Abstract
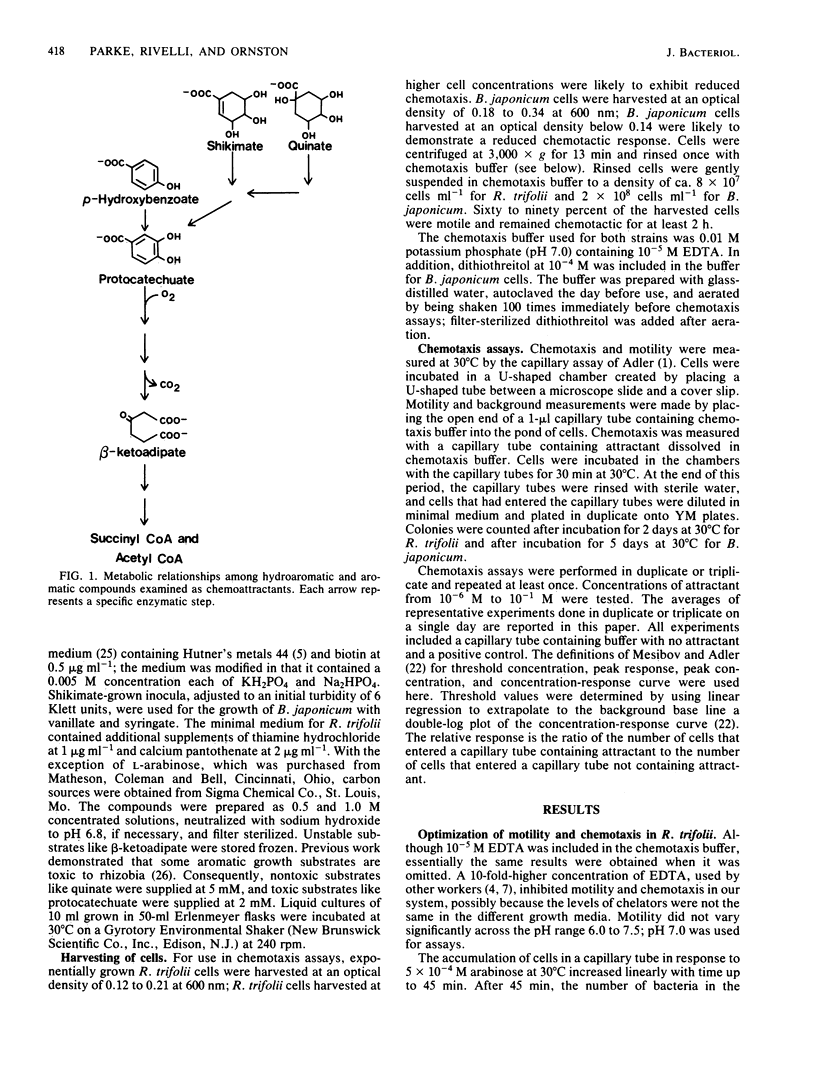
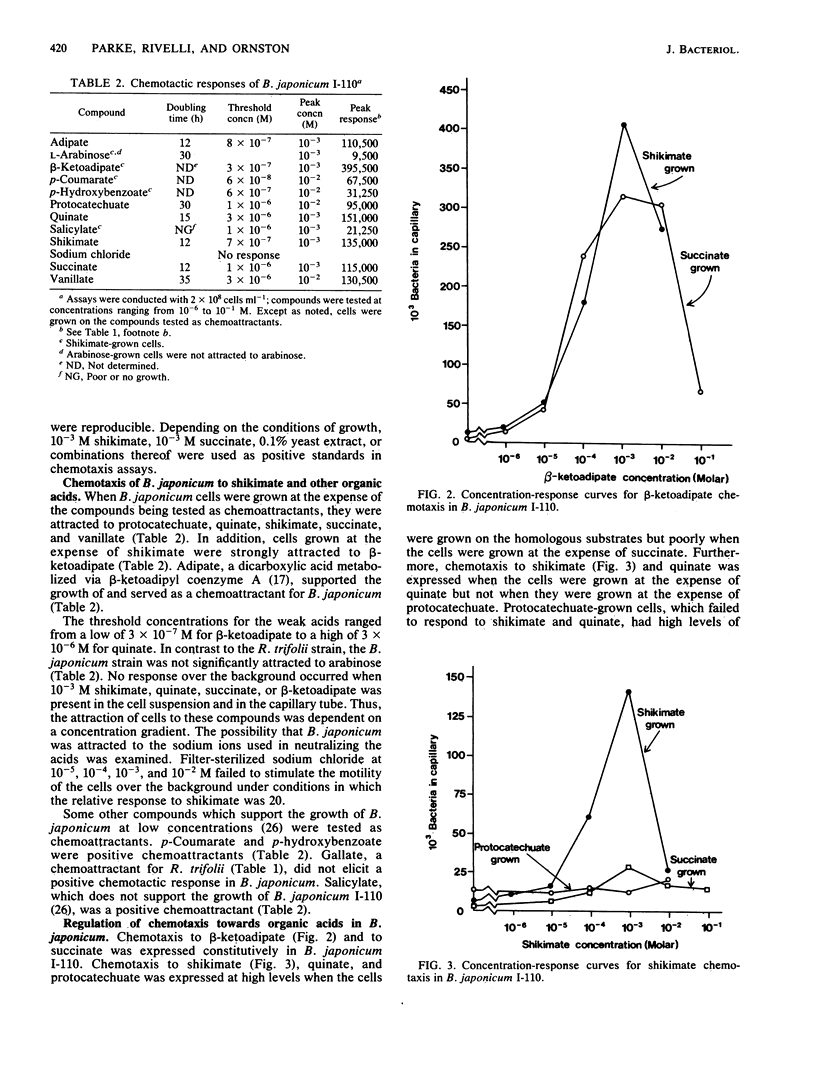
Rhizobia are bacteria well known for their ability to fix nitrogen in symbiosis with leguminous plants. Members of diverse rhizobial species grow at the expense of hydroaromatic and aromatic compounds commonly found in plant cells and plant litter. Using a quantitative capillary assay to measure chemotaxis, we tested the ability of hydroaromatic acids, selected aromatic acids, and their metabolites to serve as chemoattractants for two distantly related rhizobial species, Bradyrhizobium japonicum and Rhizobium trifolii. Slow-growing B. japonicum I-110 demonstrated positive chemotaxis to shikimate, quinate, protocatechuate, and vanillate; threshold concentrations for the compounds were as low as 10(-6) M. The dicarboxylic acids succinate and beta-ketoadipate, metabolites in the catabolism of many aromatic compounds, were positive chemoattractants with low threshold concentrations as well. Taxis to beta-ketoadipate occurred constitutively and, of the tested compounds, beta-ketoadipate gave the strongest peak response. Taxis to shikimate or quinate was induced by growth on either substrate but not by growth on protocatechuate or succinate. In contrast, fast-growing R. trifolii 2066 was only weakly attracted to quinate and other aromatic and dicarboxylic acids that were strong attractants for B. japonicum. The R. trifolii strain exhibited positive chemotaxis to shikimate, but the threshold concentration of shikimate required to elicit a response (10(-4) M) was 2 orders of magnitude higher than that for the B. japonicum strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Ames P., Bergman K. Competitive advantage provided by bacterial motility in the formation of nodules by Rhizobium meliloti. J Bacteriol. 1981 Nov;148(2):728–p. doi: 10.1128/jb.148.2.728-729.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Currier W. W., Strobel G. A. Chemotaxis of Rhizobium spp. to Plant Root Exudates. Plant Physiol. 1976 May;57(5):820–823. doi: 10.1104/pp.57.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier W. W., Strobel G. A. Chemotaxis of Rhizobium spp. to a Glycoprotein Produced by Birdsfoot Trefoil Roots. Science. 1977 Apr 22;196(4288):434–436. doi: 10.1126/science.196.4288.434. [DOI] [PubMed] [Google Scholar]
- Gulash M., Ames P., Larosiliere R. C., Bergman K. Rhizobia are attracted to localized sites on legume roots. Appl Environ Microbiol. 1984 Jul;48(1):149–152. doi: 10.1128/aem.48.1.149-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Rivelli M., Ornston L. N. Aromatic acids are chemoattractants for Pseudomonas putida. J Bacteriol. 1984 Nov;160(2):622–628. doi: 10.1128/jb.160.2.622-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet P. P., Stanier R. Y. The dissimilation of higher dicarboxylic acids by Pseudomonas fluorscens. Eur J Biochem. 1970 Mar 1;13(1):65–70. doi: 10.1111/j.1432-1033.1970.tb00899.x. [DOI] [PubMed] [Google Scholar]
- KILBY B. A. The formation of beta-ketoadipic acid by bacterial fission of aromatic rings. Biochem J. 1951 Oct;49(5):671–674. doi: 10.1042/bj0490671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimian M., Ornston L. N. Participation of the beta-ketoadipate transport system in chemotaxis. J Gen Microbiol. 1981 May;124(1):25–28. doi: 10.1099/00221287-124-1-25. [DOI] [PubMed] [Google Scholar]
- Mesibov R., Adler J. Chemotaxis toward amino acids in Escherichia coli. J Bacteriol. 1972 Oct;112(1):315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Urban J. E., Bechtel D. B. Fine Structure of Succinate-Swollen Rhizobium trifolii 0403. Appl Environ Microbiol. 1984 May;47(5):1178–1181. doi: 10.1128/aem.47.5.1178-1181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Drift C., De Jong M. H. Chemotaxis toward amino acids in Bacillus subtilis. Arch Mikrobiol. 1974 Mar 4;96(2):83–92. [PubMed] [Google Scholar]


