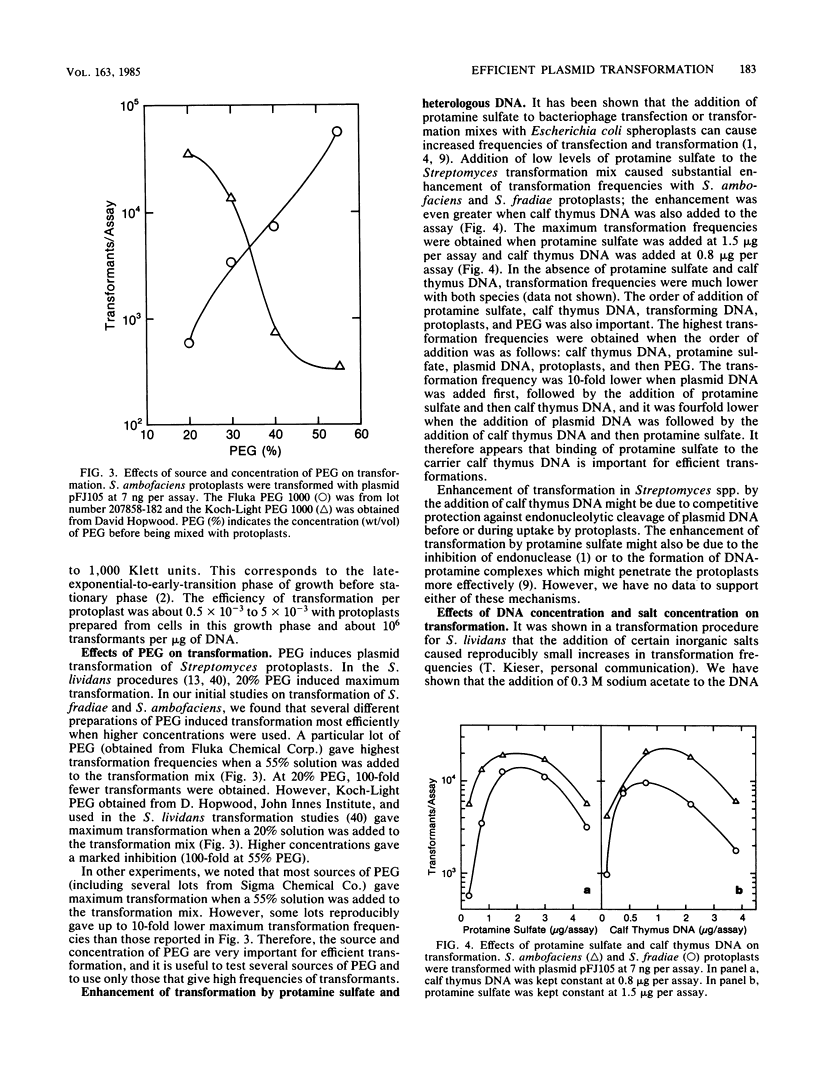
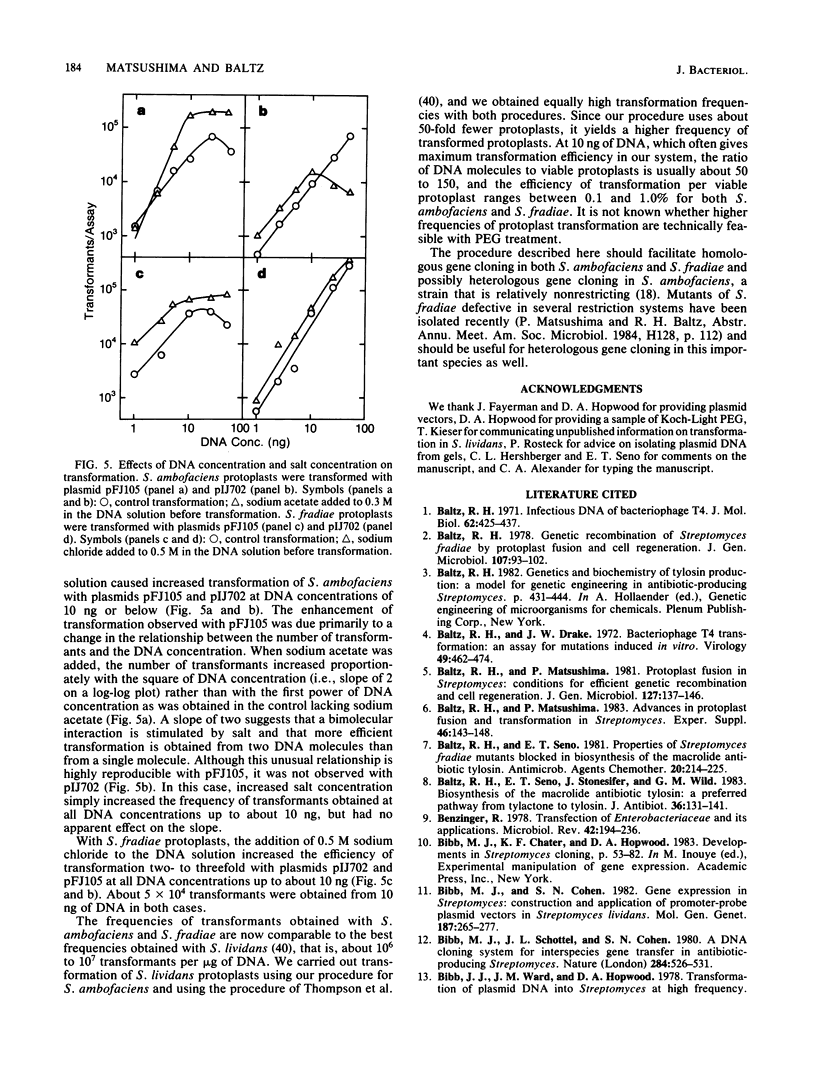
A procedure for efficient transformation of Streptomyces ambofaciens and Streptomyces fradiae protoplasts with plasmid DNA was developed. Transformation frequencies with S. fradiae protoplasts were strongly influenced by the temperatures for cell growth, protoplast formation, and protoplast regeneration. Transformation frequencies for both species were also influenced by the culture age before protoplast formation, the source and concentration of polyethylene glycol, the transformation-inducing agent, the concentration of protoplasts used in the transformation procedure, and the number of protoplasts added to regeneration plates. Transformation frequencies were substantially higher for both species when calf thymus DNA and protamine sulfate were added to the transformation mix. With S. fradiae, transformation frequencies were much lower with plasmid DNA prepared from other species than with the same plasmids prepared from S. fradiae, suggesting that S. fradiae expresses restriction and modification. With the modified transformation procedures using DNA prepared from homologous hosts, S. ambofaciens and S. fradiae are now transformed routinely at frequencies of 10(6) to 10(7) transformants per micrograms of plasmid DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H., Drake J. W. Bacteriophage T4 transformation: an assay for mutations induced in vitro. Virology. 1972 Aug;49(2):462–474. doi: 10.1016/0042-6822(72)90498-9. [DOI] [PubMed] [Google Scholar]
- Baltz R. H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978 Jul;107(1):93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- Baltz R. H. Genetics and biochemistry on tylosin production: a model for genetic engineering in antibiotic-producing Streptomyces. Basic Life Sci. 1982;19:431–444. doi: 10.1007/978-1-4684-4142-0_32. [DOI] [PubMed] [Google Scholar]
- Baltz R. H. Infectious DNA of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):425–437. doi: 10.1016/0022-2836(71)90146-x. [DOI] [PubMed] [Google Scholar]
- Baltz R. H., Matsushima P. Advances in protoplast fusion and transformation in Streptomyces. Experientia Suppl. 1983;46:143–148. doi: 10.1007/978-3-0348-6776-4_18. [DOI] [PubMed] [Google Scholar]
- Baltz R. H., Matsushima P. Protoplast fusion in Streptomyces: conditions for efficient genetic recombination and cell regeneration. J Gen Microbiol. 1981 Nov;127(1):137–146. doi: 10.1099/00221287-127-1-137. [DOI] [PubMed] [Google Scholar]
- Baltz R. H., Seno E. T. Properties of Streptomyces fradiae mutants blocked in biosynthesis of the macrolide antibiotic tylosin. Antimicrob Agents Chemother. 1981 Aug;20(2):214–225. doi: 10.1128/aac.20.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R. H., Seno E. T., Stonesifer J., Wild G. M. Biosynthesis of the macrolide antibiotic tylosin. A preferred pathway from tylactone to tylosin. J Antibiot (Tokyo) 1983 Feb;36(2):131–141. doi: 10.7164/antibiotics.36.131. [DOI] [PubMed] [Google Scholar]
- Benzinger R. Transfection of Enterobacteriaceae and its applications. Microbiol Rev. 1978 Mar;42(1):194–236. doi: 10.1128/mr.42.1.194-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Cohen S. N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187(2):265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- Bibb M., Schottel J. L., Cohen S. N. A DNA cloning system for interspecies gene transfer in antibiotic-producing Streptomyces. Nature. 1980 Apr 10;284(5756):526–531. doi: 10.1038/284526a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J., King A. A., Suarez J. E. The expression of Streptomyces and Escherichia coli drug-resistance determinants cloned into the Streptomyces phage phi C31. Gene. 1982 Jul-Aug;19(1):21–32. doi: 10.1016/0378-1119(82)90185-8. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J. Mutational cloning in Streptomyces and the isolation of antibiotic production genes. Gene. 1983 Dec;26(1):67–78. doi: 10.1016/0378-1119(83)90037-9. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Cox K. L., Baltz R. H. Restriction of bacteriophage plaque formation in Streptomyces spp. J Bacteriol. 1984 Aug;159(2):499–504. doi: 10.1128/jb.159.2.499-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Feitelson J. S., Hopwood D. A. Cloning of a Streptomyces gene for an O-methyltransferase involved in antibiotic biosynthesis. Mol Gen Genet. 1983;190(3):394–398. doi: 10.1007/BF00331065. [DOI] [PubMed] [Google Scholar]
- Gil J. A., Hopwood D. A. Cloning and expression of a p-aminobenzoic acid synthetase gene of the candicidin-producing Streptomyces griseus. Gene. 1983 Nov;25(1):119–132. doi: 10.1016/0378-1119(83)90174-9. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Hara O., Beppu T. Cloning of a pleiotropic gene that positively controls biosynthesis of A-factor, actinorhodin, and prodigiosin in Streptomyces coelicolor A3(2) and Streptomyces lividans. J Bacteriol. 1983 Sep;155(3):1238–1248. doi: 10.1128/jb.155.3.1238-1248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Kumada Y., Beppu T. Unstable genetic determinant of A-factor biosynthesis in streptomycin-producing organisms: cloning and characterization. J Bacteriol. 1984 May;158(2):481–487. doi: 10.1128/jb.158.2.481-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. H., Hopwood D. A. Molecular cloning and expression of the phenoxazinone synthase gene from Streptomyces antibioticus. J Biol Chem. 1984 Nov 25;259(22):14151–14157. [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kendall K., Cullum J. Cloning and expression of an extracellular-agarase from Streptomyces coelicolor A3(2) in Streptomyces lividans 66. Gene. 1984 Sep;29(3):315–321. doi: 10.1016/0378-1119(84)90060-x. [DOI] [PubMed] [Google Scholar]
- Kuhstoss S., Rao R. N. Expression in Streptomyces ambofaciens of an Escherichia coli K-12 gene which confers resistance to hygromycin B. Gene. 1983 Dec;26(2-3):295–299. doi: 10.1016/0378-1119(83)90200-7. [DOI] [PubMed] [Google Scholar]
- Larson J. L., Hershberger C. L. Shuttle vectors for cloning recombinant DNA in Escherichia coli and Streptomyces griseofuscus C581. J Bacteriol. 1984 Jan;157(1):314–317. doi: 10.1128/jb.157.1.314-317.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Zalacaín M., Jiménez A., Davies J. Molecular cloning and expression in streptomyces lividans of a hygromycin B phosphotransferase gene from Streptomyces hygroscopicus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):6–12. doi: 10.1016/0006-291x(83)91533-4. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Mashiko H., Ogawara H. Cloning of the kanamycin resistance gene from a kanamycin-producing Streptomyces species. J Bacteriol. 1984 Jan;157(1):79–83. doi: 10.1128/jb.157.1.79-83.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S., Nakagawa A. Chemical and biological studies on 16-membered macrolide antibiotics. J Antibiot (Tokyo) 1975 Jun;28(6):401–433. doi: 10.7164/antibiotics.28.401. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Mabe J. A., Beerman N. E., Nakatsukasa W. M., Fayerman J. T. Development of cloning vehicles from the Streptomyces plasmid pFJ103. Gene. 1982 Dec;20(3):451–457. doi: 10.1016/0378-1119(82)90214-1. [DOI] [PubMed] [Google Scholar]
- Schottel J. L., Bibb M. J., Cohen S. N. Cloning and expression in streptomyces lividans of antibiotic resistance genes derived from Escherichia coli. J Bacteriol. 1981 Apr;146(1):360–368. doi: 10.1128/jb.146.1.360-368.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno E. T., Baltz R. H. Properties of S-adenosyl-L-methionine:macrocin O-methyltransferase in extracts of Streptomyces fradiae strains which produce normal or elevated levels of tylosin and in mutants blocked in specific O-methylations. Antimicrob Agents Chemother. 1981 Sep;20(3):370–377. doi: 10.1128/aac.20.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno E. T., Bruton C. J., Chater K. F. The glycerol utilization operon of Streptomyces coelicolor: genetic mapping of gyl mutations and the analysis of cloned gylDNA. Mol Gen Genet. 1984;193(1):119–128. doi: 10.1007/BF00327424. [DOI] [PubMed] [Google Scholar]
- Suarez J. E., Chater K. F. DNA cloning in Streptomyces: a bifunctional replicon comprising pBR322 inserted into a Streptomyces phage. Nature. 1980 Jul 31;286(5772):527–529. doi: 10.1038/286527a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol. 1982 Aug;151(2):668–677. doi: 10.1128/jb.151.2.668-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]



