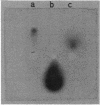
Abstract
The coupling of membrane-bound glucose dehydrogenase (EC 1.1.99.17) to the respiratory chain has been studied in whole cells, cell-free extracts, and membrane vesicles of gram-negative bacteria. Several Escherichia coli strains synthesized glucose dehydrogenase apoenzyme which could be activated by the prosthetic group pyrrolo-quinoline quinone. The synthesis of the glucose dehydrogenase apoenzyme was independent of the presence of glucose in the growth medium. Membrane vesicles of E. coli, grown on glucose or succinate, oxidized glucose to gluconate in the presence of pyrrolo-quinoline quinone. This oxidation led to the generation of a proton motive force which supplied the driving force for uptake of lactose, alanine, and glutamate. Reconstitution of glucose dehydrogenase with limiting amounts of pyrrolo-quinoline quinone allowed manipulation of the rate of electron transfer in membrane vesicles and whole cells. At saturating levels of pyrrolo-quinoline quinone, glucose was the most effective electron donor in E. coli, and glucose oxidation supported secondary transport at even higher rates than oxidation of reduced phenazine methosulfate. Apoenzyme of pyrrolo-quinoline quinone-dependent glucose dehydrogenases with similar properties as the E. coli enzyme were found in Acinetobacter calcoaceticus (var. lwoffi) grown aerobically on acetate and in Pseudomonas aeruginosa grown anaerobically on glucose and nitrate.
Full text
PDF

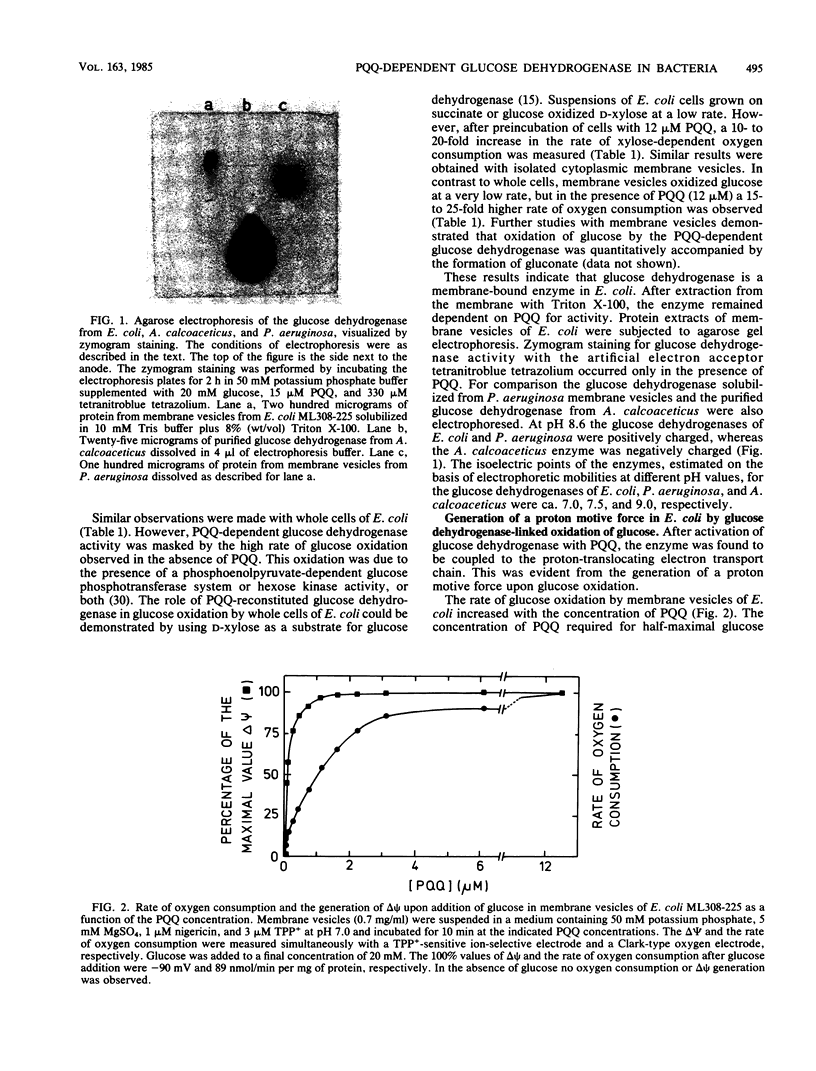
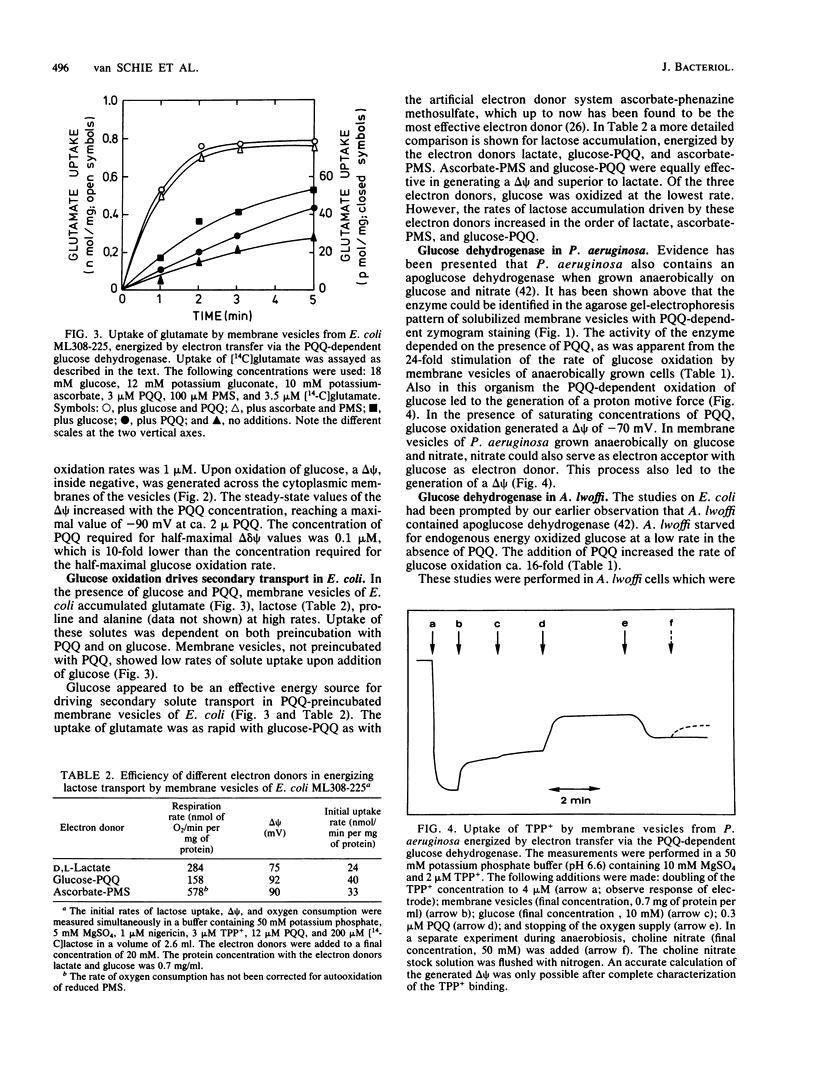
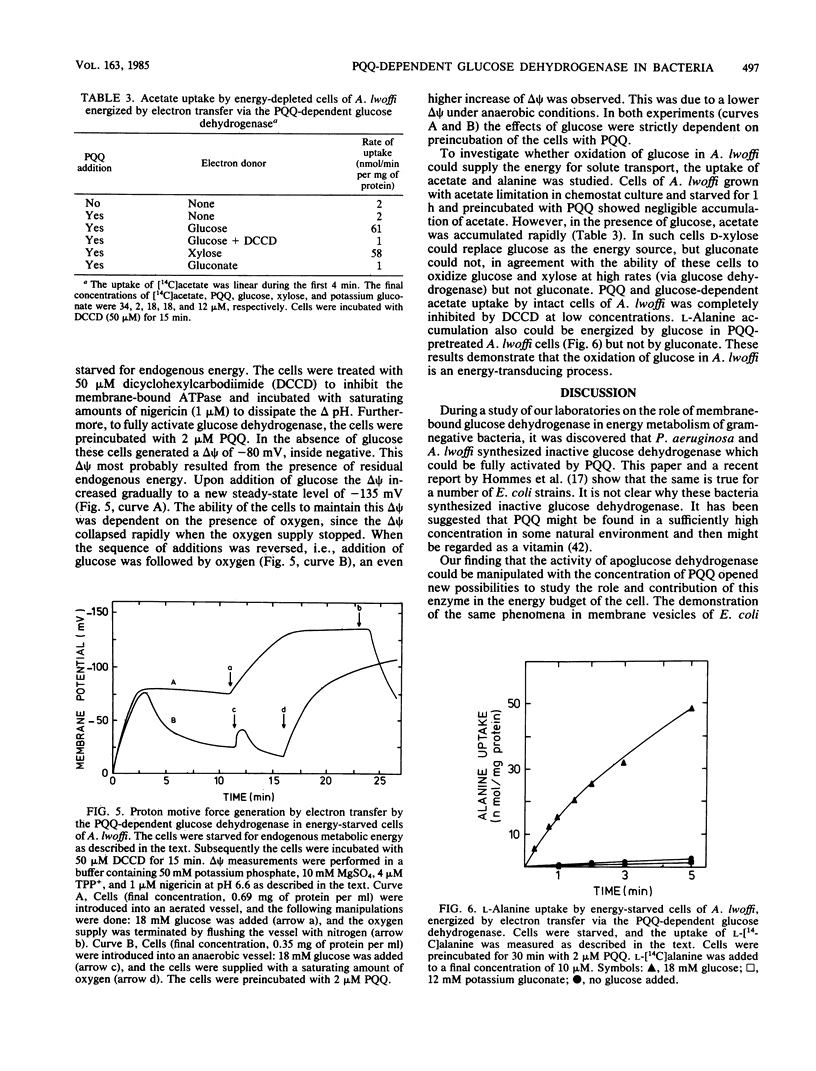


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamforth C. W., Quayle J. R. Aerobic and anaerobic growth of Paracoccus denitrificans on methanol. Arch Microbiol. 1978 Oct 4;119(1):91–97. doi: 10.1007/BF00407934. [DOI] [PubMed] [Google Scholar]
- DALBY A., BLACKWOOD A. C. Oxidation of sugars by an enzyme preparation from Aerobacter aerogenes. Can J Microbiol. 1955 Dec;1(9):733–742. doi: 10.1139/m55-087. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. C., Dobrogosz W. J. Gluconate metabolism in Escherichia coli. J Bacteriol. 1967 Mar;93(3):941–949. doi: 10.1128/jb.93.3.941-949.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink M. G., Hellingwerf K. J., Michels P. A., Seÿen H. G., Konings W. N. Immunochemical analysis of membrane vesicles and chromatophoresis of Rhodopseudomonas sphaeroides by crossed immunoelectrophoresis. FEBS Lett. 1979 Nov 15;107(2):300–307. doi: 10.1016/0014-5793(79)80395-6. [DOI] [PubMed] [Google Scholar]
- Futai M. Stimulation of transport into Escherichia coli membrane vesicles by internally generated reduced nictotinamide adenine dinucleotide. J Bacteriol. 1974 Nov;120(2):861–865. doi: 10.1128/jb.120.2.861-865.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUGE J. G. Kinetics and specificity of glucose dehydrogenase from Bacterium anitratum. Biochim Biophys Acta. 1960 Dec 4;45:263–269. doi: 10.1016/0006-3002(60)91450-5. [DOI] [PubMed] [Google Scholar]
- HAUGE J. G. Purification and properties of glucose dehydrogenase and cytochrome b from Bacterium anitratum. Biochim Biophys Acta. 1960 Dec 4;45:250–262. doi: 10.1016/0006-3002(60)91449-9. [DOI] [PubMed] [Google Scholar]
- Hellingwerf K. J., Bolscher J. G., Konings W. N. The electrochemical proton gradient generated by the fumarate-reductase system in Escherichia coli and its bioenergetic implications. Eur J Biochem. 1981 Jan;113(2):369–374. doi: 10.1111/j.1432-1033.1981.tb05075.x. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Sodium-dependent transport of L-leucine in membrane vesicles prepared from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):73–81. doi: 10.1128/jb.137.1.73-81.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Edwards C. The effect of respiratory chain composition on the growth efficiencies of aerobic bacteria. Arch Microbiol. 1977 Oct 24;115(1):85–93. doi: 10.1007/BF00427850. [DOI] [PubMed] [Google Scholar]
- Juni E. Genetics and physiology of Acinetobacter. Annu Rev Microbiol. 1978;32:349–371. doi: 10.1146/annurev.mi.32.100178.002025. [DOI] [PubMed] [Google Scholar]
- Kasprzak A. A., Steenkamp D. J. Localization of the major dehydrogenases in two methylotrophs by radiochemical labeling. J Bacteriol. 1983 Oct;156(1):348–353. doi: 10.1128/jb.156.1.348-353.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of beta-galactosides and amino acids. J Biol Chem. 1971 Oct 10;246(19):5857–5861. [PubMed] [Google Scholar]
- Konings W. N. Energization of solute transport in membrane vesicles from anaerobically grown bacteria. Methods Enzymol. 1979;56:378–388. doi: 10.1016/0076-6879(79)56035-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lockwood L. B., Tabenkin B., Ward G. E. The Production of Gluconic Acid and 2-Keto-Gluconic Acid from Glucose by Species of Pseudomonas and Phytomonas. J Bacteriol. 1941 Jul;42(1):51–61. doi: 10.1128/jb.42.1.51-61.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolkema J. S., Abbing A., Hellingwerf K. J., Konings W. N. The transmembrane electrical potential in Rhodopseudomonas sphaeroides determined from the distribution of tetraphenylphosphonium after correction for its binding to cell components. Eur J Biochem. 1983 Feb 1;130(2):287–292. doi: 10.1111/j.1432-1033.1983.tb07149.x. [DOI] [PubMed] [Google Scholar]
- Midgley M., Dawes E. A. The regulation of transport of glucose and methyl alpha-glucoside in Pseudomonas aeruginosa. Biochem J. 1973 Feb;132(2):141–154. doi: 10.1042/bj1320141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. G., Dawes E. A. The role of oxygen in the regulation of glucose metabolism, transport and the tricarboxylic acid cycle in Pseudomonas aeruginosa. J Gen Microbiol. 1982 Jan;128(1):49–59. doi: 10.1099/00221287-128-1-49. [DOI] [PubMed] [Google Scholar]
- NIEDERPRUEM D. J., DOUDOROFF M. COFACTOR-DEPENDENT ALDOSE DEHYDROGENASE OF RHODOPSEUDOMONAS SPHEROIDES. J Bacteriol. 1965 Mar;89:697–705. doi: 10.1128/jb.89.3.697-705.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, McCowen S. M., Feary T. W., Blevins W. T. Mannitol and fructose catabolic pathways of Pseudomonas aeruginosa carbohydrate-negative mutants and pleiotropic effects of certain enzyme deficiencies. J Bacteriol. 1978 Feb;133(2):717–728. doi: 10.1128/jb.133.2.717-728.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehl R. A., Feary T. W., Phibbs P. V., Jr Clustering of mutations affecting central pathway enzymes of carbohydrate catabolism in Pseudomonas aeruginosa. J Bacteriol. 1983 Dec;156(3):1123–1129. doi: 10.1128/jb.156.3.1123-1129.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Guymon L. F., Eagon R. G. A novel technique for the preparation of transport-active membrane vesicles from Pseudomonas aeruginosa: observations on gluconate transport. Biochem Biophys Res Commun. 1973 May 1;52(1):285–290. doi: 10.1016/0006-291x(73)90985-6. [DOI] [PubMed] [Google Scholar]
- Verwiel P. E., Frank J., Verwiel E. J. Characterization of the second prosthetic group in methanol dehydrogenase from hyphomicrobium X. Eur J Biochem. 1981 Aug;118(2):395–399. doi: 10.1111/j.1432-1033.1981.tb06415.x. [DOI] [PubMed] [Google Scholar]