Abstract
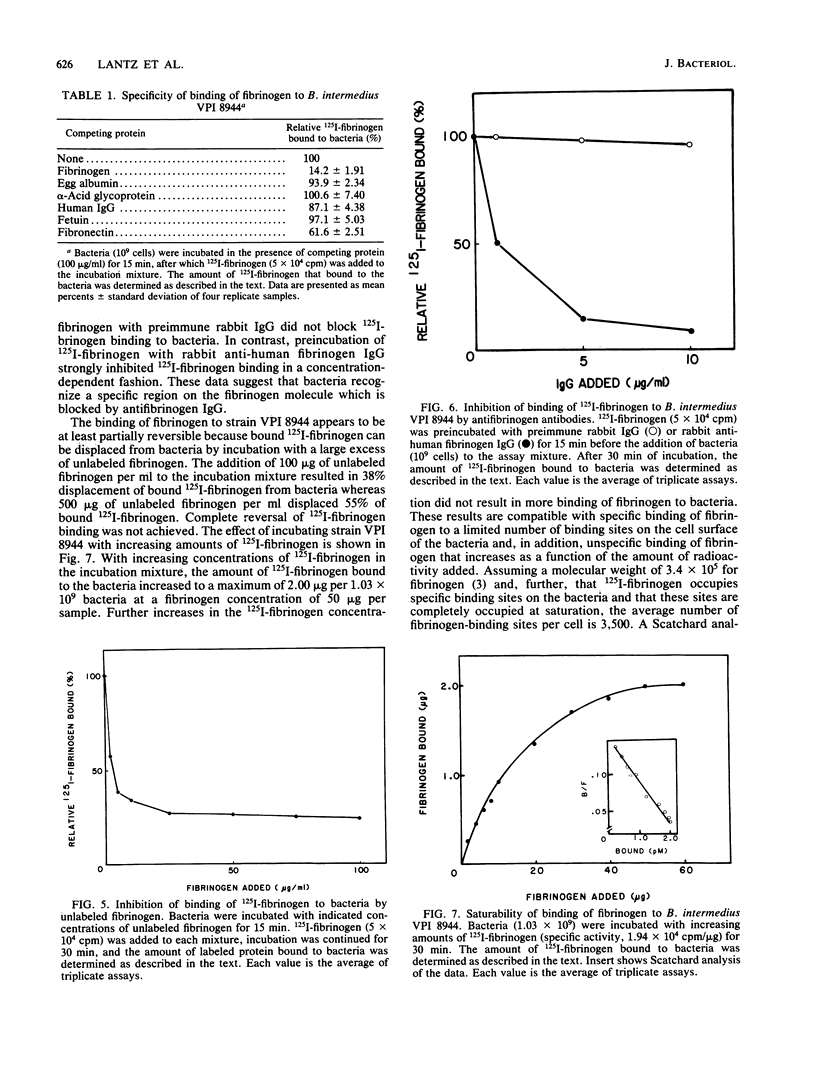
The binding of Bacteroides intermedius VPI 8944 to human fibrinogen has been characterized. The binding is time dependent, at least partially reversible, saturable, and specific. On an average, a maximum of 3,500 fibrinogen molecules bind per bacterial cell, with a dissociation constant of 1.7 X 10(-11) M. These bacteria also exhibit a fibrinogenolytic activity which can be partially inhibited by protease inhibitors. Bacteria release fibrinogenolytic activity into the surrounding medium without loss of binding activity, but more pronounced fibrinogen breakdown occurs when 125I-labeled fibrinogen is associated with the bacteria, suggesting that fibrinogen is degraded at the cell surface. Fibrinogen binding by B. intermedius might represent a mechanism of bacterial tissue adherence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M., Hayashi M. The structure of fibronectin and its role in cellular adhesion. J Supramol Struct Cell Biochem. 1981;16(4):345–348. doi: 10.1002/jsscb.1981.380160405. [DOI] [PubMed] [Google Scholar]
- Allenspach-Petrzilka G. E., Guggenheim B. Bacteroides melaninogenicus ss. intermedius invasion of rat gingival tissue. J Periodontal Res. 1982 Sep;17(5):456–459. doi: 10.1111/j.1600-0765.1982.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Gillett R., Johnson N. W. Bacterial invasion of the periodontium in a case of juvenile periodontitis. J Clin Periodontol. 1982 Jan;9(1):93–100. doi: 10.1111/j.1600-051x.1982.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Hawiger J., Timmons S., Strong D. D., Cottrell B. A., Riley M., Doolittle R. F. Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry. 1982 Mar 16;21(6):1407–1413. doi: 10.1021/bi00535a047. [DOI] [PubMed] [Google Scholar]
- Johansson S., Hök M. Substrate adhesion of rat hepatocytes: on the mechanism of attachment to fibronectin. J Cell Biol. 1984 Mar;98(3):810–817. doi: 10.1083/jcb.98.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980 Mar;15(2):111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Schönbeck C., Myhre E. Fibrinogen binding structures in beta-hemolytic streptococci group A, C, and G. Comparisons with receptors for IgG and aggregated beta 2-microglobulin. Acta Pathol Microbiol Scand B. 1979 Oct;87(5):303–310. [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Possibilities for treating periodontal disease as specific anaerobic infections. J Can Dent Assoc. 1984 Jun;50(6):467–472. [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Laughon B. E., Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982 Apr;53(4):223–230. doi: 10.1902/jop.1982.53.4.223. [DOI] [PubMed] [Google Scholar]
- Lämmler C., Chhatwal G. S., Blobel H. Binding of human fibrinogen and its polypeptide chains to group B streptococci. Med Microbiol Immunol. 1983;172(3):149–153. doi: 10.1007/BF02123799. [DOI] [PubMed] [Google Scholar]
- Manor A., Lebendiger M., Shiffer A., Tovel H. Bacterial invasion of periodontal tissues in advanced periodontitis in humans. J Periodontol. 1984 Oct;55(10):567–573. doi: 10.1902/jop.1984.55.10.567. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Baseman J. B., Alderete J. F. Treponema pallidum receptor binding proteins interact with fibronectin. J Exp Med. 1983 Jun 1;157(6):1958–1970. doi: 10.1084/jem.157.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Saglie R., Newman M. G., Carranza F. A., Jr, Pattison G. L. Bacterial invasion of gingiva in advanced periodontitis in humans. J Periodontol. 1982 Apr;53(4):217–222. doi: 10.1902/jop.1982.53.4.217. [DOI] [PubMed] [Google Scholar]
- Schmidt K. H., Gerlach D., Kühnemund O., Köhler W. Quantitative differences in specific binding of fibrinogen fragment D by M-positive and M-negative group-A streptococci. Med Microbiol Immunol. 1984;173(3):145–153. doi: 10.1007/BF02123763. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Strong D. D., Laudano A. P., Hawiger J., Doolittle R. F. Isolation, characterization, and synthesis of peptides from human fibrinogen that block the staphylococcal clumping reaction and construction of a synthetic clumping particle. Biochemistry. 1982 Mar 16;21(6):1414–1420. doi: 10.1021/bi00535a048. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Ljungh A., Rydén C., Rubin K., Hök M., Wadström T. Binding of fibronectin to the surface of group A, C, and G streptococci isolated from human infections. Eur J Clin Microbiol. 1982 Dec;1(6):381–387. doi: 10.1007/BF02019939. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M. B., Dahlén G., Linde A. Fibrinogenolytic and fibrinolytic activity in oral microorganisms. J Clin Microbiol. 1983 May;17(5):759–767. doi: 10.1128/jcm.17.5.759-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]