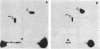
Abstract
The methylcytosine-containing sequences in the DNA of Bacillus subtilis 168 Marburg (restriction-modification type BsuM) were determined by three different methods: (i) examination of in vivo-methylated DNA by restriction enzyme digestion and, whenever possible, analysis for methylcytosine at the 5' end; (ii) methylation in vitro of unmethylated DNA with B. subtilis DNA methyltransferase and determination of the methylated sites; and (iii) the methylatability of unmethylated DNA by B. subtilis methyltransferase after potential sites have been destroyed by digestion with restriction endonucleases. The results obtained by these methods, taken together, show that methylcytosine was present only within the sequence 5'-TCGA-3'. The presence of methylcytosine at the 5' end of the DNA fragments generated by restriction endonuclease AsuII digestion and the fact that in vivo-methylated DNA could not be digested by the enzyme XhoI showed that the recognition sequences of these two enzymes contained methylcytosine. As these two enzymes recognized a similar sequence containing a 5' pyrimidine (Py) and a 3' purine (Pu), 5'-PyTCGAPu-3', the possibility that methylcytosine is present in the complementary sequences 5'-TTCGAG-3' and 5'-CTCGAA-3' was postulated. This was verified by the methylation in vitro, with B. subtilis enzyme, of a 2.6-kilobase fragment of lambda DNA containing two such sites and devoid of AsuII or XhoI recognition sequences. By analyzing the methylatable sites, it was found that in one of the two PyTCGAPu sequences, cytosine was methylated in vitro in both DNA strands. It is concluded that the sequence 5'-PyTCGAPu-3' is methylated by the DNA methyltransferase (of cytosine) of B. subtilis Marburg.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale A., d'Alarcao M., Marinus M. G. Characterization of DNA adenine methylation mutants of Escherichia coli K12. Mutat Res. 1979 Feb;59(2):157–165. doi: 10.1016/0027-5107(79)90153-2. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Bonamy C., Szulmajster J. Cloning and expression of Bacillus subtilis spore genes. Mol Gen Genet. 1982;188(2):202–210. doi: 10.1007/BF00332676. [DOI] [PubMed] [Google Scholar]
- Bron S., Murray K., Trautner T. A. Restriction and modification in B. subtilis. Purification and general properties of a restriction endonuclease from strain R. Mol Gen Genet. 1975 Dec 30;143(1):13–23. doi: 10.1007/BF00269416. [DOI] [PubMed] [Google Scholar]
- Cregg J. M., Ito J. A physical map of the genome of temperate phage phi 3T. Gene. 1979 Jul;6(3):199–219. doi: 10.1016/0378-1119(79)90058-1. [DOI] [PubMed] [Google Scholar]
- Dreiseikelmann B., Wackernagel W. Absence in Bacillus subtilis and Staphylococcus aureus of the sequence-specific deoxyribonucleic acid methylation that is conferred in Escherichia coli K-12 by the dam and dcm enzymes. J Bacteriol. 1981 Jul;147(1):259–261. doi: 10.1128/jb.147.1.259-261.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K., Swinton D., Maniloff J., Hattman S. Cytosine methylation of the sequence GATC in a mycoplasma. J Bacteriol. 1982 Sep;151(3):1420–1424. doi: 10.1128/jb.151.3.1420-1424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. T. Genetic recombination during transformation in Bacillus subtilis: appearance of a deoxyribonucleic acid methylase. J Bacteriol. 1979 Jul;139(1):270–279. doi: 10.1128/jb.139.1.270-279.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S. W., Piekarowicz A. Host specificity of DNA in Haemophilus influenzae: restriction and modification in strain Rd. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1610–1617. doi: 10.1016/0006-291x(72)90793-0. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Stein R., Cedar H., Razin A. Methylation of CpG sequences in eukaryotic DNA. FEBS Lett. 1981 Feb 9;124(1):67–71. doi: 10.1016/0014-5793(81)80055-5. [DOI] [PubMed] [Google Scholar]
- Guha S. Effect of 5-azacytidine on DNA methylation and on the enzymes of de novo pyrimidine biosynthesis in Bacillus subtilis Marburg strain. Eur J Biochem. 1984 Nov 15;145(1):99–106. doi: 10.1111/j.1432-1033.1984.tb08527.x. [DOI] [PubMed] [Google Scholar]
- Günthert U., Trautner T. A. DNA methyltransferases of Bacillus subtilis and its bacteriophages. Curr Top Microbiol Immunol. 1984;108:11–22. doi: 10.1007/978-3-642-69370-0_2. [DOI] [PubMed] [Google Scholar]
- Hattman S., Keister T., Gottehrer A. Sequence specificity of DNA methylases from Bacillus amyloliquefaciens and Bacillus brevis. J Mol Biol. 1978 Oct 5;124(4):701–711. doi: 10.1016/0022-2836(78)90178-x. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Mathews J. L. Chromosomal location of pleiotropic negative sporulation mutations in Bacillus subtilis. Genetics. 1973 Feb;73(2):215–228. doi: 10.1093/genetics/73.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa S., Shibata T., Ando T., Saito H. Genetic studies on site-specific endodeoxyribonucleases in Bacillus subtilis: multiple modification and restriction systems in transformants of Bacillus subtilis 168. Mol Gen Genet. 1980 Feb;177(3):359–368. doi: 10.1007/BF00271474. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Shibata T., Matsumoto K., Iijima T., Saito H., Ando T. Chromosomal loci of genes controlling site-specific restriction endonucleases of Bacillus subtilis. Mol Gen Genet. 1981;183(1):1–6. doi: 10.1007/BF00270129. [DOI] [PubMed] [Google Scholar]
- Jentsch S. Restriction and modification in Bacillus subtilis: sequence specificities of restriction/modification systems BsuM, BsuE, and BsuF. J Bacteriol. 1983 Nov;156(2):800–808. doi: 10.1128/jb.156.2.800-808.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., Kiss A., Venetianer P. Biochemical characterization of the restriction-modification system of Bacillus sphaericus. Eur J Biochem. 1978 Sep 1;89(2):523–529. doi: 10.1111/j.1432-1033.1978.tb12557.x. [DOI] [PubMed] [Google Scholar]
- Marinus M. G. Location of DNA methylation genes on the Escherichia coli K-12 genetic map. Mol Gen Genet. 1973 Dec 14;127(1):47–55. doi: 10.1007/BF00267782. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Primrose S. B., Ehrlich S. D. Isolation of plasmid deletion Mutants and study of their instability. Plasmid. 1981 Sep;6(2):193–201. doi: 10.1016/0147-619x(81)90066-4. [DOI] [PubMed] [Google Scholar]
- Radman M., Wagner R. Effects of DNA methylation on mismatch repair, mutagenesis, and recombination in Escherichia coli. Curr Top Microbiol Immunol. 1984;108:23–28. doi: 10.1007/978-3-642-69370-0_3. [DOI] [PubMed] [Google Scholar]
- Roy P. H., Smith H. O. DNA methylases of Hemophilus influenzae Rd. I. Purification and properties. J Mol Biol. 1973 Dec 25;81(4):427–444. doi: 10.1016/0022-2836(73)90515-9. [DOI] [PubMed] [Google Scholar]
- Saito H., Shibata T., Ando T. Mapping of genes determining nonpermissiveness and host-specific restriction to bacteriophages in Bacillus subtilis Marburg. Mol Gen Genet. 1979 Feb 26;170(2):117–122. doi: 10.1007/BF00337785. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Shibata T., Ando T. Host controlled modification and restriction in Bacillus subtilis. Mol Gen Genet. 1974;131(4):275–280. doi: 10.1007/BF00264858. [DOI] [PubMed] [Google Scholar]
- Smith H. O. Nucleotide sequence specificity of restriction endonucleases. Science. 1979 Aug 3;205(4405):455–462. doi: 10.1126/science.377492. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner T. A., Pawlek B., Bron S., Anagnostopoulos C. Restriction and modification in B. subtilis. Biological aspects. Mol Gen Genet. 1974;131(3):181–191. doi: 10.1007/BF00267958. [DOI] [PubMed] [Google Scholar]
- Uozumi T., Hoshino T., Miwa K., Horinouchi S., Beppu T., Arima K. Restriction and modification in Bacillus species: genetic transformation of bacteria with DNA from different species, part I. Mol Gen Genet. 1977 Mar 28;152(1):65–69. doi: 10.1007/BF00264941. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Belozersky A. N., Kokurina N. A., Kadirova D. X. 5-methylcytosine and 6-methylamino-purine in bacterial DNA. Nature. 1968 Jun 15;218(5146):1066–1067. doi: 10.1038/2181066a0. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Dobritsa A. P. On the nature of the cytosine-methylated sequence in DNA of Bacillus brevis var. G.-B. Biochim Biophys Acta. 1975 Sep 12;407(1):61–72. doi: 10.1016/0005-2787(75)90023-4. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Fields P. I., Andersen B. J. Properties of Bacillus subtilis 168 derivatives freed of their natural prophages. Gene. 1980 Dec;12(1-2):155–159. doi: 10.1016/0378-1119(80)90026-8. [DOI] [PubMed] [Google Scholar]