Abstract
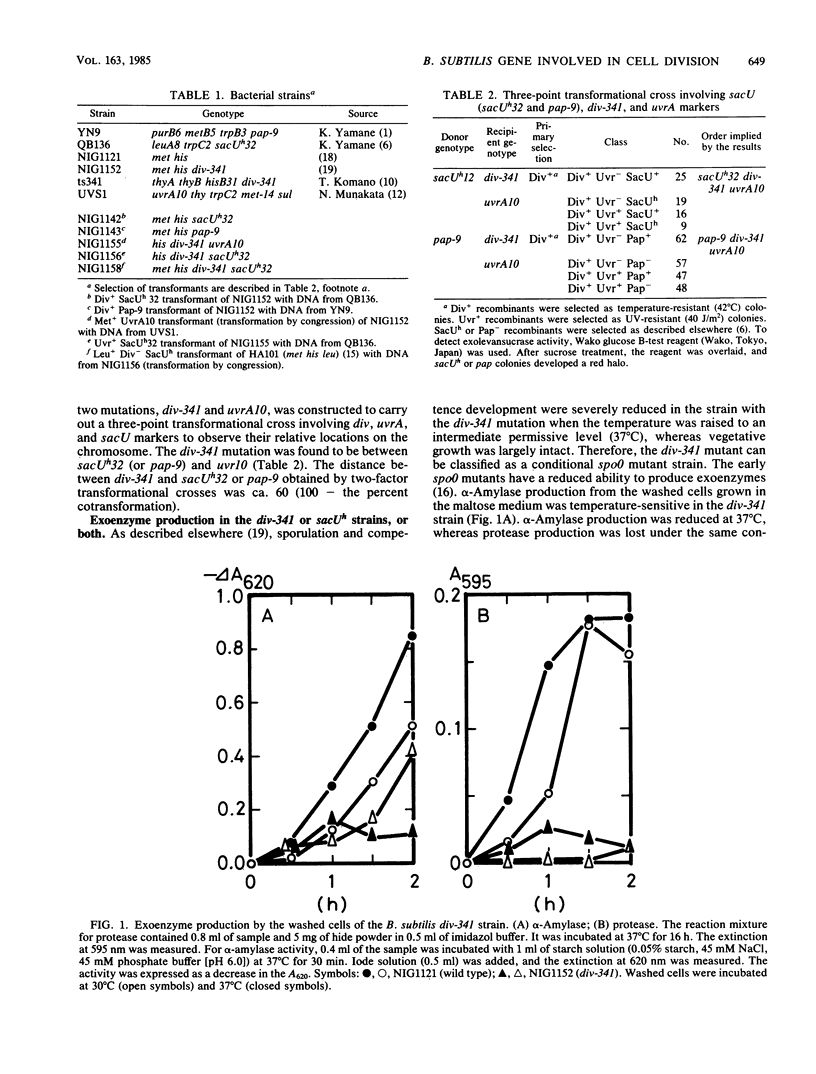
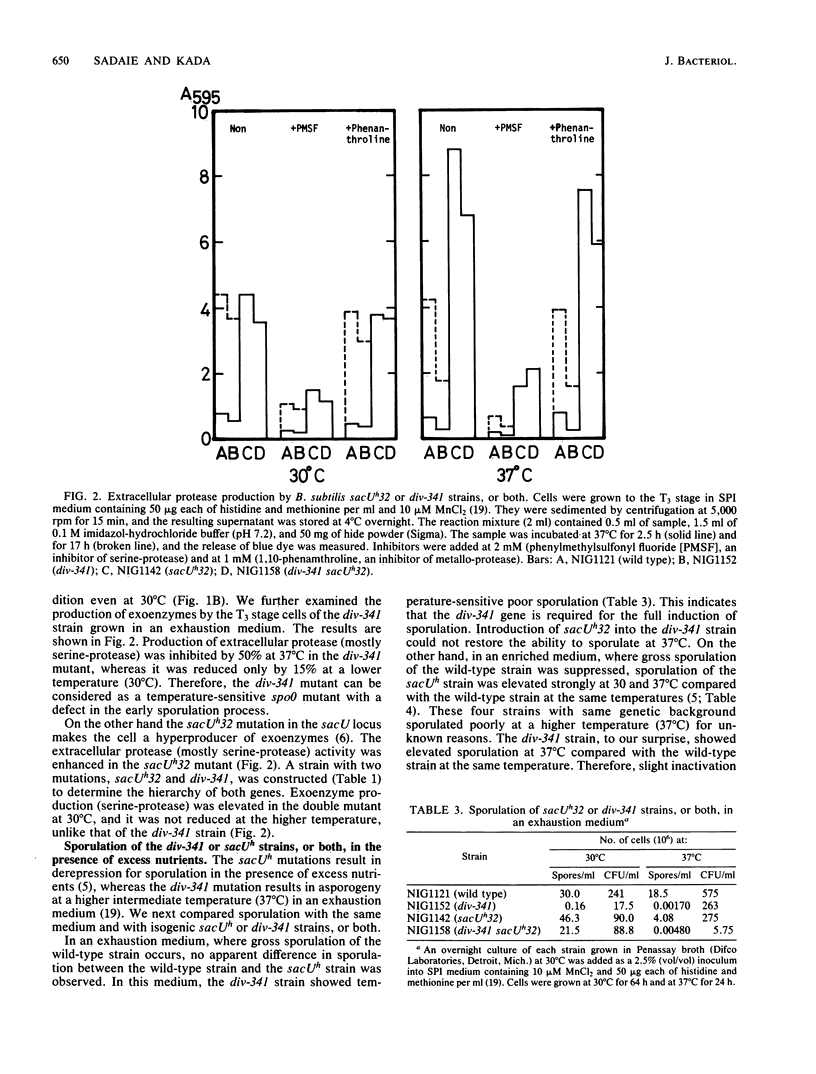
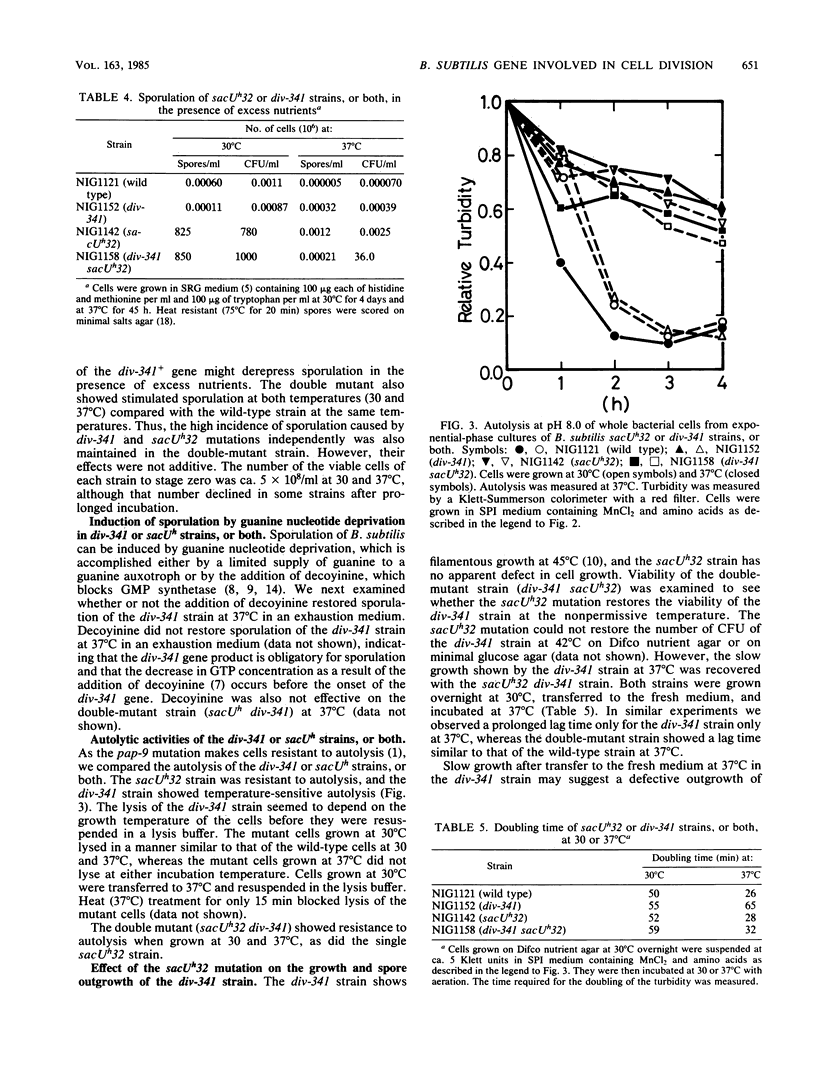
Bacillus subtilis strains carrying div-341 or sacU mutations, or both, have been characterized to reveal the roles of both genes in the initiation of sporulation, as well as in cell division and exoenzyme secretion. Both mutations were closely linked by transformation and caused the pleiotropic effects on sporulation and sporulation-associated events. Some sacU mutations (sacUh) resulted in hyperproduction of exoenzymes, reduced autolysis, and an ability to sporulate in the presence of excess nutrients. The div-341 mutation, on the other hand, resulted in filamentous growth at a higher temperature (45 degrees C) and showed spo0 properties at an intermediate permissive temperature (37 degrees C) in the usual sporulation medium. However, the div-341 strain sporulated better than wild-type strain at 37 degrees C in the presence of excess nutrients. Exoenzyme production and autolysis were reduced at 37 degrees C in the div-341 strain. A double mutant with sacUh32 and div-341 showed the complex phenotypes. It showed the sacUh32 property of autolysis and exoenyzme secretion. It showed the sacUh32 property of sporulation at 30 degrees C and the div-341 property at 37 degrees C. Slow growth and defective spore outgrowth of the div-341 strain at 37 degrees C were not observed in the double-mutant strain. Based on pleiotropic phenotypes and close linkages of both mutations, we discuss the relationship between the sacU and div-341 genes and their roles in sporulation, exoenzyme secretion, and cell division.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Yoneda Y., Yamane K., Maruo B. Pleiotropic phenomena in autolytic enzyme(s) content, flagellation, and simultaneous hyperproduction of extracellular alpha-amylase and protease in a Bacillus subtilis mutant. J Bacteriol. 1975 Oct;124(1):459–469. doi: 10.1128/jb.124.1.459-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer B. N., Mandelstam J. Criteria for categorizing early biochemical events occurring during sporulation of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):411–415. doi: 10.1128/jb.121.2.411-415.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R. H. Genetic control of sporulation. Annu Rev Genet. 1977;11:29–48. doi: 10.1146/annurev.ge.11.120177.000333. [DOI] [PubMed] [Google Scholar]
- Endo T., Ishihara H., Freese E. Properties of a Bacillus subtilis mutant able to sporulate continually during growth in synthetic medium. J Gen Microbiol. 1983 Jan;129(1):17–30. doi: 10.1099/00221287-129-1-17. [DOI] [PubMed] [Google Scholar]
- Kunst F., Pascal M., Lepesant-Kejzlarova J., Lepesant J. A., Billault A., Dedonder R. Pleiotropic mutations affecting sporulation conditions and the syntheses of extracellular enzymes in Bacillus subtilis 168. Biochimie. 1974;56(11-12):1481–1489. doi: 10.1016/s0300-9084(75)80270-7. [DOI] [PubMed] [Google Scholar]
- Lepesant J. A., Kunst F., Lepesant-Kejzlarová J., Dedonder R. Chromosomal location of mutations affecting sucrose metabolism in Bacillus subtilis Marburg. Mol Gen Genet. 1972;118(2):135–160. doi: 10.1007/BF00267084. [DOI] [PubMed] [Google Scholar]
- Lopez J. M., Dromerick A., Freese E. Response of guanosine 5'-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J Bacteriol. 1981 May;146(2):605–613. doi: 10.1128/jb.146.2.605-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. M., Marks C. L., Freese E. The decrease of guanine nucleotides initiates sporulation of Bacillus subtilis. Biochim Biophys Acta. 1979 Oct 4;587(2):238–252. doi: 10.1016/0304-4165(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Mitani T., Heinze J. E., Freese E. Induction of sporulation in Bacillus subtilis by decoyinine or hadacidin. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1118–1125. doi: 10.1016/s0006-291x(77)80094-6. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Komano T., Maruyama Y. Timed action of the gene products required for septum formation in the cell cycle of Bacillus subtilis. J Bacteriol. 1982 Feb;149(2):673–680. doi: 10.1128/jb.149.2.673-680.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K., Freese E. A decrease in S-adenosylmethionine synthetase activity increases the probability of spontaneous sporulation. J Bacteriol. 1982 Oct;152(1):400–410. doi: 10.1128/jb.152.1.400-410.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K., Kandala J., Freese E. Evidence that Bacillus subtilis sporulation induced by the stringent response is caused by the decrease in GTP or GDP. J Bacteriol. 1982 Aug;151(2):1062–1065. doi: 10.1128/jb.151.2.1062-1065.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Yanagida T. Isolation of a suppressor mutant in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):1187–1188. doi: 10.1128/jb.95.3.1187-1188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Typist: effect of glucose and cyclic nucleotides on the transcription of alpha-amylase mRHA in Bacillus subtilis. Biochem Biophys Res Commun. 1975 Apr 7;63(3):606–610. doi: 10.1016/s0006-291x(75)80427-x. [DOI] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Formation of competent Bacillus subtilis cells. J Bacteriol. 1983 Feb;153(2):813–821. doi: 10.1128/jb.153.2.813-821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotsu H., Kawamura F., Kobayashi Y., Saito H. Early sporulation gene spo0F: nucleotide sequence and analysis of gene product. Proc Natl Acad Sci U S A. 1983 Feb;80(3):658–662. doi: 10.1073/pnas.80.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Kunst F., Dedonder R. Mapping of mutations affecting synthesis of exocellular enzymes in Bacillus subtilis. Identity of the sacUh, amyB and pap mutations. Mol Gen Genet. 1976 Nov 17;148(3):281–285. doi: 10.1007/BF00332902. [DOI] [PubMed] [Google Scholar]
- Uratani B., Lopez J. M., Freese E. Effect of decoyinine on peptidoglycan synthesis and turnover in Bacillus subtilis. J Bacteriol. 1983 Apr;154(1):261–268. doi: 10.1128/jb.154.1.261-268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


