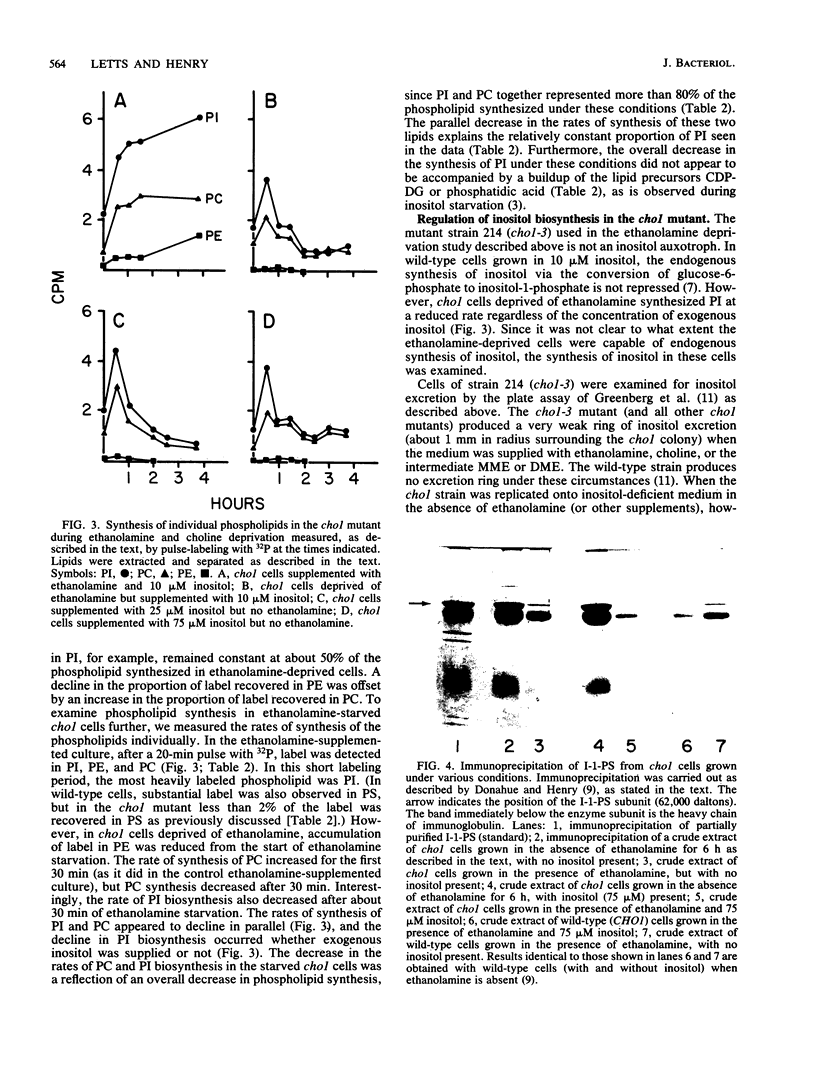
Abstract
chol mutants of Saccharomyces cerevisiae are deficient in the synthesis of the phospholipid phosphatidylserine owing to lowered activity of the membrane-associated enzyme phosphatidylserine synthase. chol mutants are auxotrophic for ethanolamine or choline and, in the absence of these supplements, cannot synthesize phosphatidylethanolamine or phosphatidylcholine (PC). We exploited these characteristics of the chol mutants to examine the regulation of phospholipid metabolism in S. cerevisiae. Macromolecular synthesis and phospholipid metabolism were examined in chol cells starved for ethanolamine. As expected, when chol mutants were starved for ethanolamine, the rates of synthesis of the phospholipids phosphatidylethanolamine and PC declined rapidly. Surprisingly, however, coupled to the decline in PC biosynthesis was a simultaneous decrease in the overall rate of phospholipid synthesis. In particular, the rate of synthesis of phosphatidylinositol decreased in parallel with the decline in PC biosynthesis. The results obtained suggest that the slowing of PC biosynthesis in ethanolamine-starved chol cells leads to a coordinated decrease in the synthesis of all phospholipids. However, under conditions of ethanolamine deprivation in chol cells, the cytoplasmic enzyme inositol-1-phosphate synthase could not be repressed by exogenous inositol, and the endogenous synthesis of the phospholipid precursor inositol appeared to be elevated. The implications of these findings with respect to the coordinated regulation of phospholipid synthesis are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K. D., Jensen B., Kolat A. I., Storm E. M., Henry S. A., Fogel S. Yeast mutants auxotrophic for choline or ethanolamine. J Bacteriol. 1980 Feb;141(2):558–564. doi: 10.1128/jb.141.2.558-564.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson K., Fogel S., Henry S. A. Yeast mutant defective in phosphatidylserine synthesis. J Biol Chem. 1980 Jul 25;255(14):6653–6661. [PubMed] [Google Scholar]
- Becker G. W., Lester R. L. Changes in phospholipids of Saccharomyces cerevisiae associated with inositol-less death. J Biol Chem. 1977 Dec 10;252(23):8684–8691. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carson M. A., Atkinson K. D., Waechter C. J. Properties of particulate and solubilized phosphatidylserine synthase activity from Saccharomyces cerevisiae. Inhibitory effect of choline in the growth medium. J Biol Chem. 1982 Jul 25;257(14):8115–8121. [PubMed] [Google Scholar]
- Carson M. A., Emala M., Hogsten P., Waechter C. J. Coordinate regulation of phosphatidylserine decarboxylase activity and phospholipid N-methylation in yeast. J Biol Chem. 1984 May 25;259(10):6267–6273. [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae: properties of a repressible enzyme system in extracts of wild-type (Ino+) cells. J Bacteriol. 1976 Apr;126(1):232–242. doi: 10.1128/jb.126.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Henry S. A. myo-Inositol-1-phosphate synthase. Characteristics of the enzyme and identification of its structural gene in yeast. J Biol Chem. 1981 Jul 10;256(13):7077–7085. [PubMed] [Google Scholar]
- Greenberg M. L., Klig L. S., Letts V. A., Loewy B. S., Henry S. A. Yeast mutant defective in phosphatidylcholine synthesis. J Bacteriol. 1983 Feb;153(2):791–799. doi: 10.1128/jb.153.2.791-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. L., Reiner B., Henry S. A. Regulatory mutations of inositol biosynthesis in yeast: isolation of inositol-excreting mutants. Genetics. 1982 Jan;100(1):19–33. doi: 10.1093/genetics/100.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Klig L. S., Loewy B. S. The genetic regulation and coordination of biosynthetic pathways in yeast: amino acid and phospholipid synthesis. Annu Rev Genet. 1984;18:207–231. doi: 10.1146/annurev.ge.18.120184.001231. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Brody S. Glycerophospholipid variation in choline and inositol auxotrophs of Neurospora crassa. Internal compensation among zwitterionic and anionic species. J Biol Chem. 1975 Sep 25;250(18):7173–7181. [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- Letts V. A., Dawes I. W. Mutations affecting lipid biosynthesis of Saccharomyces cerevisiae: isolation of ethanolamine auxotrophs [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):976–977. doi: 10.1042/bst0070976. [DOI] [PubMed] [Google Scholar]
- Letts V. A., Klig L. S., Bae-Lee M., Carman G. M., Henry S. A. Isolation of the yeast structural gene for the membrane-associated enzyme phosphatidylserine synthase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7279–7283. doi: 10.1073/pnas.80.23.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegren G., Hwang Y. L., Oshima Y., Lindegren C. C. Genetical mutants induced by ethyl methanesulfonate in Saccharomyces. Can J Genet Cytol. 1965 Sep;7(3):491–499. doi: 10.1139/g65-064. [DOI] [PubMed] [Google Scholar]
- Loewy B. S., Henry S. A. The INO2 and INO4 loci of Saccharomyces cerevisiae are pleiotropic regulatory genes. Mol Cell Biol. 1984 Nov;4(11):2479–2485. doi: 10.1128/mcb.4.11.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Differential regulation of the N-methyl transferases responsible for phosphatidylcholine synthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):401–410. doi: 10.1016/0003-9861(73)90637-1. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Oshima A., Nikawa J., Hosaka K. Regulation of the phosphatidylethanolamine methylation pathway in Saccharomyces cerevisiae. Eur J Biochem. 1982 Nov 15;128(2-3):589–595. doi: 10.1111/j.1432-1033.1982.tb07005.x. [DOI] [PubMed] [Google Scholar]