Abstract
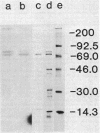
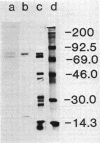
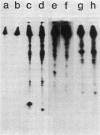
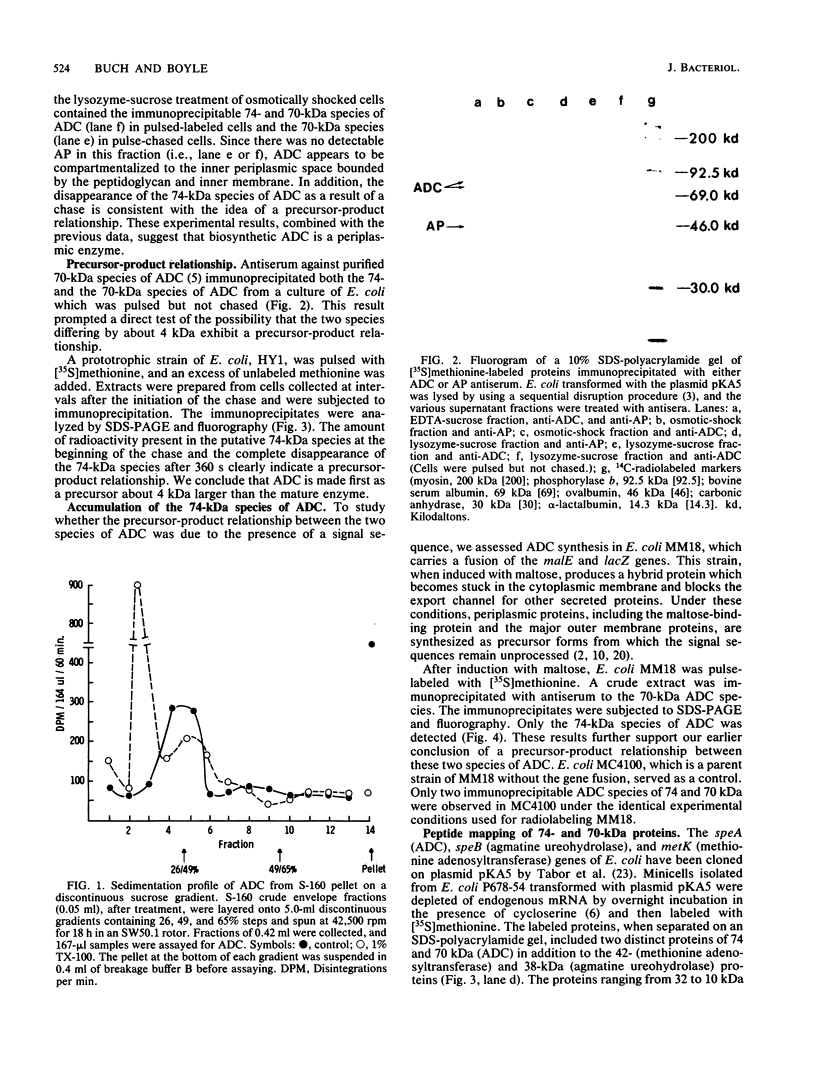
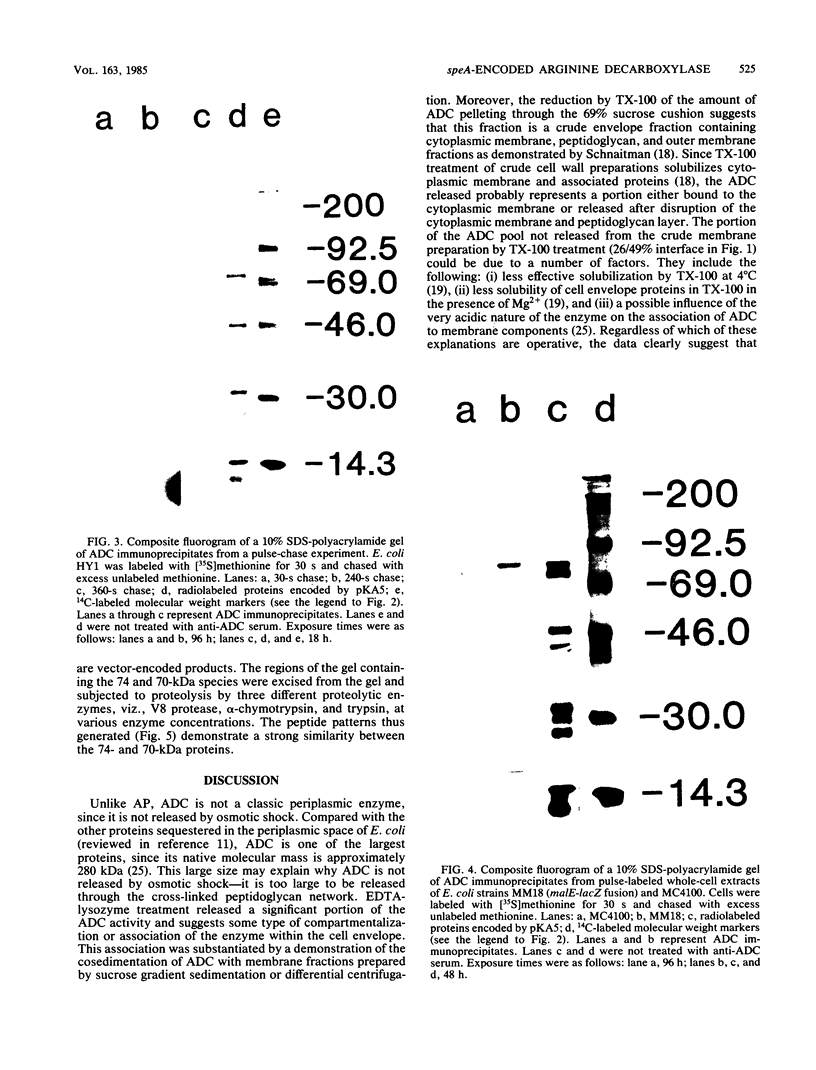
The biosynthetic form of arginine decarboxylase (ADC) catalyzes the synthesis of agmatine, a precursor of putrescine, in Escherichia coli. Selective disruption of the cell envelope and an assessment of ADC activity or immunoprecipitable ADC in various fractions demonstrated its location between the cytoplasmic membrane and peptidoglycan layer. Expression in minicells of the speA gene encoding ADC resulted in the production of two immunoprecipitable species (74 and 70 kilodaltons). Studies in vivo with a pulse and chase of radiolabeled amino acid into the two species suggest a precursor-product relationship. This relationship was corroborated by demonstrating the accumulation of the 74-kilodalton species in a strain of E. coli unable to process signal sequences. Peptide mapping experiments with V8 protease, trypsin, and alpha-chymotrypsin demonstrated that the two species of ADC were very similar except for a minor difference. These data were used to substantiate the compartmentalization hypothesis as to how exogenous arginine can be channeled preferentially into putrescine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick M. A., Lo T. C. Localization of the dicarboxylate binding protein in the cell envelope of Escherichia coli K12. Can J Biochem. 1980 Oct;58(10):885–897. doi: 10.1139/o80-123. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., Adachi K. Biosynthetic ornithine and arginine decarboxylases: correlation of rates of synthesis with activities in Escherichia coli during exponential growth and following nutritional shift-up. Can J Microbiol. 1982 Aug;28(8):945–950. doi: 10.1139/m82-142. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., Markham G. D., Hafner E. W., Wright J. M., Tabor H., Tabor C. W. Expression of the cloned genes encoding the putrescine biosynthetic enzymes and methionine adenosyltransferase of Escherichia coli (speA, speB, speC and metK). Gene. 1984 Oct;30(1-3):129–136. doi: 10.1016/0378-1119(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W., Markham G. D., Boyle S. M. Cloning of the Escherichia coli genes for the biosynthetic enzymes for polyamines. Methods Enzymol. 1983;94:117–121. doi: 10.1016/s0076-6879(83)94019-3. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Partial separation of two pools of arginine in Escherichia coli; preferential use of exogenous rather than endogenous arginine for the biosynthesis of 1,4-diaminobutane. J Biol Chem. 1969 Dec 10;244(23):6383–6387. [PubMed] [Google Scholar]
- Wright J. M., Boyle S. M. Negative control of ornithine decarboxylase and arginine decarboxylase by adenosine-3':5'-cyclic monophosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):482–487. doi: 10.1007/BF00337952. [DOI] [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]