Abstract
The beta-lactamases of Bacillus cereus have attracted interest because they are secreted efficiently, because multiple enzymes are frequently present, and because their regulation has unusual features. beta-Lactamase I of strain 5/B is produced constitutively at a high level, and the exoenzyme appears to be several thousand daltons larger than the corresponding product of strain 569/H. We have cloned the gene for 5/B beta-lactamase I in Escherichia coli and B. subtilis and have sequenced the structural portion and the regulatory regions. The 5/B enzyme is produced at a low level in E. coli RR1(pRWY200) and remains cellbound. In B. subtilis it is formed in large amounts, and over 90% of it is released into the medium. There is a large degree of homology between the promoter and leader peptide regions of the 5/B and 569/H genes; both utilize UUG as the translation initiation codon (P. S. F. Mézes, R. W. Blacher, and J. O. Lampen, (J. Biol. Chem. 260:1218-1223, 1985). Although there are significant differences in the peptide segment where processing would be expected to occur, the NH2 terminus of the major 5/B product from B. subtilis BD170(pRWY215) is His-44, which is the same as the NH2 terminus of the major 569/H product from B. subtilis BD170(pRWM5).
Full text
PDF

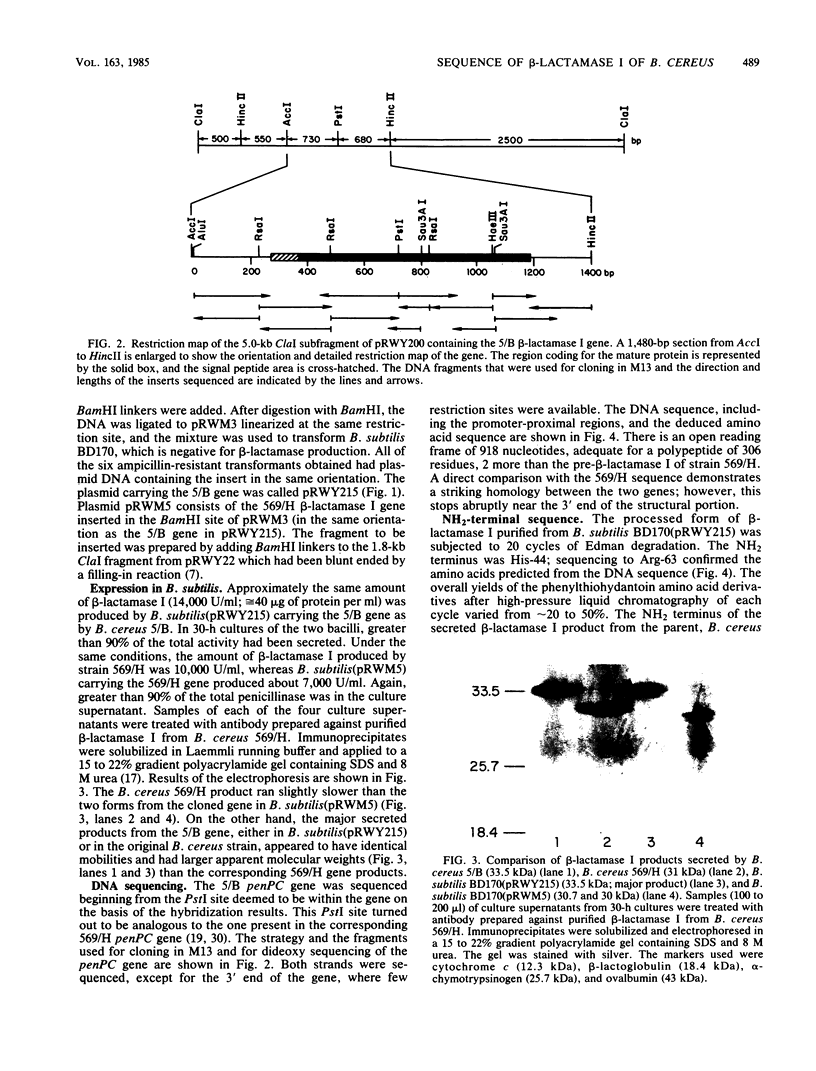



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly A. K., Waley S. G. Characterization of the membrane beta-lactamase in Bacillus cereus 569/H/9. Biochemistry. 1983 Sep 27;22(20):4647–4651. doi: 10.1021/bi00289a006. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Izui K., Nielsen J. B., Caulfield M. P., Lampen J. O. Large exopenicillinase, initial extracellular form detected in cultures of Bacillus licheniformis. Biochemistry. 1980 Apr 29;19(9):1882–1886. doi: 10.1021/bi00550a023. [DOI] [PubMed] [Google Scholar]
- KOGUT M., POLLOCK M. R., TRIDGELL E. J. Purification of penicillin-induced penicillinase of Bacillus cereus NRRL 569: a comparison of its properties with those of a similarly purified penicillinase produced spontaneously by a constitutive mutant strain. Biochem J. 1956 Mar;62(3):391–401. doi: 10.1042/bj0620391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Mézes P. S., Blacher R. W., Lampen J. O. Processing of Bacillus cereus 569/H beta-lactamase I in Escherichia coli and Bacillus subtilis. J Biol Chem. 1985 Jan 25;260(2):1218–1223. [PubMed] [Google Scholar]
- Mézes P. S., Wang W., Yeh E. C., Lampen J. O. Construction of penP delta 1, Bacillus licheniformis 749/C beta-lactamase lacking site for lipoprotein modification. Expression in Escherichia coli and Bacillus subtilis. J Biol Chem. 1983 Sep 25;258(18):11211–11218. [PubMed] [Google Scholar]
- Mézes P. S., Yang Y. Q., Hussain M., Lampen J. O. Bacillus cereus 569/H beta-lactamase I: cloning in Escherichia coli and signal sequence determination. FEBS Lett. 1983 Sep 19;161(2):195–200. doi: 10.1016/0014-5793(83)81006-0. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Beta-lactamase III of Bacillus cereus 569: membrane lipoprotein and secreted protein. Biochemistry. 1983 Sep 27;22(20):4652–4656. doi: 10.1021/bi00289a007. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. The cell-bound penicillinase of Bacillus cereus. J Gen Microbiol. 1956 Aug;15(1):154–169. doi: 10.1099/00221287-15-1-154. [DOI] [PubMed] [Google Scholar]
- SNEATH P. H. Proof of the spontaneity of a mutation to penicillinase production in Bacillus cereus. J Gen Microbiol. 1955 Dec;13(3):561–568. doi: 10.1099/00221287-13-3-561. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt D. J., Collins J. F. Analysis by transformation of the penicillinase system in Bacillus licheniformis. J Gen Microbiol. 1973 May;76(1):217–230. doi: 10.1099/00221287-76-1-217. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Sloma A., Gross M. Molecular cloning and nucleotide sequence of the type I beta-lactamase gene from Bacillus cereus. Nucleic Acids Res. 1983 Jul 25;11(14):4997–5004. doi: 10.1093/nar/11.14.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R. The partial amino acid sequence of the extracellular beta-lactamase I of Bacillus cereus 569/H. Biochem J. 1975 May;147(2):313–326. doi: 10.1042/bj1470313. [DOI] [PMC free article] [PubMed] [Google Scholar]




