Abstract
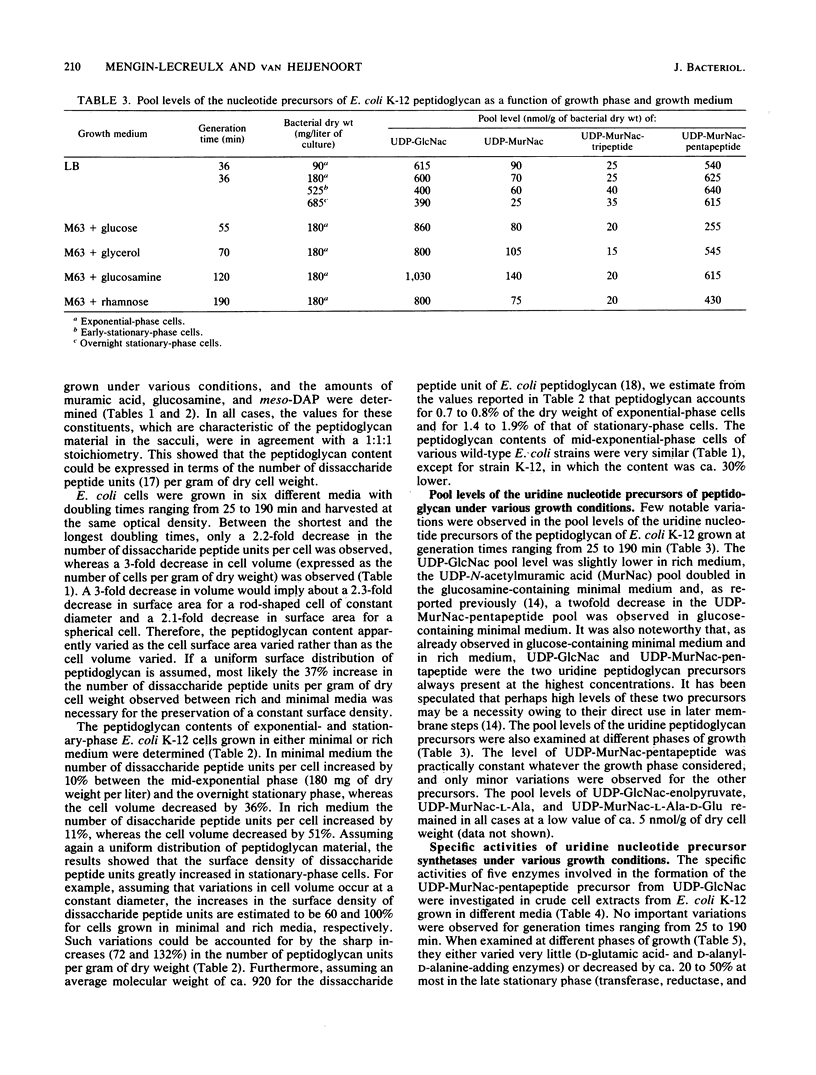
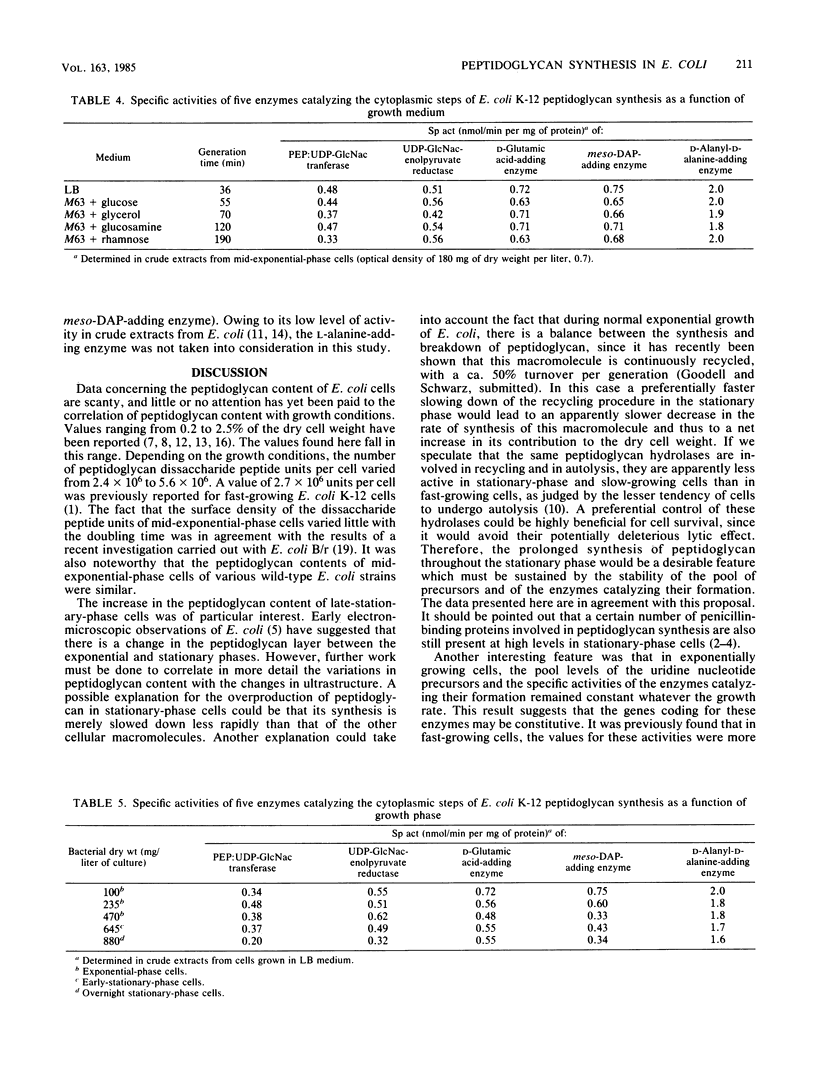
In an attempt to bring some insight into how peptidoglycan synthesis is controlled in Escherichia coli, simple parameters, such as cell peptidoglycan content, the pool levels of its seven uridine nucleotide precursors, and the specific activities of five enzymes involved in their formation, were investigated under different growth conditions. When exponential-phase cells with generation times ranging from 25 to 190 min were examined, the peptidoglycan content apparently varied as the cell surface area changed, and no important variations in the pool levels of the nucleotide precursors or in the specific activities of the five enzymes considered were observed. The peptidoglycan of exponential-phase cells accounted for 0.7 to 0.8% of the dry cell weight, whereas that of stationary-phase cells accounted for 1.4 to 1.9%. Depending on the growth conditions, the number of peptidoglycan disaccharide peptide units per cell varied from 2.4 X 10(6) to 5.6 X 10(6). The levels of the nucleotide precursor pools as well as the specific activities of the D-glutamic acid- and D-alanyl-D-alanine-adding enzymes varied little with the growth phase. The specific activities of UDP-N-acetylglucosamine transferase, UDP-N-acetylglucosamine-enolpyruvate reductase, and the diaminopimelic acid-adding enzymes decreased by 20 to 50% at most in the late stationary phase. The results are discussed in terms of the possible importance for cell survival of the maintenance of a high capacity for peptidoglycan synthesis, whatever its rate under various growth conditions, and of a balance between the synthesis and breakdown of peptidoglycan during active growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Sowell M. O. Synthesis of penicillin-binding protein 6 by stationary-phase Escherichia coli. J Bacteriol. 1982 Jul;151(1):491–494. doi: 10.1128/jb.151.1.491-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank H., Dekegel D. Electron microscopical studies on the localisation of the different components of cell walls of gram-negative bacteria. Folia Microbiol (Praha) 1967;12(3):227–233. doi: 10.1007/BF02868736. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Cleavage and resynthesis of peptide cross bridges in Escherichia coli murein. J Bacteriol. 1983 Oct;156(1):136–140. doi: 10.1128/jb.156.1.136-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle B. D., Beveridge T. J. Metal binding by the peptidoglycan sacculus of Escherichia coli K-12. Can J Microbiol. 1984 Feb;30(2):204–211. doi: 10.1139/m84-031. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Involvement of the relA gene product and feedback inhibition in the regulation of DUP-N-acetylmuramyl-peptide synthesis in Escherichia coli. J Bacteriol. 1978 Sep;135(3):766–774. doi: 10.1128/jb.135.3.766-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., Kasra R., van Heijenoort J. Induction and control of the autolytic system of Escherichia coli. J Bacteriol. 1982 Oct;152(1):26–34. doi: 10.1128/jb.152.1.26-34.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J. Studies on Escherichia coli enzymes involved in the synthesis of uridine diphosphate-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1972 Apr;110(1):26–34. doi: 10.1128/jb.110.1.26-34.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. Preparation and properties of the mucopeptides of cell walls of gram-negative bacteria. Biochem J. 1962 Aug;84:294–299. doi: 10.1042/bj0840294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1109–1117. doi: 10.1128/jb.151.3.1109-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Pool levels of UDP N-acetylglucosamine and UDP N-acetylglucosamine-enolpyruvate in Escherichia coli and correlation with peptidoglycan synthesis. J Bacteriol. 1983 Jun;154(3):1284–1290. doi: 10.1128/jb.154.3.1284-1290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Hespell R. B. Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1158–1165. doi: 10.1128/jb.121.3.1158-1165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L., Mirelman D. Constant peptidoglycan density in the sacculus of Escherichia coli B/r growing at different rates. FEBS Lett. 1979 Feb 1;98(1):29–32. doi: 10.1016/0014-5793(79)80144-1. [DOI] [PubMed] [Google Scholar]



