Abstract
The pathogenesis of tuberculous meningitis, a devastating complication of tuberculosis in man, is poorly understood. We previously reported that rabbits with experimental tuberculous meningitis were protected from death by a combination of antibiotics and thalidomide therapy. Survival was associated with inhibition of tumor necrosis factor α (TNF-α) production by thalidomide. To test whether cerebrospinal fluid (CSF) levels of TNF-α correlated with pathogenesis, the response of rabbits infected in the central nervous system (CNS) with various mycobacterial strains was studied. CNS infection with Mycobacterium bovis Ravenel, M. bovis bacillus Calmette–Guérin (BCG) Pasteur, and M. bovis BCG Montreal were compared. M. bovis Ravenel induced the highest levels of TNF-α in the CSF in association with high leukocytosis, protein accumulation, and severe meningeal inflammation. BCG Pasteur had intermediate effects, and BCG Montreal was the least virulent. In addition, M. bovis Ravenel numbers were highest in the brain and CSF and the bacilli also disseminated more efficiently to distant organs, compared with BCG Pasteur and BCG Montreal. In subsequent experiments, rabbits were infected with either recombinant M. bovis BCG Montreal (vector), or BCG Montreal expressing the murine gene for TNF-α (BCG mTNF-α). BCG Montreal was rendered virulent by the expression of murine TNF-α, as demonstrated by high CSF leukocytosis, high protein accumulation, severe meningeal inflammation, persistent bacillary load, and progressive clinical deterioration. Taken together, these results demonstrate that the level of TNF-α produced during mycobacterial CNS infection determines, at least in part, the extent of pathogenesis.
Tuberculosis of the central nervous system (CNS) is one of the most serious presentations of the disease. In children, tuberculous meningitis (TBM) is associated with about 50% mortality, and most of the survivors have permanent neurologic sequelae and experience considerable disability (1–4). In spite of the severity of the clinical presentation, the cellular and molecular mechanisms that underlie the pathogenesis of mycobacterial disease in the CNS are poorly understood.
We previously reported on a rabbit model of experimental TBM (5). When rabbits were infected with high doses of mycobacteria in the CNS, they succumbed to the infection within a few days. In this model, treatment of infected rabbits with standard antituberculous antibiotics did not protect the animals from meningitis-associated death. However, the combination of antituberculous drugs with the immunomodulatory drug thalidomide resulted in a dramatic improvement in outcome, and the infected rabbits survived. The beneficial effect of thalidomide was associated with inhibition of tumor necrosis factor α (TNF-α) production, reduced leukocytosis in the cerebrospinal fluid (CSF), and attenuation of the inflammatory response in the meninges.
TNF-α is one of the cytokines involved in the protective cell-mediated immune response to mycobacteria (6). Besides its protective role, TNF-α production has been associated with the development of pathology in vivo, including tissue necrosis and cachexia (wasting) (7, 8). In the CNS, TNF-α is involved in induction of a fever response, activates the hypothalamus–pituitary–adrenal axis, and triggers the release of other cytokines (9–12). TNF-α may also influence transport of compounds into the brain by “opening” the blood–brain barrier (BBB) (10). In patients with bacterial meningitis, high TNF-α levels in the CSF correlate with increased levels of IL-6 and protein, as well as low glucose levels (13). In addition, TNF-α and IL-1β levels are associated with prolonged fever, seizures, spasticity, and death (13–15).
To test whether the levels of TNF-α in the CSF determine the extent of pathogenesis, we infected rabbits in the CNS with different strains of Mycobacterium bovis, including virulent M. bovis Ravenel and avirulent M. bovis bacillus Calmette–Guérin (BCG). The persistence of the infecting organisms, TNF-α levels, leukocytosis, and protein levels in the CSF were monitored, and the clinical course of the infection was followed. To directly evaluate the contribution of TNF-α to pathogenesis, rabbits were infected with a recombinant strain of M. bovis BCG that secretes murine TNF-α. We evaluated the inflammatory parameters, disease progression, and brain pathology in these rabbits, and compared these to control rabbits inoculated with M. bovis BCG carrying the plasmid vector only.
MATERIALS AND METHODS
Infecting Organisms.
M. bovis strains used were: (i) M. bovis Ravenel (Trudeau Mycobacterial Culture Collection, TMC no.401), known to be highly virulent in rabbits (16, 17); (ii) M. bovis BCG Pasteur (Trudeau Mycobacterial Culture Collection, TMC no. 1011) which is avirulent in rabbits; (iii) Recombinant M. bovis BCG strain Montreal, genetically engineered to secrete murine TNF-α (BCG mTNF-α); and (iv) the control strain of M. bovis BCG Montreal, carrying the plasmid vector only [BCG Montreal (v)] (18). The genetic manipulations of the BCG did not affect their growth rates: both strains [BCG mTNF-α and BCG Montreal (v)] exhibited similar growth in liquid and on solid medium. Also, the two strains showed similar growth rates in the lungs after intravenous infection of B6x129 mice (L.-G. Bekker and G. Kaplan, unpublished data). Mycobacteria were grown in Middlebrook 7H9 broth (Difco) followed by culture in Proskauer and Beck medium containing 0.01% Tween 80 as described (19). Recombinant mycobacteria were grown as above with 20 μg/ml kanamycin (Sigma) as described (18). Mycobacteria were stored frozen in aliquots and then thawed and subjected to brief ultrasonication to break up aggregates. Final single-cell suspensions were prepared to achieve an inoculum of 5 × 105 or 2 × 107 colony-forming units (cfu) per 0.1–0.2 ml.
Induction of Meningitis.
New Zealand white rabbits, ≈2.5 kg (Charles River Breeding Laboratories) were used as described (5). Anesthetized rabbits were placed in a stereotaxic frame, a spinal needle was introduced into the cisterna magna for CSF sampling, and 0.1–0.2 ml of live mycobacteria or pyrogen-free saline was injected intracisternally (4–6 animals per experiment). At 2 h after inoculation, a sample of CSF was obtained, diluted, and plated onto 7H10 agar to determine the number of cfu injected. In some experiments, CSF samples were withdrawn at 0, 2, 4, 6, and 8 days after inoculation. In other experiments, the samples were obtained on day 0, 1, 3, 7, 14, and 21. After euthanasia, half of the brain and a segment of the lung, liver, and spleen was collected aseptically, homogenized, and used for evaluation of bacillary load. The other half of the brain and the rest of the organs were removed and fixed in 10% (vol/vol) buffered formalin acetate (Fisher) and prepared for histopathology. This protocol was approved by the Rockefeller University Animal Care and Use Committee.
In addition, recombinant murine TNF-α (4 μg in 200 μl of saline; Endogen, Cambridge, MA) was injected into the cisterna magna of rabbits. Leukocytosis was evaluated at 0, 4, 6, 8, 10, and 24 h as described below.
CSF Samples.
Immediately after collection, duplicate CSF samples were analyzed for numbers of white blood cells (Coulter), and 100 μl of CSF was removed for determination of the bacterial load. The remainder of the CSF was centrifuged at 10,000 × g for 5 min, and the supernatant was stored at −70°C for TNF-α and protein assays. CSF protein concentration was determined by using the bicinchoninic acid method (BCA kit, Pierce) as described by the manufacturer. The cellular sediment was used for differential cell counts (Diff-Quick, Baxter) and for electron microscopy.
Blood Samples.
Blood was collected at different time points from the ear artery with a heparinized syringe and centrifuged at 10,000 × g. Plasma was separated and frozen at −70°C for TNF evaluation.
TNF Assay.
Because a TNF-α ELISA kit for rabbits is currently unavailable, TNF biologic activity (TNF-α and TNF-β) in CSF and plasma was assayed by using murine L929 fibroblasts as target cells in a cytotoxicity assay, as described (20). The minimal detection level was 16 units/ml.
Determination of Murine TNF-α Levels.
The ability of the recombinant mycobacteria to secrete murine TNF-α was verified by measuring the concentration of the cytokine in the bacterial culture supernatants by ELISA (Endogen, Cambridge, MA). Levels of murine TNF-α in the rabbit CSF samples were also determined by ELISA.
cfu Assay.
Bacterial loads in the CSF, brains, lungs, livers, and spleens of the infected rabbits were evaluated by plating 10-fold serial dilutions of the CSF and of the organ homogenates onto Middlebrook 7H10 agar plates (Difco) as well as onto Middlebrook 7H10 agar supplemented with 20 μg/ml of kanamycin (Sigma). Plates were incubated at 37°C for 2–3 weeks. Colonies were counted and expressed as cfu.
Histopathology.
After fixation in 10% formalin (Fisher), organ specimens were embedded in paraffin (TissuePrep 2, Fisher) and then sectioned and stained with hematoxylin and eosin and acid fast stain (Kinyouns). The brains were cut transversely in serial 2- to 3-mm sections from rostral to caudal. Sections were selected representing the fore-, mid-, and hindbrains.
Electron Microscopy.
The CSF cellular sediment and a part of the brain including the meninges were processed for transmission electron microscopy as described (21). Sections were stained and examined with a JEM 100CX transmission electron microscope (JEOL).
Statistical Analysis.
Results are presented as means of values obtained for all animals evaluated at each time point. Data were analyzed by using an independent Student’s t test to compare the different groups of animals. P < 0.05 was considered significant.
RESULTS
Responses of Rabbits After CNS Infection with Different M. bovis Strains.
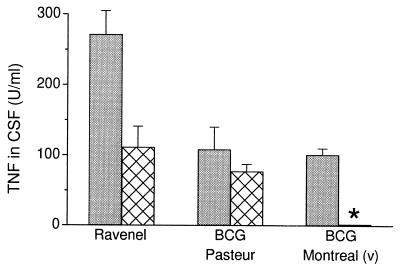
The response of rabbits infected with M. bovis Ravenel was compared with the response to either of two strains of M. bovis BCG. Intracisternal inoculation of virulent M. bovis Ravenel at either a low (5 × 105 cfu) or high (2 × 107 cfu) dose induced relatively high dose-dependent levels of TNF in the CSF, which peaked 2 h after infection (Fig. 1). Significant levels were observed until day 8 (data not shown). TNF was also detected systemically in the plasma of rabbits infected with the high dose of Ravenel (data not shown). In contrast, rabbits inoculated with BCG Pasteur had lower TNF levels in the CSF at 2 h and barely detectable levels at later time points postinfection (Fig. 1). No TNF was detected in the plasma of these rabbits at any time. Rabbits infected with BCG Montreal (v) had lower levels (high dose) or no TNF in the CSF (low dose) even at peak (2 h). Thus, Ravenel induced more TNF then the BCG strains, which induced either lower levels or none at all.
Figure 1.
TNF levels in CSF 2 h after infection. Rabbits were inoculated intracisternally with a high (2 × 107 cfu) (shaded bars) or low (5 × 105 cfu) (cross-hatched bars) dose of mycobacteria. Significantly lower TNF levels were observed in rabbits infected with the low dose of M. bovis BCG Montreal (v) compared with M. bovis Ravenel (P = 0.02). Values are means ± SEM of results obtained from 4–6 rabbits per group. ∗ denotes statistical significance.
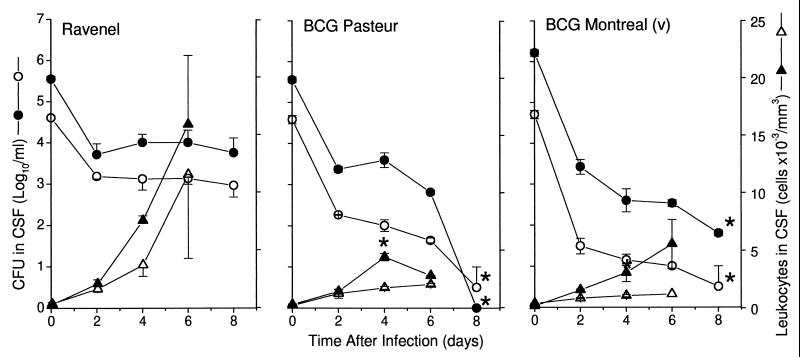
Significant differences also were observed in the leukocyte response in the CSF (Fig. 2). Ravenel induced high leukocyte accumulation, which progressively increased, indicating that the BBB had been breached. On day 6, cell counts reached >11,000 cells per mm3 and >15,000 cells per mm3 in the rabbits infected with the low dose and high dose, respectively. In contrast, BCG Pasteur and Montreal (v) failed to induce remarkable leukocytosis at any time (Fig. 2). In all rabbits, examination of the differential counts in the CSF revealed that >90% of the cells were mononuclear (monocytes and lymphocytes).
Figure 2.
Leukocyte numbers and cfu in CSF of infected rabbits. Rabbits were inoculated intracisternally with a high (filled symbols) or low (open symbols) dose of either M. bovis Ravenel, BCG Pasteur, or BCG Montreal (v). Leukocytosis was reduced in rabbits infected with BCG Pasteur and BCG Montreal (v) compared with Ravenel on day 4 (P < 0.03). cfu were reduced at day 8 in rabbits inoculated with BCG Pasteur and BCG Montreal (v) compared with Ravenel (P < 0.05). Values are means ± SEM for 4–6 rabbits per group. ∗ denotes statistical significance.
Mycobacterial Levels After Infection with Different M. bovis Strains.
After infection, viable mycobacteria were isolated from the CSF at different time points (Fig. 2). The number of cfu in the CSF of Ravenel-infected animals remained high to day 8 postinfection. However, in rabbits inoculated with BCG Pasteur or Montreal (v), there was a progressive decrease in cfu in the CSF, and by day 8, significantly reduced numbers of organisms were isolated (P ≤ 0.05). Comparably high numbers of cfu were found in the brains of all infected rabbits on day 8 postinfection regardless of the strain of mycobacteria used for infection (Table 1). Ravenel cfu were high in the lungs, livers, and spleens. BCG Pasteur and Montreal (v) cfu were significantly lower in the lungs and also in the spleens and livers at the low-dose inoculation (Table 1). These results suggest that once the BBB was disrupted after infection, the bacilli crossed in an unrestricted manner from the subarachnoid space into the blood, and the infection was spread hematogenously to other organs.
Table 1.
Mycobacterial levels in organs of infected rabbits (day 8)
| M. bovis strain | Brain | Lungs | Liver | Spleen |
|---|---|---|---|---|
| Ravenel | ||||
| 2 × 107† | 5.1 ± 0.05 | 4.6 ± 0.09 | 5.7 ± 0.33 | 4.9 ± 0.33 |
| 5 × 105 | 4.7 ± 0.25 | 4.3 ± 0.55 | 5.6 ± 0.03 | 4.7 ± 0.05 |
| Pasteur | ||||
| 2 × 107 | 4.5 ± 0.11* | 2.0 ± 2.00 | 5.1 ± 0.22 | 3.3 ± 1.47 |
| 5 × 105 | 4.4 ± 0.10 | 0* | 1.8 ± 1.80 | 2.4 ± 0.10* |
| Montreal (v) | ||||
| 2 × 107 | 5.2 ± 0.01 | 0* | 4.7 ± 0.28 | 3.9 ± 0.35 |
| 5 × 105 | 4.1 ± 0.17 | 0* | 0* | 1.2 ± 1.10* |
cfu are expressed as log10. Results are means of data from four rabbits per group ± SEM.
, cfu were significantly lower compared to Ravenel (P < 0.05).
, infecting inoculum.
Meningitis After Infection with Different M. bovis Strains.
The inflammatory parameters and bacterial load correlated with the clinical course of meningitis. Rabbits infected with high-dose Ravenel developed acute progressive meningitis with severe neurologic signs, including somnolence or irritability, loss of coordination, hemiparesis, or hemiplegia and survived 4–8 days. Low-dose Ravenel produced a subacute inflammatory response, which resulted in delayed-onset, less severe symptoms and survival of at least 8 days. Animals inoculated with BCG Pasteur or Montreal (v) did not develop any symptoms and showed no clinical signs of CNS disease.
Response of Rabbits After CNS Infection with Recombinant BCG Secreting Murine TNF-α.
The results described above suggested that the peak TNF-α levels at 2 h postinoculation determined the pathogenesis and course of mycobacterial CNS infection. To directly address the role of TNF-α, we infected rabbits with a low dose (5 × 105 cfu) of a recombinant strain of BCG Montreal that expresses the murine gene for TNF-α (BCG mTNF-α) or the control strain BCG Montreal (v). Animals were monitored for 3 weeks.
To confirm that BCG mTNF-α were secreting the murine cytokine during the infection, mycobacterial colonies recovered from the CSF were grown in 7H9 broth supplemented with 20 μg/ml of kanamycin for 5 days, and the supernatants were tested for TNF-α by using ELISA. TNF-α concentrations were in the range of 10,000–40,000 pg/ml at all time points, indicating that the recombinant BCG produced murine TNF-α throughout the experiment.
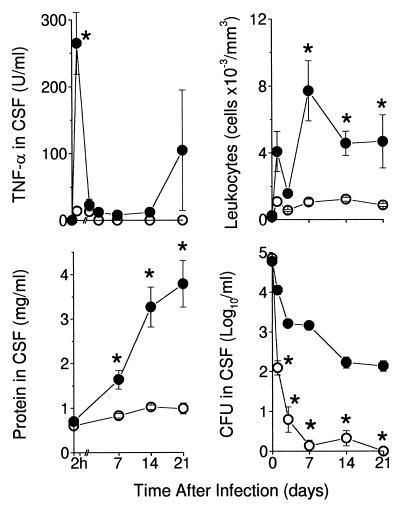
Infection with BCG Montreal (v) resulted in barely detectable levels of TNF in the CSF at 2 h and 24 h, which disappeared at later time-points (Fig. 3). In rabbits infected with BCG mTNF-α the peak levels at 2 h were significantly higher (P < 0.001). By 24 h, TNF concentrations were reduced, although they were detectable to day 14. Later secondary peaks of TNF were observed at day 21, when the rabbits were severely sick. TNF detected at 2 h in the CSF of rabbits infected with BCG mTNF-α was predominantly murine TNF-α, as determined by a specific ELISA against murine TNF-α. At later time points (14–21 days), the TNF assayed in the CSF was predominantly rabbit TNF (detected by bioassay and less so by ELISA), suggesting that the murine TNF-α in the CSF stimulated the production of endogenous rabbit TNF.
Figure 3.
Response of rabbits infected with BCG mTNF-α. Rabbits were infected intracisternally with 5 × 105 cfu of BCG mTNF-α (filled symbols) (n = 10) or BCG Montreal (v) (open symbols) (n = 9) and monitored for 21 days. (Upper Left) TNF concentration in CSF of infected rabbits. Levels of TNF at 2 h were higher in the rabbits inoculated with BCG mTNF-α compared with BCG Montreal (v) (P < 0.001). (Upper Right) Leukocyte density in CSF. Higher leukocyte counts in rabbits infected with BCG mTNF-α versus BCG Montreal (v) were observed on day 7 (P < 0.005), day 14 (P = 0.001) and day 21 (P = 0.04). (Lower Left) Protein levels. Protein induced in the CSF by BCG mTNF-α was significantly higher from day 7 (P = 0.008) and continued to increase to day 21 (P < 0.003). (Lower Right) cfu in the CSF. BCG Montreal (v) cfu were reduced from day 1 (P = 0.001), continued to decrease and disappeared by day 21 (P = 0.001). All values are means ± SEM for the group of rabbits studied. ∗ denotes statistical significance.
To confirm that murine TNF-α is biologically active in rabbits, rabbits were injected intrathecally with 4 μg of recombinant murine TNF-α. Significant leukocytosis (2,095 ± 1,900 cells per mm3) was observed in the CSF 24 h after cytokine administration. This result is similar to results obtained in a previous study on the effects of exogenously administered rabbit TNF-α (11). Our finding indicates that rabbits respond to murine TNF-α.
We next examined the leukocyte response in the CSF. BCG Montreal (v) did not induce significant leukocytosis (Fig. 3). Cell counts were <1,000 cells per mm3 throughout the experiment. In contrast, BCG mTNF-α induced an extensive leukocyte influx into the CSF. High leukocyte counts persisted to day 21.
Another feature characteristic of BBB injury is protein accumulation in the CSF. Rabbits inoculated with BCG Montreal (v) had CSF protein levels within the normal range (<1 mg/ml) (Fig. 3). BCG mTNF-α induced significantly higher CSF protein at day 7 (P = 0.008), which continued to increase up to day 21 (P < 0.003).
To compare the course of infection, mycobacteria were cultured from the CSF and quantitated by the cfu assay (Fig. 3). Already at day 1, a significant reduction in BCG Montreal (v) cfu was observed compared with BCG mTNF-α (P = 0.001). BCG Montreal (v) cfu continued to decrease and completely disappeared by day 21 (P = 0.001). A two-order-of-magnitude reduction in BCG mTNF-α cfu was observed by day 3, after which the numbers remained relatively high throughout the experiment, suggesting impaired bacillary clearance in the CSF similar to the lack of clearance of infection when Ravenel was used.
Systemic Response After Infection with Recombinant BCG mTNF-α.
Mycobacterial cfu in the brains, lungs, livers, and spleens were evaluated on day 21 after infection. High cfu were recovered from the brains of all rabbits infected with BCG mTNF-α (Table 2). Significantly lower cfu were isolated from 50% of animals inoculated with BCG Montreal (v) (P = 0.001); no cfu were detected in the other animals. BCG mTNF-α spread to the lungs, livers, and spleens, whereas BCG Montreal (v) was not observed in the lungs and livers. Only 16% of rabbit spleens had any detectable BCG Montreal (v).
Table 2.
CFU in organs of infected rabbits (day 21)
| M. bovis strain | n | Brain | Lungs | Liver | Spleen |
|---|---|---|---|---|---|
| BCG mTNF-α | 10 | 4.2 ± 0.3 | 3.8 ± 0.2 | 0.6 ± 0.6 | 1.4 ± 0.6 |
| BCG Montreal (v) | 9 | 1.5 ± 0.6* | 0* | 0* | 0.5 ± 0.4* |
cfu are expressed as log10. Results are means ± SEM for the group of animals studied.
, cfu were significantly lower in organs of rabbits inoculated with BCG Montreal (v) compared to BCG mTNF-α (P = 0.001).
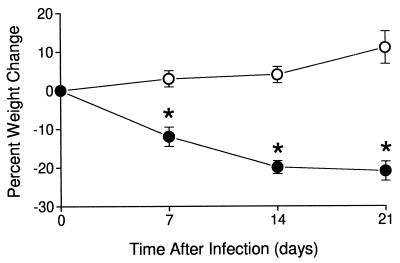
These responses correlated with the clinical course of experimental mycobacterial meningitis. Rabbits infected with BCG Montreal (v) developed well, gained weight (Fig. 4), and showed no signs of disease. Rabbits infected with BCG mTNF-α lost weight and by day 21 had lost >20% of the starting body weight (P < 0.001). During week 3 of observation, these rabbits became somnolent and manifested some of the neurologic symptoms characteristic of meningitis, including loss of coordination, paresis, and paralysis.
Figure 4.
Body weight of rabbits infected with 5 × 105 cfu of BCG mTNF-α (●) (n = 10) or BCG Montreal (v) (○) (n = 9). Rabbits infected with BCG mTNF-α lost weight by day 7 (P = 0.002) and continued losing weight until day 21 (P < 0.001). ∗ denotes statistical significance.
Histologic and electron microscopy examination of the brains revealed few inflammatory cells scattered within the subarachnoid space in rabbits infected with BCG Montreal (v) (Fig. 5 and Fig. 6). In contrast, BCG mTNF-α induced marked meningeal inflammation, with thickening of the leptomeninges and large numbers of mononuclear cells. Vasculitis in the meninges as well as in the brain (neuropil) was observed in these animals. Acid-fast bacilli were seen in macrophages in the CSF and the meninges, consistent with cfu data (Fig. 3, Table 2, and Fig. 6).
Figure 5.
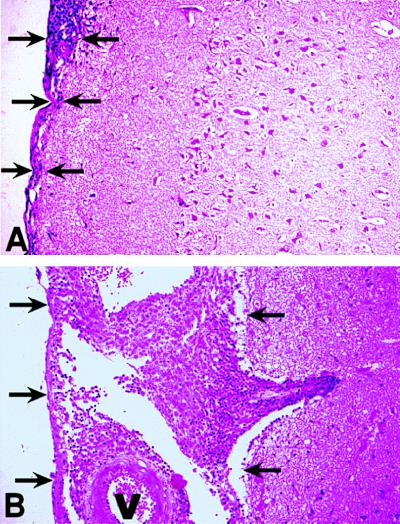
Histopathology of meninges and brain of infected rabbits (21 days). (A) Rabbit infected with BCG Montreal (v). Mild focal inflammatory response is seen within the meninges (arrows). There is no evidence of perivascular tissue damage or leukocytic infiltration within the neuropil. (B) Rabbit infected with BCG mTNF-α. Marked thickening of the leptomeninges and distension of the arachnoid space by large numbers of inflammatory cells are observed (arrows). Note extension of perivascular inflammation to neuropil. Sections are stained with hematoxylin and eosin. Magnification ×25. Blood vessels are labeled V.
Figure 6.
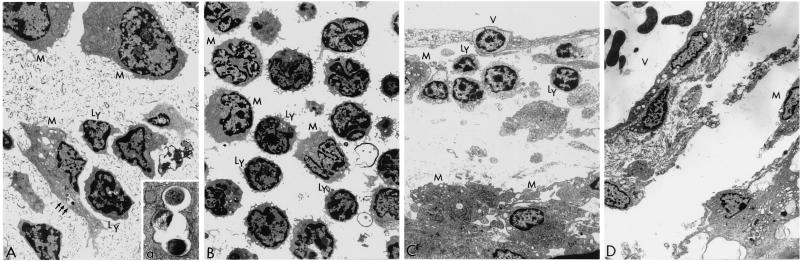
Electron micrographs of the CSF cellular sediment (day 14) (A and B) and the meninges (day 21) (C and D) of rabbits infected with BCG mTNF-α (A and C) or control BCG Montreal (v) (B and D). (A) Cellular sediment from the CSF of a rabbit infected with BCG mTNF-α. Mononuclear cells, including enlarged irregular macrophages (M) and lymphocytes (Ly), are observed. Note mycobacteria inside a tight vacuole (arrows) in the cytoplasm of a parasitized macrophage. Magnification ×3,600. (a) Higher magnification of the mycobacteria-containing vacuole. Magnification ×20,000. (B) Cellular sediment from the CSF of a rabbit infected with BCG Montreal (v). Round lymphocytes (Ly) and monocytes (M) are observed. Phagocytosed mycobacteria were not detected in these cells. Magnification ×2,900. (C) The meninges of a rabbit infected with BCG mTNF-α. A portion of a meningeal area containing a capillary vessel (V) is shown, with lymphocytes (Ly) and monocytes (M) in the perivascular space. Note a migrating lymphocyte crossing the endothelial monolayer at the top of the micrograph. Magnification ×2,900. (D) The meninges of a rabbit infected with BCG Montreal (v). A meningeal vessel (V) is shown. In the perivascular space few migrating macrophages (M) are seen. Magnification ×2,200.
DISCUSSION
The experiments described here demonstrate that TNF-α is a determinant of mycobacterial pathogenicity in the CNS. Several lines of evidence support this conclusion. When rabbits are infected with a mycobacterial strain that induces high levels of TNF-α in the CSF, they develop severe acute meningitis and die within a few days. This is not observed when rabbits are infected with strains of mycobacteria that do not induce much TNF-α release. In addition, we have previously shown that in infected rabbits, inhibition of TNF-α production by thalidomide treatment blocks the development of meningitis (5). Finally, when the nonvirulent strain is genetically engineered to contain a functional gene for TNF-α, the nonvirulent strain is rendered virulent. These results suggest that pathogenicity of mycobacteria in the CNS depends at least in part on the induction of TNF-α, resulting in breach of the BBB and triggering of the inflammatory cascade. These alterations lead to brain edema, cerebrovascular changes, and increased intracranial pressure (22).
TNF-α production in the CNS may be so deleterious because it affects vascular endothelium by inducing procoagulant activity (23), formation of thrombi, and production of nitric oxide synthase (24–26), thus causing endarteritis. Occlusions of large or small vessels are the most common reason for cranial nerve palsies, hemiparesis, and paralysis. In addition, it has been reported that injection of recombinant human TNF-α into the cisterna magna of rabbits results in an acute reduction in cerebral oxygen uptake and a prolonged reduction in cerebral blood flow (24). Even small amounts of TNF-α can exert harmful effects on capillaries already sensitized by mycobacterial products (27). Our morphologic studies on the brain and meninges of rabbits infected with BCG mTNF-α demonstrate directly the impairment of the vasculature by the local production of TNF-α (Fig. 5 and 6).
In the present experiments, expression of TNF-α appeared to block control of the mycobacterial infection because significantly more cfu were isolated from the CSF of rabbits infected with mycobacterial strains that induced or expressed TNF-α. The mechanism by which TNF-α affects mycobacterial numbers is still undefined and controversial. In our previous in vivo studies, partial TNF-α inhibition by thalidomide did not affect the number of cfu in the rabbit CSF (5). Similarly, in a mouse model of tuberculosis aerosol infection, thalidomide immunomodulation reduced the levels of TNF-α and concomitant lung granuloma size but had no effect on the bacillary load in the lungs (28). However, in our in vitro infection studies we found that enhanced TNF-α production by human monocytes was associated with faster growth of the intracellular mycobacteria. When TNF-α was neutralized in vitro by addition of mAb and recombinant human IL-10 to the infected cultures, the growth rate of the mycobacteria in the monocytes was reduced (unpublished data). Also, induction of higher levels of TNF-α production by infected monocytes is associated with more virulent or faster growing mycobacterial strains (29). Additional studies are clearly necessary to define the effect of TNF-α on intracellular mycobacterial growth and survival.
It is possible that the effects of TNF-α on mycobacterial numbers within the infected cell are mediated by regulation of apoptosis. We recently reported that induction of apoptosis of mycobacteria-infected macrophages with ATP4− or H2O2 was associated with killing of the intracellular bacilli (21, 30). We are currently investigating whether the levels of TNF-α determine the extent of apoptosis of mycobacteria-infected human monocytes in vitro and also whether cytokine-modulated apoptosis is associated with killing of intracellular bacilli in the rabbit infection model.
In the present studies, we observed dissemination of the mycobacteria from the CSF across the blood brain barrier. However, we used an artificial model of infection, in which the infecting bacteria were introduced directly into the CNS. When the BBB was breached, the bacteria seeded distant organs, including lungs, liver, and spleen. In the human infection, the opposite transiting of bacteria occurs. The primary infection is respiratory, and usually the lung is the first site of infection. When bacteria disseminate, presumably within infected monocytes traveling through the blood stream, they somehow cross the BBB and seed the meninges. The likelihood that this will occur is much higher in very young children and in immunocompromised adults (1–4, 31–33), suggesting that an immune mechanism(s) contributes to control of dissemination of infected monocytes and/or to the integrity of the BBB. How the BBB is breached in the tuberculosis-infected patient is unknown. Past studies have attempted unsuccessfully to induce breach of the BBB to cause dissemination of mycobacteria into the CNS from experimental systemic infection (34, 35). Because it appears that TNF-α induces leaks in the BBB, we can now use exogenously added TNF-α to attempt to recreate the natural direction of infection of the CNS. These studies will help us to understand the factors regulating pathogenesis in TBM, identify individuals at risk for CNS infection, and suggest how we may intervene to prevent this serious complication of tuberculosis infection in children and immunocompromised adults.
Acknowledgments
We thank Wilhelmine Hellmann for her expert assistance with the electron microscopy studies, Judy Adams for preparation of the figures, and Marguerite Nulty for secretarial assistance. This work was supported in part by Celgene Corporation (Warren, NJ).
ABBREVIATIONS
- CNS
central nervous system
- TBM
tuberculous meningitis
- TNF-α
tumor necrosis factor α
- CSF
cerebrospinal fluid
- BCG
bacillus Calmette-Guérin
- BBB
blood–brain barrier
- cfu
colony-forming units
References
- 1.Leonard J M, Des Prez R M. Infect Dis Clin North Am. 1990;174:483–489. [PubMed] [Google Scholar]
- 2.Bishburg E, Sunderham G, Reihman L B, Kapila R A. Intern Med. 1986;105:210–213. doi: 10.7326/0003-4819-105-2-210. [DOI] [PubMed] [Google Scholar]
- 3.Udani P M. Indian J Pediatr. 1994;61:451–462. doi: 10.1007/BF02751703. [DOI] [PubMed] [Google Scholar]
- 4.Gutman L T. Semin Ped Inf Dis. 1993;4:250–260. [Google Scholar]
- 5.Tsenova L, Sokol K, Freedman V H, Kaplan G. J Infect Dis. 1998;177:1563–1572. doi: 10.1086/515327. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan G, Freedman V H. Res Immunol. 1996;147:565–572. doi: 10.1016/s0923-2494(97)85223-6. [DOI] [PubMed] [Google Scholar]
- 7.Beutler B, Cerami A. Adv Immunol. 1998;42:213–231. doi: 10.1016/s0065-2776(08)60846-9. [DOI] [PubMed] [Google Scholar]
- 8.Tracey K J, Cerami A. Am J Trop Med Hyg. 1992;47:2–7. doi: 10.4269/ajtmh.1992.47.2. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Ishikawa Y, Goto F, Bando T, Sakakibara Y, Iriki M. Brain Res. 1991;540:217–223. doi: 10.1016/0006-8993(91)90510-3. [DOI] [PubMed] [Google Scholar]
- 10.De Vries H E, Kuiper J, De Boer A G, Van Berkel T J C, Breimer D D. Pharmacol Rev. 1997;49:143–155. [PubMed] [Google Scholar]
- 11.Ramilo O, Saez-Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkaware S, Nariuchi H, McCracken G H., Jr J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low P S, Lee B W, Yap H K, Tay J S, Lee W L, Seah C C, Ramzan M M. Ann Trop Pediatr. 1995;15:55–59. doi: 10.1080/02724936.1995.11747749. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa M M, Lebel M H, Ramilo O, Olsen K D, Reisch J S, Beutler B, McCracken G H., Jr J Pediatr. 1989;115:208–213. doi: 10.1016/s0022-3476(89)80067-8. [DOI] [PubMed] [Google Scholar]
- 15.Sharief M K, Ciardi M, Thompson E J. J Infect Dis. 1992;166:350–358. doi: 10.1093/infdis/166.2.350. [DOI] [PubMed] [Google Scholar]
- 16.Lurie M B, Abramson S, Swartz J B, Heppleston A G. Am Rev Tuberc. 1950;61:765–797. [PubMed] [Google Scholar]
- 17.Converse P J, Dannenberg A M, Jr, Shigenaga T, McMurray D N, Phalen S W, Stanford J L, Rook G A W, Koru-Sengul T, Abbey H, Estep J E, Pitt M L M. Clin Diagn Lab Immunol. 1998;5:871–881. doi: 10.1128/cdli.5.6.871-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray P J, Aldovani A, Young R A. Proc Natl Acad Sci USA. 1996;93:934–939. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atlas R M. In: Handbook of Microbiological Media. Parks L C, editor. Boca Raton, FL: CRC; 1993. p. pp.734. [Google Scholar]
- 20.Burroughs M H, Tsenova-Berkova L, Sokol K, Ossig J, Tuomanen E, Kaplan G. Microb Pathog. 1995;19:245–255. doi: 10.1016/s0882-4010(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 21.Molloy A, Laochumroonvorapong P, Kaplan G. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quagliarello V J, Scheld W M. N Engl J Med. 1992;327:864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 23.Beliqua M P, Pober J S, Majeau G R, Fiers W, Cotran R S, Gimbrone M A. Proc Natl Acad Sci USA. 1986;83:4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tureen J. J Clin Invest. 1995;95:1086–1091. doi: 10.1172/JCI117755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard C, Tedgui A. Eur Cytokine Netw. 1992;3:19–33. [PubMed] [Google Scholar]
- 26.Yoshizumi M, Perrella M A, Burnett J C, Jr, Lee M E. Circ Res. 1993;73:205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- 27.Rook G A W, Al Attiyah R, Foby N. Lymphokine Res. 1989;8:323–328. [PubMed] [Google Scholar]
- 28.Moreira A L, Tsenova-Berkova L, Wang J, Laochumroonvorapong P, Freeman S, Freedman V H, Kaplan G. Tuber Lung Dis. 1997;78:47–55. doi: 10.1016/s0962-8479(97)90015-0. [DOI] [PubMed] [Google Scholar]
- 29.Silver R F, Li Q, Ellner J J. Infect Immun. 1998;66:1190–1199. doi: 10.1128/iai.66.3.1190-1199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laochumroonvorapong P, Paul S, Elkon K B, Kaplan G. Infect Immun. 1996;64:452–459. doi: 10.1128/iai.64.2.452-459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idriss Z H, Sinno A A, Kronfol N M. Am J Dis Child. 1976;130:364–367. doi: 10.1001/archpedi.1976.02120050022004. [DOI] [PubMed] [Google Scholar]
- 32.Smith A L. Med J Aust. 1975;1:57–60. doi: 10.5694/j.1326-5377.1975.tb111231.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith S, Jacobs R F, Wilson C B. J Pediatr. 1997;131:16–26. doi: 10.1016/s0022-3476(97)70120-3. [DOI] [PubMed] [Google Scholar]
- 34.Rich A R, McCordock H A. Bull Johns Hopkins Hosp. 1933;52:5–37. [Google Scholar]
- 35.MacGregor A R, Green C A. J Pathol Bacteriol. 1937;45:613–645. [Google Scholar]