Abstract
Taxol, a plant-derived antitumor agent, stabilizes microtubules. Taxol also elicits cell signals in a manner indistinguishable from bacterial lipopolysaccharide (LPS). LPS-like actions of Taxol are controlled by the lps gene and are independent of binding to the known Taxol target, β-tubulin. Using biotin-labeled Taxol, avidin-agarose affinity chromatography, and peptide mass fingerprinting, we identified two Taxol targets from mouse macrophages and brain as heat shock proteins (Hsps) of the 70- and 90-kDa families. Geldanamycin, a specific inhibitor of the Hsp 90 family, blocked the nuclear translocation of NF-κB and expression of tumor necrosis factor in macrophages treated with Taxol or with LPS. Geldanamycin did not block microtubule bundling by Taxol or macrophage activation by tumor necrosis factor. Thus, Taxol binds Hsps, and Hsp 90 helps mediate the activation of macrophages by Taxol and by LPS.
Taxol’s antitumor action is ascribed to its ability to block mitosis by binding and stabilizing microtubules (1, 2). However, Taxol also triggers cellular responses that mimic those induced by a potent activator of the innate immune system, bacterial lipopolysaccharide (LPS), such as the induction of cytokines and the activation of kinases and transcription factors (3–6). Such responses are seen in macrophages and carcinoma and myeloma cells, and in cells of both mouse and human origin (7, 8).
Three lines of evidence suggest the LPS-mimetic activity of Taxol is independent of binding to its known target, β-tubulin. First, macrophages from C3H/HeJ mice, an LPS-hyporesponsive strain that carries a defective allele of the lps gene, have a normal response to Taxol’s microtubule-bundling but not its LPS-mimetic activity (3–5). In recombinant inbred lines between C3H/HeJ and C57BL/6J (an LPS-normoresponsive strain), responsiveness to the LPS-mimetic effects of Taxol cosegregates with responses to LPS itself, even though Taxol neither contains nor structurally resembles LPS (3). Second, the LPS-like effects are cell cycle-independent and are not induced by certain analogs of Taxol, such as taxotere (Docetaxel), which bind β-tubulin with high affinity (9, 10). Third, LPS antagonists inhibit Taxol’s LPS-like actions but not its ability to bind microtubules (9). In addition, Taxol does not bind CD14, a well characterized cell-surface LPS receptor (11). Thus, Taxol is likely to bind a target(s) other than β-tubulin or CD14 that lies on a signaling pathway shared with LPS.
In low concentrations, LPS is the most potent known alarm to the immune system. In higher amounts, LPS induces systemic inflammatory response syndrome and the hallmarks of septic shock. Convergence of the LPS and Taxol signaling pathways provides an opportunity to gain insight into Taxol’s actions. At the same time, identification of a shared component of these convergent paths may lead to better understanding of cellular responses to LPS. Evidence presented below identifies members of the heat shock protein (Hsp) 90 and Hsp 70 families as Taxol targets and implicates the Hsp 90 family in Taxol’s LPS-mimetic effects as well as in LPS-mediated macrophage activation.
MATERIALS AND METHODS
Materials.
Recombinant human tumor necrosis factor (TNF) was from Genentech, I-κB antibody was from Santa Cruz Biotechnology, monoclonal antibody against α-tubulin was from ICN, and ImmunoPure monomeric avidin agarose beads were from Pierce. Tritiated LPS from Escherichia coli K12 (Rb chemotype, specific activity 2 × 106 dpm/μg) was from List Biological Laboratories (Campbell, CA). All other reagents were from Sigma. Macrophages were obtained as in ref. 12. II-45, an analog of geldanamycin in which the N—C1 bond underwent methanolysis and an aminobutyl group was substituted at position 17, was synthesized and kindly provided by S. Kuduk and S. Danishefsky, Memorial Sloan–Kettering Cancer Center.
Biotinylation of Taxol.
7-β-Alanyltaxol was prepared by the method of Nicolaou and co-workers (13) and reacted with 1.1 equivalents of N-hydroxysuccinimide biotin reagent, Biotin-XX-NHS (Calbiochem), in dimethylformamide containing 1.1 equivalents of triethylamine at room temperature for 12 h. Solvent was removed under reduced pressure, and the product was purified by preparative centrifugal chromatography on a Harrison Chromatotron, with precoated silica on aluminum sheets with gypsum (95:5 vol/vol CH2Cl2/MeOH), visualized with 5% phosphomolybdic acid in absolute ethanol, and characterized by standard analytical methods, including 1H NMR and 13C NMR (Bruker AMX-400) (data available on request).
Affinity Purification of Taxol-Binding Proteins.
RAW 264.7 lysate was prepared by freezing and thawing of 109 cells in 20 mM Tris⋅HCl, pH 8.0/140 mM NaCl/10% (vol/vol) glycerol/0.05% Triton X-100 containing 1 mM each of EDTA, dithiothreitol (DTT), and phenylmethylsulfonyl fluoride (PMSF) and 5 μg/ml each of aprotinin, leupeptin, pepstatin, and chymostatin (protease inhibitors); clarified at 15,000 × g; and precleared over a monomeric avidin column. Portions of precleared lysate (5.2 mg of protein each) were incubated with 100 μg/ml each Taxol (117 μM), biotinylated Taxol (73 μM), or biotin (400 μM). Complexes formed overnight at 4°C were applied to avidin-agarose columns, washed with phosphate-buffered saline (PBS) followed by PBS/0.6 M NaCl, reequilibrated with PBS, eluted with 4 mM biotin in PBS, and concentrated. Mouse brains were homogenized in 0.1 M Pipes, pH 7.0/10% glycerol containing 1 mM each of EGTA, MgSO4, Na3VO4, PMSF, and DTT and 5 μg/ml each of protease inhibitors; centrifuged; clarified; and precleared. Precleared homogenate (25 mg of protein) was used for affinity purification as above.
Mass Spectrometry.
Chromatographically isolated Taxol-binding polypeptides were separated by SDS/PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell). Bands visualized by amido black stain were subjected to tryptic digestion in situ (14). The resulting peptide mixture was loaded onto 2 μl of Poros 50 R2 (PerSeptive Biosystems) reversed-phase beads packed into an Eppendorf gel-loading tip and eluted stepwise with 4 μl of 16% (vol/vol) (and then with 4 μl of 30%) acetonitrile/0.1% formic acid. The “16%” and “30%” peptide pools were each analyzed twice by matrix-assisted laser desorption ionization-reflectron time-of-flight mass spectrometry (MALDI-reTOF MS), in the presence and absence of peptide calibrants (15), using a Reflex III (Brüker–Franzen) instrument equipped with a gridless pulse-extraction ion source with a 2-GHz digitizer and operated in reflector mode. Spectra were obtained by averaging 150 scans under constant irradiance. After recalibration with internal standards, monoisotopic masses were assigned for all prominent peaks and a peptide mass list was generated to search a nonredundant protein database (NRDB; European Bioinformatics Institute, Hinxton, U.K.) using the PeptideSearch (16) algorithm. For the 86-kDa polypeptide, only the “30%” pool was used because the masses obtained from the “16%” pool overlapped.
Taxol Binding by Pure Hsps.
Human Hsp 90α was expressed in insect cells from a baculovirus vector. Hsc 73 was purified from bovine brain (17). Ten micrograms of protein was incubated with 10 μM biotinylated Taxol in binding buffer (20 mM Tris⋅HCl, pH 7.4/140 mM NaCl/10% glycerol/1 mM EDTA/1 mM DTT) in a total volume of 50 μl for 6 h on ice (input) before addition of 20 μl of monomeric avidin beads and incubation overnight at 4°C. The beads were washed with 20 vol of binding buffer four times and eluted with 80 μl of binding buffer plus 2 mM biotin at room temperature for 3 h (elute). The samples were fractionated by SDS/PAGE and silver-stained.
Electrophoretic Mobility-Shift Assay (EMSA).
HeNC2 macrophages (12) were preincubated with geldanamycin, II-45, or matching concentrations of DMSO vehicle for 4 h before incubation with 10 μM Taxol, 10 ng/ml LPS, or 100 ng/ml human recombinant TNF for 30 min. Nuclear extracts were prepared and incubated with a probe prepared from the promoter of inducible nitric oxide synthase (18).
Northern Analysis.
Total RNA (25 μg) from RAW macrophages was pretreated with or without geldanamycin or II-45 (5 μM each) for 2 h, followed by a 90-min incubation with 10 μM Taxol or 10 ng/ml LPS. Samples were electrophoresed, transferred to nylon membranes (NEN), and hybridized with 32P-labeled TNF and β-actin probes sequentially.
Immunoblotting.
HeNC2 cells were incubated with 10 μM geldanamycin or corresponding concentrations of DMSO for the indicated times, lysed in RIPA buffer with protease inhibitors, separated by SDS/PAGE, transferred to nitrocellulose membranes, and blotted with antibodies for p50, p65, and I-κB.
Microtubule Bundling.
Thioglycolate-elicited mouse peritoneal macrophages were cultured on coverslips with or without 10 μM geldanamycin for 1 h followed by 10 μM Taxol for 2 h. Coverslips were fixed in 100% ice-cold methanol and stained with an anti-α-tubulin antibody followed by donkey anti-mouse IgG conjugated with Texas red.
Native PAGE.
Proteins (1 μg) and sonicated [3H]LPS (50 ng) were mixed in PBS with 1 mM EDTA and incubated at 37°C overnight. Samples were separated on 8% Tris-glycine polyacrylamide gels with running buffer containing 24 mM Tris/192 mM glycine, pH 8.3. Gels were fixed in 30% methanol/8% acetic acid/62% water for 1 h, soaked in EN3HANCE (DuPont NEN) for 1 h, rinsed, dried, and exposed to Kodak XAR film. Alternatively, gels were silver-stained.
RESULTS
To identify Taxol-binding polypeptides, we generated a site on Taxol that allowed elution from a solid-phase matrix independent of the interaction between Taxol and its binding polypeptide(s). Biotin was linked covalently to Taxol through a spacer at position 7, the only position at which substitutions permit retention of LPS-mimetic activity (10). Biotinylated Taxol (Fig. 1A), biotin, or Taxol was incubated with lysates of a macrophage cell line (RAW 264.7) or with mouse brain homogenates and passed through a monomeric avidin column (Fig. 1B). After extensive washing, specific Taxol-binding polypeptides were eluted with biotin. Polypeptides of apparent molecular mass of 90, 70, 50, and 35 kDa from mouse macrophages and these plus several additional species from mouse brain were visualized in silver-stained SDS gels of eluates from the mixtures containing biotinylated Taxol (Fig. 1C). All these polypeptides were absent from elutions of columns loaded with mixtures of lysates plus unmodified Taxol or biotin, ruling out that we had purified agarose-, avidin-, or biotin-binding proteins.
Figure 1.
Purification of Taxol-binding proteins. (A) Structure of biotinylated Taxol. (B) Scheme for purification of Taxol-binding proteins. BT, biotinylated Taxol; B, biotin; T, Taxol. (C) Profiles of isolated Taxol-binding polypeptides from mouse brain (Upper) and macrophage cell line (Lower) on silver-stained SDS/PAGE. Species with apparent molecular masses (kDa) in common are indicated by arrows. L, lysate or homogenate; P, precleared lysate or homogenate.
Major bands from mouse brain were subjected to tryptic digestion in situ (14). The resulting peptide mixtures were microcolumn purified and subjected to MALDI-reTOF MS (14–16). The 16 major peptide masses obtained from the spectra of the 70-kDa polypeptide (Fig. 2 A and B) were used as a fingerprint to query a nonredundant protein sequence database with requirements of a mass accuracy of 50 ppm or better and no more than one missed cleavage site. A single match was made, with 31% sequence coverage, conclusively identifying the 70-kDa polypeptide as the heat shock cognate 71-kDa protein (Hsp 71, Swiss-Prot P08109). The 90-kDa polypeptide was identified by using the same approach (Fig. 2C); 11 major peptide masses obtained were used to query the database and yielded one match, the tumor-specific transplantation antigen-presenting 86-kDa protein (Hsp 86, Swiss-Prot P07901) with 14% sequence coverage. A minor 55-kDa species was identified as β-tubulin, the only known target for Taxol (19). Hsp 71, Hsp 86, and β-tubulin were also identified from mouse macrophages by MS (Hsp 71) and by immunoblotting (Hsp 71, Hsp 86, β-tubulin) (data not shown). Proteins constituting most of the other bands purified by this technique were also identified by peptide mass fingerprinting, but only for Hsp 71 and 86 have we confirmed that their binding to Taxol is direct, and for Hsp 86 consequential, as shown below.
Figure 2.
Identification of the 70- and 90-kDa Taxol-binding polypeptides: Mass spectra for the 70-kDa polypeptide pools “16%” (A) and “30%” (B), and for the 90-kDa polypeptide pool “30%” (C). CAL, calibrant; M, matrix ion signal; T, trypsin autolytic product.
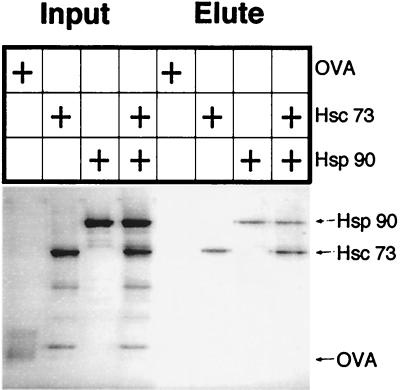
Hsp 71 and Hsp 86 are constitutively highly expressed members of the Hsp 70 and Hsp 90 heat shock protein families. In cells, they form complexes with each other and additional proteins (20). Therefore, it was important to see whether Hsp 71 and Hsp 86 each bound Taxol independently or by means of bridging proteins (20–22). Purified bovine Hsc 73, the homolog of Hsp 71 (86% amino acid identity), and pure, recombinant human Hsp 90α, the homolog of Hsp 86 (99% amino acid identity), were incubated alone or together in the presence of biotinylated Taxol, and the complexes were purified on monomeric avidin-agarose beads. Hsp 90α and Hsc 73, but not ovalbumin, each bound independently to Taxol and were eluted specifically with biotin (Fig. 3).
Figure 3.
Independent interaction of Taxol with Hsp 90α and Hsc 73. Hsc 73, Hsp 90α, and ovalbumin (OVA) input proteins and specific biotin eluates, indicated with arrows, were visualized on a silver-stained SDS gel.
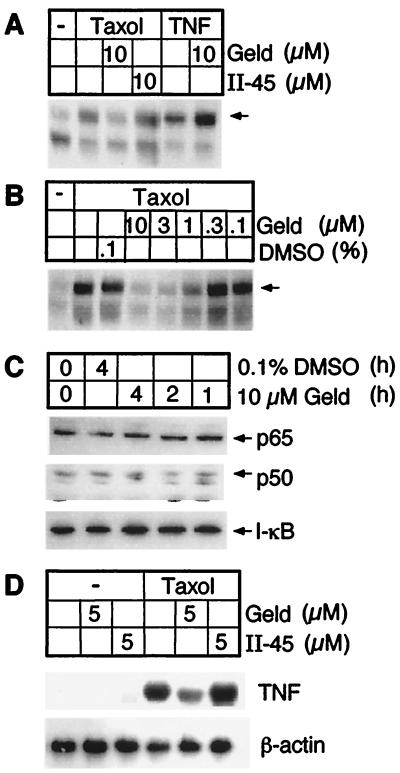
The Hsp 90 family is the only known specific target for the antitumor antibiotic geldanamycin (23), and human Hsp 90α has been crystallized with this inhibitor (24). Geldanamycin inhibits processes that require functional Hsp 90, such as regulation of endothelial nitric oxide synthase (25) and activation of src-tyrosine kinases (26) and steroid receptors (27). If Hsp 90 were important in Taxol signaling, geldanamycin might block Taxol-induced macrophage activation. Indeed, geldanamycin blocked the ability of Taxol to activate macrophage NF-κB (Fig. 4A), and this effect was dose-dependent (Fig. 4B). In contrast, even at 10 μM, geldanamycin did not inhibit activation of NF-κB by TNF (Fig. 4A), a stimulus that uses a different signal transduction pathway. This excludes that the effects of geldanamycin were toxic or nonspecific. An inactive analog of geldanamycin, II-45, did not inhibit Taxol-induced NF-κB activity (Fig. 4A). Moreover, inhibition of NF-κB activation by geldanamycin was not due to degradation of NF-κB, because incubation with geldanamycin did not affect cellular levels of p65, p50, or I-κB (Fig. 4C). Another LPS-mimetic activity of Taxol, induction of TNF expression in macrophages, was also inhibited by geldanamycin but not its analog II-45 (Fig. 4D).
Figure 4.
Geldanamycin inhibits Taxol-induced macrophage activation. (A) Taxol- but not TNF-induced NF-κB activation is inhibited by geldanamycin as analyzed by EMSA. The arrow indicates the active NF-κB complex p65/p50. (B) Geldanamycin’s effect on Taxol-induced NF-κB activation is dose dependent. (C) NF-κB signaling components are not degraded after geldanamycin treatment. Immunoblot of lysates from macrophages that had been incubated with geldanamycin or DMSO vehicle, blotted for NF-κB components p50, p65, as well as I-κB. (D) Geldanamycin inhibits Taxol-induced TNF gene expression as assessed by Northern blotting.
Pretreatment with 10 μM geldanamycin had no effect on Taxol-induced microtubule bundling in macrophages (Fig. 5). This showed that the inhibitory effect of geldanamycin was restricted to the LPS-mimetic subset of Taxol’s actions and argued that geldanmycin was unlikely to interact with Taxol directly.
Figure 5.
Geldanamycin does not inhibit Taxol’s bundling of microtubules. Macrophages were treated with geldanamycin (G), Taxol (T), or both, or left untreated and stained for α-tubulin. Arrowheads indicate bundled microtubules.
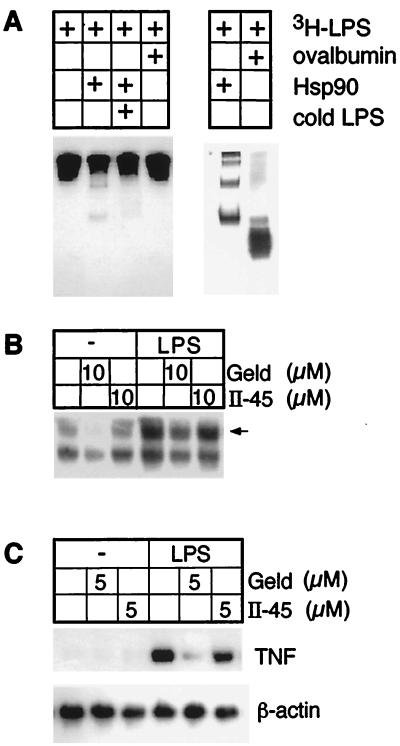
Since Hsp 90 was necessary for Taxol-induced macrophage activation, we asked whether Hsp 90 bound to LPS and whether it was involved in mediating macrophage activation by LPS. Radiolabeled LPS bound directly to Hsp 90, and unlabeled LPS competed for this binding (Fig. 6A). Moreover, geldanamycin, but not II-45, inhibited LPS-induced NF-κB activation (Fig. 6B) and TNF gene induction (Fig. 6C).
Figure 6.
Hsp 90 is involved in LPS-induced macrophage activation. (A) LPS binds Hsp 90 but not ovalbumin. Autoradiograph (Left) and silver stain (Right) of native gels for complexes of 3H-labeled LPS and Hsp 90 or ovalbumin with or without competition by unlabeled LPS in 100-fold excess. (B and C) Geldanamycin inhibits LPS-induced NF-κB activation (B) and TNF gene expression (C) as assessed by Northern blotting.
DISCUSSION
Hsps have numerous functions, such as helping proteins fold and presenting peptides to the immune system (28, 29). The Hsp 90 family appears to be unique, in that most of its known cellular targets are signal transducers—tyrosine kinases, serine/threonine kinases, transcription factors, and steroid receptors—whose structural instability is intrinsic to their action as molecular switches. The inactive forms are protected by binding to Hsp 90, whereas the active forms are stabilized by binding to specific activators (26, 27, 30). The interaction of geldanamycin with Hsp 90 causes some signaling molecules to be displaced and adopt an inactivatable conformation (31, 32). Alternatively, geldanamycin may stabilize target protein–Hsp 90 complexes, thereby preventing completion of maturation or refolding of the target (33). Our identification of the Hsp 90 family member Hsp 86 as a cellular target for Taxol, and the effects of geldanamycin on Taxol-induced activation of NF-κB and expression of TNF, indicate that Hsp 86 mediates LPS-mimetic actions of Taxol. Hsp 86 may likewise be involved in some of the other signaling elicited by Taxol.
Because Taxol’s LPS-mimetic actions are lps gene-dependent, and Hsp 86 is important for macrophage activation by both Taxol and LPS itself, it seems likely that Hsp 86 may interact in a functionally significant way with a pathway that involves the lps gene product. Identification of the mouse lps gene product as Toll-like receptor 4 (34, 35) has both clarified and raised many questions about LPS signaling. Extracellular LPS, whether self-associated in micelles, adsorbed to lipoproteins, or bound to soluble CD14, is transferred monomerically to cell-surface CD14 by the enzyme LPS-binding protein (36–38). Three processes ensue whose mutual relationship is undefined, although each is essential for LPS signaling and each is lps gene-dependent. First, monomeric LPS is rapidly internalized into nonlysosomal vesicles that subsequently converge on the Golgi complex (39–42). Second, numerous kinases and other enzymes are activated and transcription factors are mobilized (43). Third, the IL-1 receptor-like region of the intracellular domain of Toll-like receptors binds MyD88 and IL-1 receptor-associated kinase(s), leading to activation of TRAF6, NF-κB, and AP-1 (44–46). The present work suggests that Hsps may provide a link among these processes. One possibility under investigation is that Hsps could participate in LPS trafficking and signaling. Thus, Hsps on the surface and/or in the cytosol of mammalian cells might bind bacterial LPS, just as Hsps on the surface of bacteria bind certain mammalian glycolipids (47, 48). In this view, Hsps, which are highly conserved, may function bidirectionally in bacterial–eukaryotic recognition.
Structural studies of Taxol’s interaction with Hsps will be of interest to help explain how two antitumor drugs, geldanamycin and Taxol, can each bind specifically to Hsp 90 family members and exert predominantly inhibitory and predominantly stimulatory cellular actions, respectively. For example, geldanamycin blocks the action of endothelial nitric oxide synthase (49), whereas Taxol enhances the expression of inducible nitric oxide synthase (50). It will also be important to determine whether LPS interacts with Hsp 90 in the same manner as Taxol.
In sum, Hsp 71 and Hsp 86 are cellular targets for Taxol. Hsp 86 mediates at least some of the LPS-mimetic actions of Taxol and the activation of macrophages by LPS itself. These findings enlarge Taxol’s molecular pharmacology, suggest an additional function for Hsps, and open a fresh line of investigation into the mechanism of action of LPS.
Acknowledgments
We thank Scott Kuduk and Samuel Danishefsky (Sloan–Kettering Cancer Center) for II-45 synthesis, Jenny Zhang for technical assistance, and Hsiou-Chi Liou (Weill Medical College) for providing antibodies. We are indebted to Lynne Lacomis for help with mass spectrometry and to Prof. Matthias Mann (Odense University, Denmark) for the PeptideSearch program. This work was supported by National Institutes of Health grants to A.D. and C.F.N. and by a Cancer Research Institute predoctoral fellowship to C.A.B.
ABBREVIATIONS
- EMSA
electrophoretic mobility-shift assay
- Hsp
heat shock protein
- LPS
bacterial lipopolysaccharide
- MALDI-reTOF MS
matrix-assisted laser-desorption/ionization reflectron time-of-flight mass spectrometry
- TNF
tumor necrosis factor
Note Added in Proof
After this paper was submitted, Rodi et al. (51) reported using phage-displayed peptides to identify Bcl-2 as a Taxol-binding protein. Bcl-2 was not among the proteins identified in the present study.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Wani M C, Taylor H L, Wall M E. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.Schiff P B, Horwitz S B. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding A, Porteu F, Sanchez E, Nathan C F. Science. 1990;248:370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- 4.Manthey C L, Brandes M E, Perera P Y, Vogel S N. J Immunol. 1992;149:2459–2465. [PubMed] [Google Scholar]
- 5.Ding A, Sanchez E, Nathan C. J Immunol. 1993;151:5596–5602. [PubMed] [Google Scholar]
- 6.Hwang S, Ding A. Cancer Biochem Biophys. 1995;14:265–272. [PubMed] [Google Scholar]
- 7.Lee L F, Schuerer-Maly C C, Lofquist A K, van Haaften-Day C, Ting J P, White C M, Martin B K, Haskill J S. Cancer Res. 1996;56:1303–1308. [PubMed] [Google Scholar]
- 8.White C M, Martin B K, Lee L F, Haskill J S, Ting J P Y. Cancer Immunol Immunother. 1998;46:104–112. doi: 10.1007/s002620050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manthey C L, Qureshi N, Stutz P, Vogel S. J Exp Med. 1993;178:695–702. doi: 10.1084/jem.178.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhart C A, Berman J W, Swindell C S, Horwitz S B. Cancer Res. 1994;54:5779–5782. [PubMed] [Google Scholar]
- 11.Kirikae F, Kirikae T, Qureshi N, Takayama K, Morrison D C, Nakano M. Infect Immun. 1995;63:486–497. doi: 10.1128/iai.63.2.486-497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin F Y, Nathan C, Radzioch D, Ding A. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 13.Guy R K, Scott Z A, Sloboda R D, Nicolaou K C. Chem Biol. 1996;3:1021–1031. doi: 10.1016/s1074-5521(96)90168-4. [DOI] [PubMed] [Google Scholar]
- 14.Lui M, Tempst P, Erdjument-Bromage H. Anal Biochem. 1996;241:156–166. doi: 10.1006/abio.1996.0393. [DOI] [PubMed] [Google Scholar]
- 15.Erdjument-Bromage H, Lui M, Lacomis L, Grewal A, Annan R S, McNulty D E, Carr S A, Tempst P. J Chromatogr. 1998;826:167–181. doi: 10.1016/s0021-9673(98)00705-5. [DOI] [PubMed] [Google Scholar]
- 16.Mann M, Hojrup P, Roepstorff P. Biol Mass Spectrom. 1993;22:338–345. doi: 10.1002/bms.1200220605. [DOI] [PubMed] [Google Scholar]
- 17.Schlossman D M, Schmid S L, Braell W A, Rothman J E. J Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Q-w, Kashiwabara Y, Nathan C. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 19.Rao S, Horwitz S B, Ringel I. J Natl Cancer Inst. 1992;82:785–788. doi: 10.1093/jnci/84.10.785. [DOI] [PubMed] [Google Scholar]
- 20.Smith D F, Sullivan W P, Marion T N, Zaitsu K, Madden B, McCormick D J, Toft D O. Mol Cell Biol. 1993;13:869–876. doi: 10.1128/mcb.13.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez C, Padilla R, Paciucci R, Zabala J, Avila J. Arch Biochem Biophys. 1994;310:428–432. doi: 10.1006/abbi.1994.1188. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez E, Redmond T, Scherrer L, Bresnick E, Welsh M, Pratt W. Mol Endocrinol. 1988;2:756–760. doi: 10.1210/mend-2-8-756. [DOI] [PubMed] [Google Scholar]
- 23.Grenert J P, Sullivan W P, Fadden P, Haystead T A J, Clark J, Mimnaugh E, Krutzsch H, Ochel H J, Schulte T W, Sausville E, et al. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 24.Stebbins C E, Russo A A, Schneider C, Rosen N, Hartl F U, Pavletich N P. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Cardeña G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa W C. Nature (London) 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Lindquist S. Proc Natl Acad Sci USA. 1993;90:7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picard D, Khursheed B, Garabedian M J, Fortin M G, Lindquist S, Yamamoto K R. Nature (London) 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 28.Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava P K, Menoret A, Basu S, Binder R J, McQuade K L. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 30.Rutherford S L, Lindquist S. Nature (London) 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- 31.Whitesell L, Mimnaugh E G, De Costa B, Myers C E, Neckers L M. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitesell L, Cook P. Mol Endocrinol. 1996;10:705–712. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]
- 33.Schneider C, Sepp-Lerenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl F. Proc Natl Acad Sci USA. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, et al. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi S T, Larivière L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wurfel M M, Hailman E, Wright S D. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurfel M M, Wright S D. J Immunol. 1997;158:3925–3934. [PubMed] [Google Scholar]
- 39.Detmers P A, Thieblemont N, Vasselon T, Pironkova R, Miller D S, Wright S D. J Immunol. 1996;157:5589–5596. [PubMed] [Google Scholar]
- 40.Thieblemont N, Wright S D. J Exp Med. 1997;185:2095–2100. doi: 10.1084/jem.185.12.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitchens R L, Wang P-y, Munford R S. J Immunol. 1998;161:5534–5545. [PubMed] [Google Scholar]
- 42.Thieblemont N, Thieringer R, Wright S D. Immunity. 1998;8:771–777. doi: 10.1016/s1074-7613(00)80582-8. [DOI] [PubMed] [Google Scholar]
- 43.Sweet M J, Hume D A. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 44.Medzhitov R, Prestonhurlburt P, Kopp E, Stadlen A, Chen C Q, Ghosh S, Janeway C A. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 45.Yang R-B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Nature (London) 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 46.Kirschning C J, Wesche H, Ayres T M, Rothe M. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erkeller-Yuksel F M, Isenberg D A, Dhillon V B, Latchman D S, Lydyard P M. J Autoimmun. 1992;5:803–814. doi: 10.1016/0896-8411(92)90194-u. [DOI] [PubMed] [Google Scholar]
- 48.Huesca M, Goodwin A, Bhagwansingh A, Hoffman P, Lingwood C A. Infect Immun. 1998;66:4061–4067. doi: 10.1128/iai.66.9.4061-4067.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joly G A, Ayres M, Kilbourn R. FEBS Lett. 1997;403:40–44. doi: 10.1016/s0014-5793(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 50.Manthey C L, Perera P Y, Salkowski C A, Vogel S N. J Immunol. 1994;152:825–831. [PubMed] [Google Scholar]
- 51.Rodi D J, Janes R W, Sanganee H J, Holton R A, Wallace B A, Makowski L. J Mol Biol. 1999;285:197–203. doi: 10.1006/jmbi.1998.2303. [DOI] [PubMed] [Google Scholar]