Abstract
Mice lacking the chemokine stromal cell-derived factor/pre-B cell growth stimulating factor or its primary physiological receptor CXCR4 revealed defects in B lymphopoiesis and bone marrow myelopoiesis during embryogenesis. We show here that adoptive transfer experiments reveal a deficiency in long-term lymphoid and myeloid repopulation in adult bone marrow by CXCR4−/− fetal liver cells, although stromal cell-derived factor/pre-B cell growth stimulating factor−/− fetal liver cells yield normal multilineage reconstitution. These findings indicate that CXCR4 is required cell autonomously for lymphoid and myeloid repopulation in bone marrow. In addition, CXCR4−/− fetal liver cells generated much more severely reduced numbers of B cells relative to other lineages in bone marrow. Furthermore, the repopulation of c-kit+ Sca-1+ linlow/− cells by CXCR4−/− fetal liver cells was less affected compared with c-kit+ Sca-1− linlow/− cells. By previous studies, it has been shown that c-kit+ Sca-1+ linlow/− cells are highly purified primitive hematopoietic progenitors and that c-kit+ Sca-1− linlow/− cells are more committed hematopoietic progenitors in mice. Thus, CXCR4 may play an essential role in generation and/or expansion of early hematopoietic progenitors within bone marrow.
Hematopoiesis is regulated by cytokines in hematopoietic microenvironment. Although several cytokines that act on hematopoietic precursors have been shown to be essential for hematopoiesis, relatively little is known regarding the cytokines that regulate the functions of primitive or early hematopoietic progenitors in a normal in vivo context. Stromal cell-derived factor/pre-B cell growth stimulating factor (SDF-1/PBSF) (1) is a member of chemokine, which is a large family of structurally related low molecular weight cytokines (2). SDF-1/PBSF was isolated as the molecule that carried signal sequences (3) or as the molecule that stimulated the proliferation of cloned B cell progenitors (4). A G-protein-coupled receptor, CXCR4, also referred to as HIV-1 entry coreceptor (5), is a primary physiological receptor for SDF-1 (6–11). Although most members of chemokine have been designated inducible inflammatory mediators, SDF-1/PBSF and CXCR4 have been shown to be responsible for B lymphopoiesis and bone marrow myelopoiesis during embryogenesis (9–12), as well as embryonic viability (9–12), vascular formation (9), cardiogenesis (9, 10, 12), and neurogenesis (10, 11). Because SDF-1/PBSF−/− or CXCR4−/− mice have much more affected myelopoiesis in bone marrow compared with myelopoiesis in fetal liver, SDF-1/PBSF and CXCR4 were thought to be involved in colonization of bone marrow by hematopoietic progenitors during embryogenesis. In addition, several studies have shown that SDF-1/PBSF is a chemoattractant for hematopoietic progenitors (13–17). On the other hand, it is possible that SDF-1 and CXCR4 are required for the formation of hematopoietic microenvironment but not the hematopoietic cells themselves by analogy with their multiple functions. Moreover, the involvement of those molecules in adult lymphoid and myeloid cell development has yet to be elucidated.
In this paper, to determine a cell-autonomous effect of the CXCR4 mutation in adult hematopoiesis, we generated chimeric mice by using fetal liver cells from SDF-1/PBSF−/− or CXCR4−/− mice. Our results indicate that CXCR4 is required for lymphoid and myeloid repopulation of adult bone marrow in cell-autonomous manner and suggest that CXCR4 plays an essential role in generation and/or expansion of early hematopoietic progenitors, including B cell progenitors within bone marrow.
MATERIALS AND METHODS
Mice.
The generation of SDF-1/PBSF−/− and CXCR4−/− mice has been described (9, 12). Mutant mice were backcrossed several times with C57BL/6-Ly5.2 mice. C57BL/6-Ly5.1 mice were a gift from M. Osawa and H. Nakauchi (University of Tsukuba, Tsukuba, Japan).
Antibodies.
Antibodies used in this study were as follows: FITC-conjugated anti- Ly5.2 (104); phycoerythrin-conjugated anti-Sca-1 (E13–161.7), anti-Mac-1 (M1/70), anti-Gr-1 (RB6–8C5), anti-CD61 (2C9.G2), anti-erythroid lineage cells (TER119), anti-CD4 (H129.19), anti-c-kit (ACK45), anti-CD43 (S7), and anti-Ig M (IgM) (AF6–78); and Peridinin Chlorophyll protein-conjugated anti-B220 (RA3–6B2). All of these antibodies were purchased from PharMingen. The anti-c-kit antibody [ACK-2; donated by S.-I. Nishikawa (Kyoto University, Kyoto)] was labeled with Cyanine 5 (Cy5) (Biological Detection Systems). Biotinylated anti-B220 (PharMingen), anti-Mac-1 (PharMingen), anti-Gr-1 (PharMingen), anti-Thy-1.2 (5a-8, Caltag, South San Francisco, CA), and TER119 (PharMingen) antibodies were used for lineage marker and were visualized with RED670-conjugated streptavidin (GIBCO).
Adoptive Transfer.
Recipient C57BL/6-Ly5.1 mice were irradiated with 1,100 rad. Donor cells were prepared as single cell suspension (2 × 107/ml) in DMEM supplemented with 2% fetal calf serum from embryonic day 16.5 (E16.5) fetal liver. Recipient animals then were injected i.v. with 2 × 106 donor cells. After transplantation, the animals were maintained in autoclaved cages on sterilized food and acidified sterile water.
Secondary Transfer.
Secondary recipient C57BL/6-Ly5.1 mice were irradiated as described above. Primary recipients that had been injected with E16.5 fetal liver cells were selected at 5 weeks posttransplant. Donor cells were prepared from bone marrow of primary recipients and were transferred into secondary mice as described above. These recipients were analyzed after 11 weeks.
Flow Cytometry.
Flow cytometry analysis was performed on single cell suspensions of fetal liver, bone marrow, spleen, and thymus. Cells were stained with FITC-, phycoerythrin-, PerCP-, RED670-, or Cy5-conjugated antibodies and were analyzed by using flow cytometry with FACScan, FACSVantage, and cellquest software (Becton Dickinson).
RESULTS
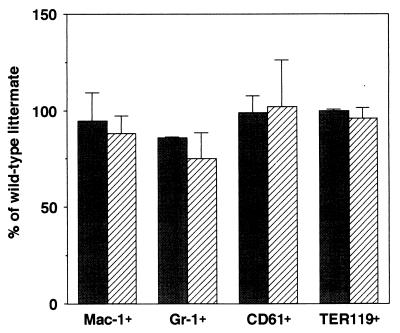
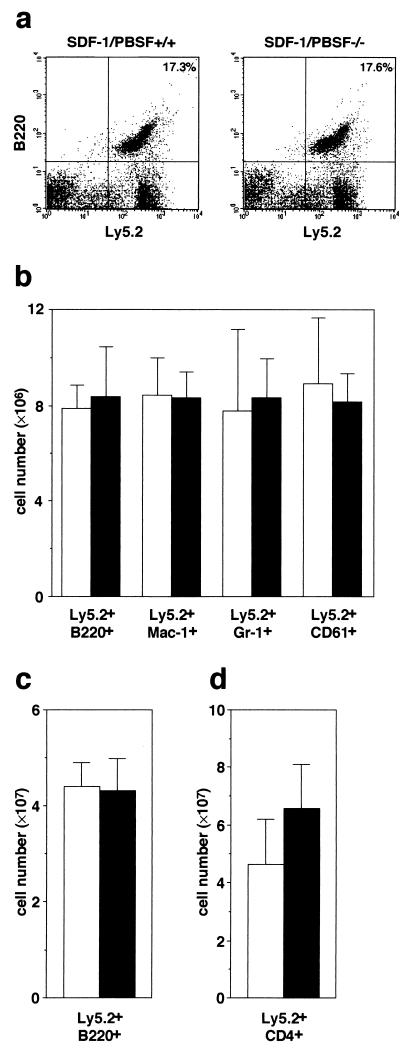
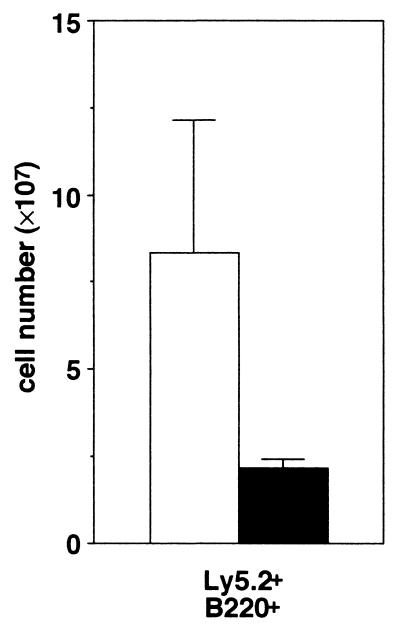
Previous studies revealed that all phenotypes found in CXCR4−/− mice were identical to those in SDF-1/PBSF−/− mice, indicating that CXCR4 is a primary physiological receptor for SDF-1/PBSF (9, 10). In agreement with the results, flow cytometry analysis revealed that the numbers of Mac-1+ granulocytic-monocytic cells, Gr-1+ granulocytic cells, Ter119+ erythroid cells, and CD61+ megakaryocytic cells within E16.5 fetal liver were indistinguishable between SDF-1/PBSF−/− and CXCR4−/− mice (Fig. 1). Then we first tested whether E16.5 fetal liver cells in the SDF-1/PBSF−/− mutants can give rise to hematopoietic reconstitution in the adult bone marrow. SDF-1/PBSF+/− mice were backcrossed several times with C57BL/6-Ly5.2 mice. We transferred fetal liver cells from E16.5 SDF-1/PBSF−/− mice or wild-type littermates into lethally irradiated C57BL/6-Ly5.1 mice. We can quantitate the contribution of the donor-derived cells in C57BL/6-Ly5.1 chimeric mice because these cells expressed the Ly5.2 allele of leukocyte common antigen Ly5 whereas host hematopoietic cells did not. After 12 weeks, the numbers of donor-derived B220+ B cells, Mac-1+ granulocytic-monocytic cells, Gr-1+ granulocytic cells, and CD61+ megakaryocytic cells in bone marrow (Fig. 2 a and b), donor-derived B220+ B cells in spleen (Fig. 2c), and donor-derived CD4+ T cells in thymus (Fig. 2d) were indistinguishable between control wild-type chimeras and SDF-1/PBSF null chimeras as shown by flow cytometry. These results demonstrated that E16.5 fetal liver in the mice that lack the signal provided by SDF-1/PBSF contained hematopoietic progenitors that were capable of reconstituting normal lymphoid and myeloid cell population in normal hematopoietic microenvironment.
Figure 1.
Myeloid, megakaryocytic, and erythroid lineage cells in E16.5 fetal liver of size-matched SDF-1/PBSF−/− (shaded columns) or size-matched CXCR4−/− mice (hatched columns). Shown is the percentage of Mac-1+ granulocytic-monocytic cells, Gr-1+ granulocytic cells, CD61+ megakaryocytic cells, and Ter119+ erythroid lineage cells in total fetal liver cells relative to control animals.
Figure 2.
Lymphoid and myeloid repopulating ability of SDF-1/PBSF mutant fetal liver cells. Shown is flow cytometric analysis of bone marrow (a and b), spleen (c), or thymus (d) from C57BL/6-Ly5.1 radiation chimeras transferred with wild-type (open columns) or SDF-1/PBSF−/− (solid columns) fetal liver cells 12 weeks after transplantation. (a) Bone marrow cells stained with anti-B220 and anti-Ly5.2. (b) Quantification of numbers of donor-derived B220+ B cells, Mac-1+ granulocytic-monocytic cells, Gr-1+ granulocytic cells, and CD61+ megakaryocytic cells found in two femurs. (c) Quantification of numbers of donor- derived B220+ B cells. (d) Quantification of numbers of donor-derived CD4+ T cells.
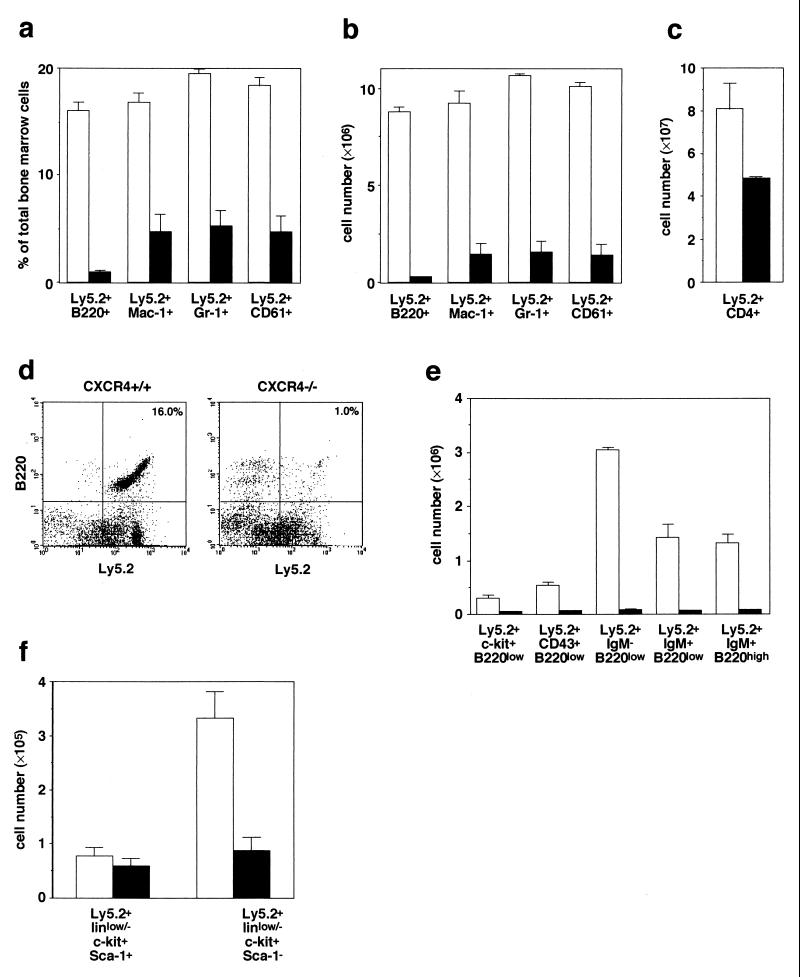
Then, to determine the functions of CXCR4 on these hematopoietic cells, fetal liver cells from E16.5 CXCR4−/− mice were transferred into lethally irradiated C57BL/6-Ly5.1 mice. The bone marrow or thymus from CXCR4 null or wild-type/C57BL/6-Ly5.1 chimeras were analyzed by flow cytometry 12 weeks after reconstitution. In contrast to normal reconstitution in SDF-1/PBSF null chimeras, CXCR4 null chimeras contained substantially reduced numbers of donor-derived B220+ B cells, Mac-1+ granulocytic-monocytic cells, Gr-1+ granulocytic cells, and CD61+ megakaryocytic cells in bone marrow, and CD4+ thymocytes (Fig. 3 a–d). Thus, CXCR4 in hematopoietic cells is essential for long-term lymphoid and myeloid reconstitution. Of note, the numbers of donor-derived B cells in the bone marrow of CXCR4 null chimeras were much more severely reduced compared with the reduction of other lineage cell numbers, suggesting that mutation of CXCR4 in fetal liver cells affected generation and/or expansion of B-cell progenitors (Fig. 3 a, b, and d). B cells in bone marrow can be divided into several fractions based on differential expression of c-kit, CD43, and IgM on the surface. An ordering of these stages were suggested, from B220low c-kit+ pro-B cells (18) to B220low c-kit− CD43+ pro-B cells to B220low CD43− IgM− pre-B cells to B220low IgM+ immature B cells and thence to B220high IgM+ mature B cells (19). The numbers of cells in all fractions were reduced in the bone marrow of CXCR4 null chimeric mice (Fig. 3e).
Figure 3.
Long-term repopulating ability of CXCR4 mutant fetal liver cells. Shown is flow cytometric analysis of staining for lymphoid, myeloid, and megakaryocytic cells of bone marrow (a, b, and d–f) or thymus (c) from C57BL/6-Ly5.1 radiation chimeras reconstituted with control (open columns) or CXCR4−/− (solid columns) fetal liver cells 12 weeks (a–e) or 9 weeks (f) after transplantation. Shown is the relative percentage (a) or cell numbers (b) of donor-derived B220+ B cells, Mac-1+ granulocytic-monocytic cells, Gr-1+ granulocytic cells and CD61+ megakaryocytic cells found in two femurs. (c) Quantification of numbers of donor-derived CD4+ T cells in thymus. (d) Bone marrow cells stained with anti-B220 and anti-Ly5.2. (e) Quantification of numbers of donor-derived B220low c-kit+ pro-B cells, B220low CD43+ pro-B cells, B220low IgM− pro-B and pre-B cells, B220low IgM+ immature B cells, and B220high IgM+ mature B cells found in two femurs. (f) Quantification of numbers of donor-derived c-kit+ Sca-1+ linlow/− cells and c-kit+ Sca-1− linlow/− cells found in two femurs.
To determine where the development of lymphoid and myeloid cells was affected in CXCR4 null chimeric mice, we examined the numbers of c-kit+ Sca-1+ linlow/− and c-kit+ Sca-1− linlow/− cells in their bone marrow. It has been shown that c-kit+ Sca-1+ linlow/− cells are highly purified primitive hematopoietic progenitors and that c-kit+ Sca-1− linlow/− cells are more committed hematopoietic progenitors in mice (20). CXCR4 null chimeras contained approximately comparable numbers of c-kit+ Sca-1+ linlow/− cells but substantially reduced numbers of c-kit+ Sca-1− linlow/− cells (Fig. 3f). These results raise the possibility that CXCR4 is involved in the transition of primitive hematopoietic progenitors to more committed hematopoietic progenitors and/or survival and/or expansion of those committed progenitors within bone marrow.
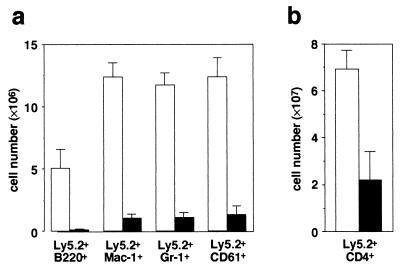
To investigate further the reconstituting ability of donor-derived hematopoietic progenitors in bone marrow, bone marrow cells from the primary transplant recipient were transferred into secondary mice. These recipients were analyzed after 11 weeks. The abilities of the mutant stem cells to repopulate secondary recipients were severely affected compared with wild-type cells. However, three of five mice that received mutant cells exhibited myeloid, megakaryocytic, and T lineage cell reconstitution, although the contribution of mutant cells was reduced (Fig. 4a and b). In these mice, donor-derived B cells were virtually absent. Thus, long-term repopulating stem cells were present in bone marrow of CXCR4 null chimeras, and generation of B cells from those cells may be impaired.
Figure 4.
Flow cytometric analysis of bone marrow (a) or thymus (b) from secondary recipients reconstituted with control (open columns) or CXCR4−/− (solid columns) bone marrow cells obtained from primary recipients 11 weeks after transplantation. (a) Quantification of numbers of donor-derived B220+ B cells, Mac-1+ granulocytic-monocytic cells, Gr-1+ granulocytic cells, and CD61+ megakaryocytic cells found in two femurs. (b) Quantification of numbers of donor-derived CD4+ T cells in thymus.
Finally, we analyzed B lymphoid cellularity of secondary lymphoid organs from CXCR4 null chimeras. Flow cytometry analysis of spleen revealed a 2- to 4-fold decrease in donor-derived mature B cell numbers in CXCR4 null/C57BL/6-Ly5.1 chimeras as compared with control chimeras 12 weeks after transplantation (Fig. 5). Despite the dramatic decrease in B cell progenitors in the bone marrow of CXCR4 null chimeras, a reduction of mature B lymphocytes in the periphery was relatively mild, suggesting that CXCR4 was not required for expansion and maintenance of the long-lived pool of peripheral mature B cells (21).
Figure 5.
Analysis of B lymphoid cellularity of spleen from C57BL/6-Ly5.1 radiation chimeras reconstituted with control (open columns) or CXCR4−/− (solid columns) fetal liver cells. Quantification of numbers of donor-derived B220+ B cells in total splenocytes.
DISCUSSION
Although previous studies have implicated a variety of cytokines act on hematopoietic progenitors, relatively little information has been available regarding cytokines that control the functions of primitive or early hematopoietic progenitor cells in a normal in vivo context. Bone marrow or fetal liver cells from flk-2−/− (22), c-kit−/− (23), or c-mpl−/− (24) mice have been shown to fail to compete effectively with normal cells for long-term repopulation of the hematopoietic organs. In this study, we demonstrated that the long-term myeloid and lymphoid repopulation by CXCR4 null fetal liver cells was affected. The defect was not merely a consequence of reduced numbers of hematopoietic stem cells in E16.5 CXCR4 −/− fetal liver cells transferred because E16.5 fetal liver cells from its ligand SDF-1/PBSF −/− mice yielded normal reconstitution. Because CXCR4 null chimeric mice contained approximately comparable numbers of c-kit+ Sca-1+ linlow/− cells, which have been thought to be highly purified primitive hematopoietic progenitors, CXCR4 may be involved in generation, survival, and/or expansion of more committed progenitor cells in bone marrow. Further characterization about the c-kit+ Sca-1+ linlow/− cells in CXCR4 null chimeras will be needed to address this issue. On the other hand, our data raised the possibility that CXCR4 plays a role in migration of long-term repopulating hematopoietic stem cells from peripheral blood into the bone marrow or their homing or expansion in a cell-autonomous manner. Recently, treatment of human CD34+-enriched hematopoietic cells with anti-CXCR4 antibodies has been shown to prevent their erythroid or myeloid reconstitution of nonobese diabetic/severe combined immunodeficient mice (25). However, the utilized assay system, that is useful in measuring the repopulating capacity of human stem cells, shares the several disadvantages for studying the biology of the hematopoietic system. There are species-specific differences between donors and recipients and multiple defects in recipient nonobese diabetic/severe combined immunodeficient mice. Also, transplanted human cells compete with endogenous murine hematopoietic cells in recipient bone marrow.
Furthermore, our results suggested that mutation of CXCR4 in hematopoietic cells also affected specifically the generation and/or expansion of B-cell progenitors in bone marrow. Thus, it is important to compare the functions of CXCR4 to those of IL-7 or IL-7 receptor, which is essential for B cell development (26–29). IL-7 or IL-7 receptor is also responsible for expansion of the long-lived pool of peripheral mature B cells as well as B cell progenitors (26–29). However, the mice reconstituted by CXCR4−/− fetal liver cells have a mild reduction in splenic mature B cell numbers relative to a reduction in bone marrow B cell numbers, suggesting that functions of CXCR4 in B cell development are relatively organ specific. Of interest is the previous study demonstrating that mice expressing dominant negative form of Ras in B lymphocytes revealed similar defects (30). It is interesting to determine whether Ras is implicated in signaling through CXCR4. Why do SDF-1/PBSF and CXCR4 have specific functions for B cell development in bone marrow? B cells may be, in fact, different from other lineages during ontogeny in bone marrow.
On the other hand, CXCR4 functions as a coreceptor for strains of HIV-1 that are dominant during progression to immunodeficiency (5). Because inhibitors of CXCR4 are intended to block viral entry (31–33), our study, which determined the functions of CXCR4 in adult hematopoiesis, is also important for HIV-1 therapeutic research. Together, our examinations of cell-autonomous effects of CXCR4 mutation in regulation of lymphoid and myeloid cell development add to previous studies showing the defects of hematopoiesis in SDF-1/PBSF or CXCR4−/− embryos.
Acknowledgments
We thank T. Usami, R. Okamoto, Y. Shiozaki, and M. Ninomiya for technical assistance and H. Nakauchi, M. Osawa, and M. Ogawa for technical advice and helpful discussions. This work was supported by grants from the Ministry of Education of Japan.
ABBREVIATIONS
- SDF-1
stromal cell-derived factor 1
- PBSF
pre-B cell growth stimulating factor
- E16.5
embryonic day 16.5
References
- 1.Nagasawa T, Tachibana K, Kawabata K. Adv Immunol. 1999;71:211–228. doi: 10.1016/s0065-2776(08)60403-4. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 3.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 4.Nagasawa T, Kikutani H, Kishimoto T. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 6.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodoroski J, Springer T A. Nature (London) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 7.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J, Arenzana-Seisdedos F, Schwartz O, Heard J, Clark-Lewis I, Legler D F, et al. Nature (London) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 8.Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul C C, Yoshie O, Matsushima K, Yoshida N, Springer T A, Kishimoto T. Proc Natl Acad Sci USA. 1996;93:14726–14729. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S-I, et al. Nature (London) 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y-Z, Kottman A H, Kuroda M, Taniuchi I, Littman D R. Nature (London) 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 11.Ma Q, Jones D, Borghesani P R, Segal R A, Nagasawa T, Kishimoto T, Bronson R T, Springer T A. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S-I, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Nature (London) 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 13.Aiuti A, Webb I J, Bleul C C, Springer T A, Gutierrez-Ramos J C. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Apuzzo M, Rolink A, Loetscher M, Hoxie J A, Clark-Lewis I, Melcher F, Baggiolini M, Moser B. Eur J Immunol. 1997;27:1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- 15.Kim C H, Broxmeyer H E. Blood. 1998;91:100–110. [PubMed] [Google Scholar]
- 16.Wang J F, Liu Z-Y, Groopman J E. Blood. 1998;92:756–764. [PubMed] [Google Scholar]
- 17.Hamada T, Möhle R, Hesselgesser J, Hoxie J, Nachman R L, Moore M A S, Rafii S. J Exp Med. 1998;188:539–548. doi: 10.1084/jem.188.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolink A, Grawunder U, Winkler T H, Karasuyama H, Melchers F. Int Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 19.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S-I, Miura Y, Suda T. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 21.Förster I, Rajewsky K. Proc Natl Acad Sci USA. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda S, Shimizu T, Rodewald H-R. Blood. 1997;89:518–525. [PubMed] [Google Scholar]
- 23.Mackarehtschian K, Hardin J D, Moore K A, Boast S, Goff S P, Lemischka I R. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 24.Kimura S, Roberts A W, Metcalf D, Alexander W S. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 26.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, Nishikawa S-I. Proc Natl Acad Sci USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabstein K H, Waldschmidt T J, Finkelman F D, Hess B W, Alpert A R, Boiani N E, Namen A E, Morrissey P J. J Exp Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peschon J J, Morrissey P J, Grabstein K H, Ramsdell F J, Maraskovsky E, Gliniak B C, Park L S, Ziegler S F, Williams D E, Ware C B, et al. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Freeden-Jeffry U, Vieira P, Lucian L A, McNeil T, Burdach S E, Murray R. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iritani B M, Forbush K A, Farrar M A, Perlmutter R M. EMBO J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schols D, Struyf S, Van Damme J, Este J A, Henson G, De Clercq E. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, et al. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doranz B J, Grovit-Ferbas K, Sharron M P, Mao S-H, Goetz M B, Daar E S, Doms R W, O’Brien W A. J Exp Med. 1997;186:1395–1400. doi: 10.1084/jem.186.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donzella G A, Schols D, Lin S W, Este J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, et al. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]