Abstract
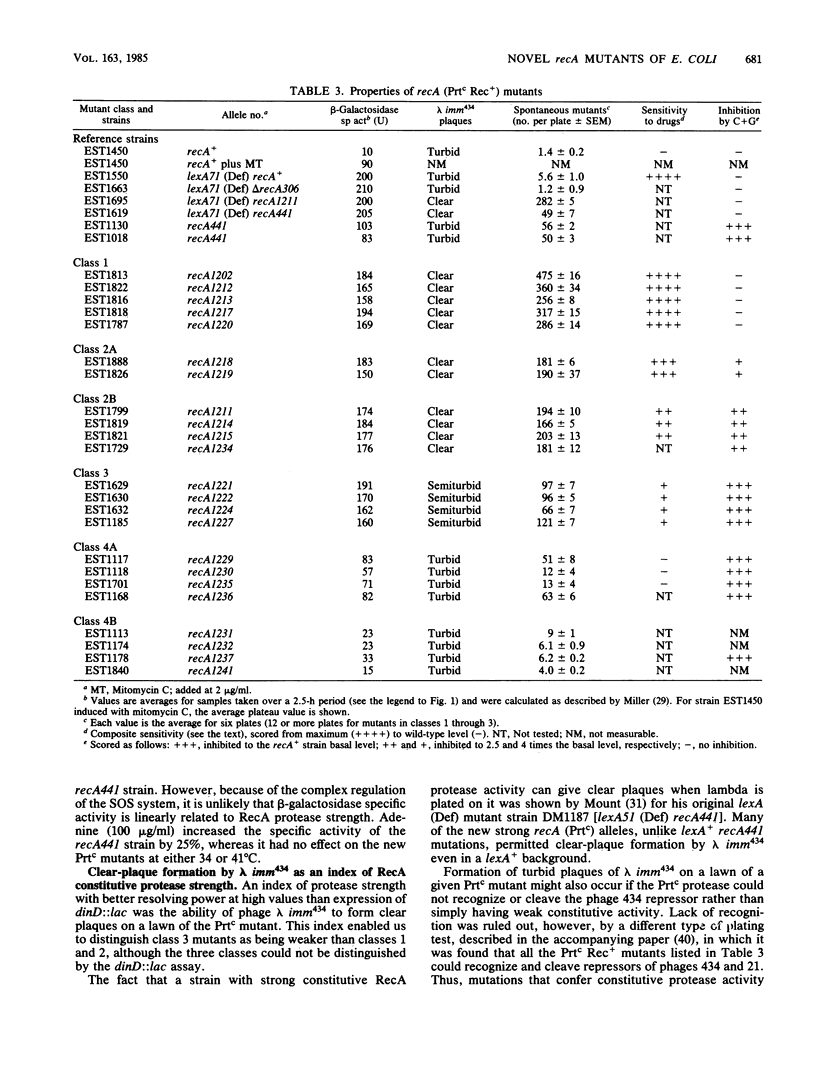
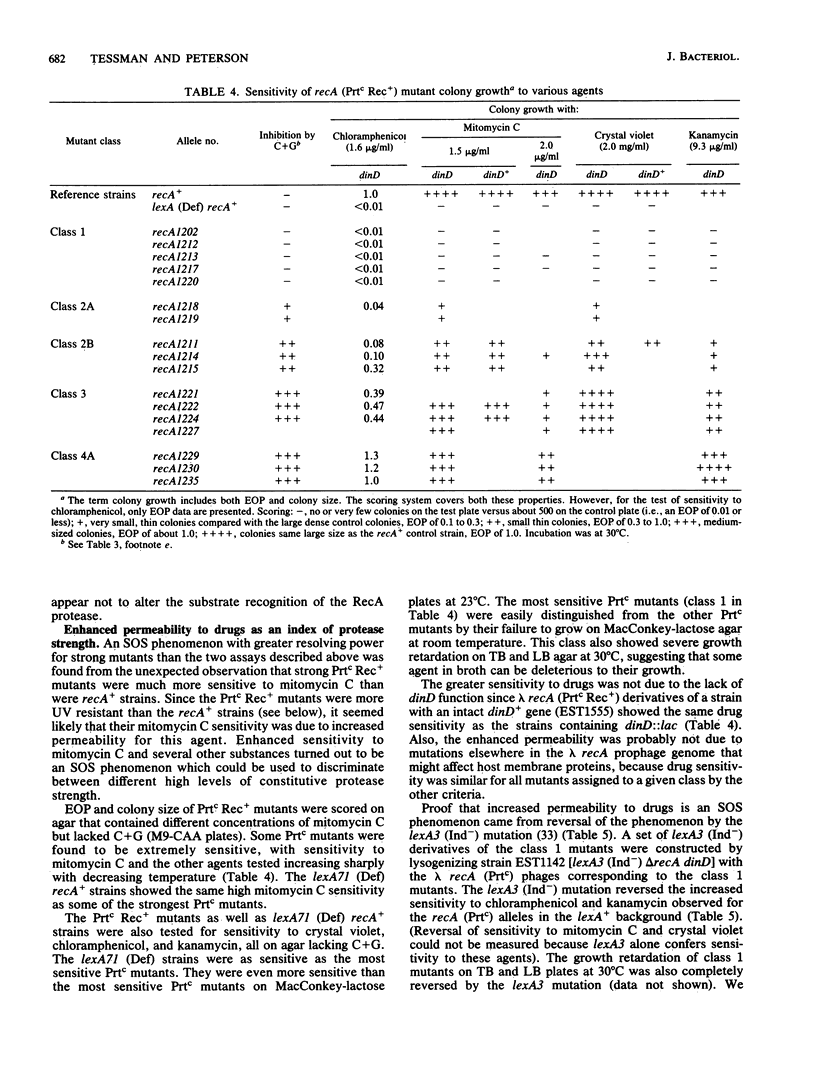
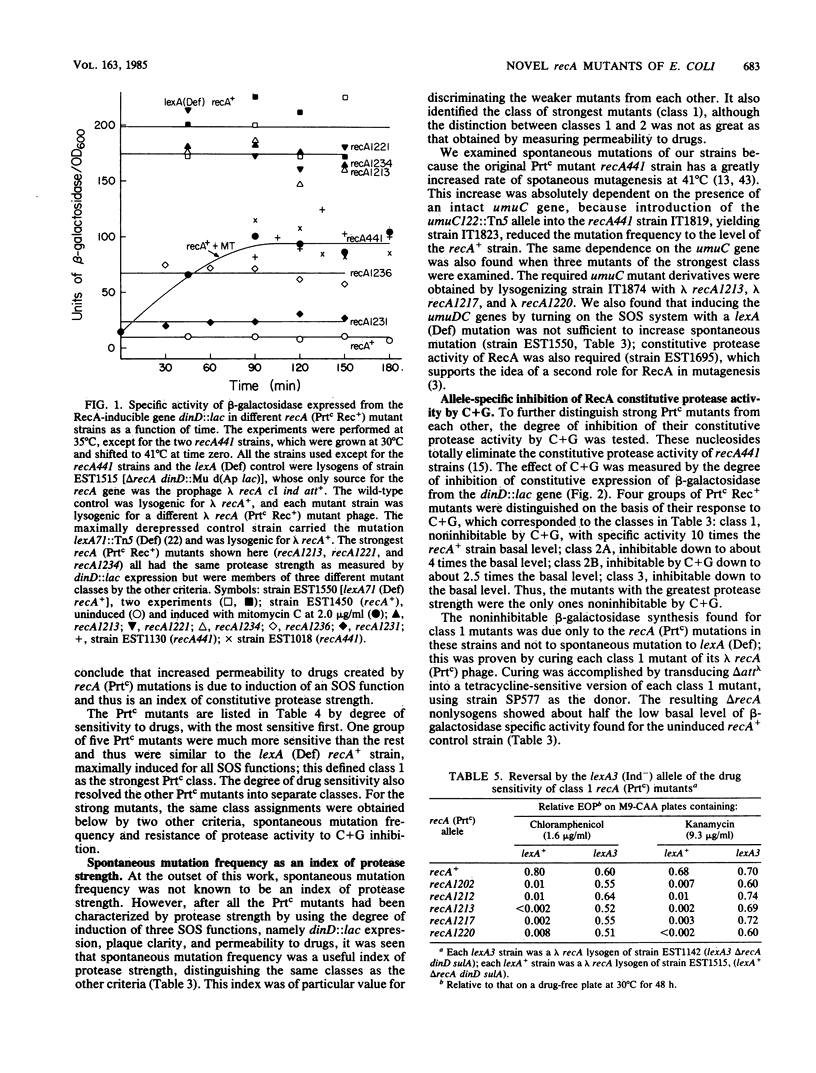
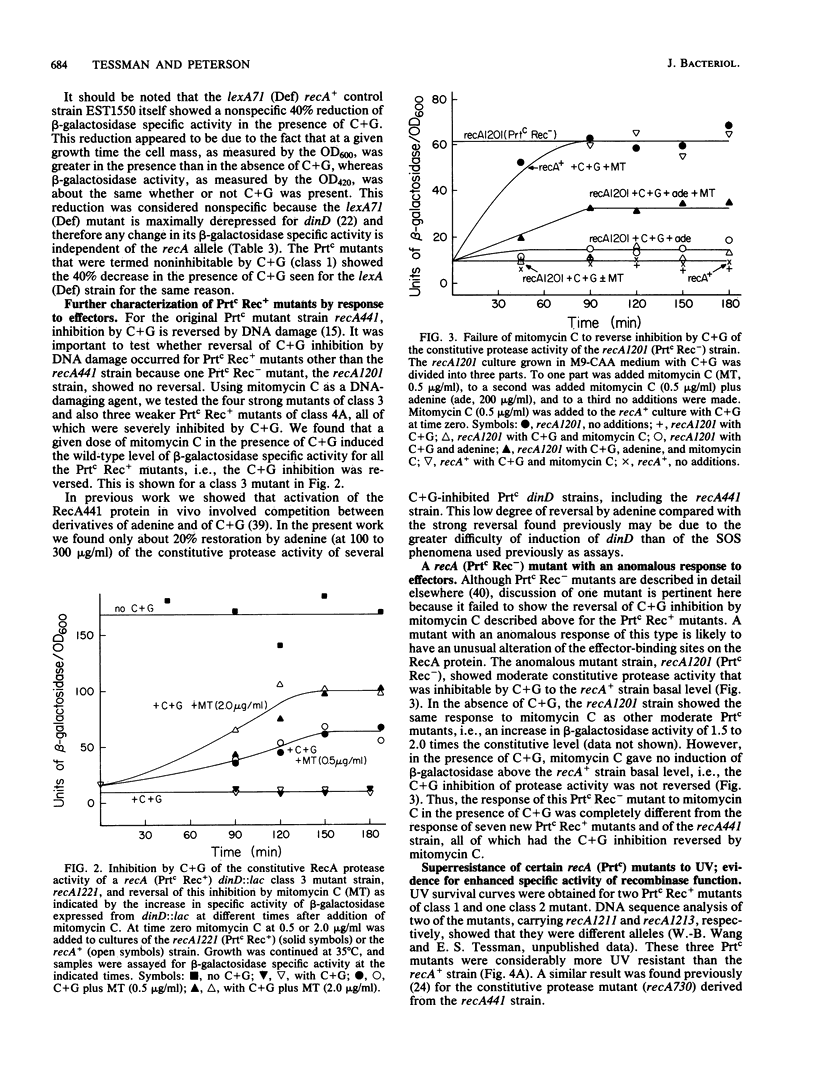
As a prerequisite to mutational analysis of functional sites on the RecA protein of Escherichia coli, a method was developed for rapid isolation of recA mutants with altered RecA protease function. The method involves plating mutagenized lambda recA+ cI ind on strains deleted for recA and containing, as indicators of RecA protease activity, Mu d(Ap lac) fusions in RecA-inducible genes. The lambda recA phages were recognized by their altered plaque colors, and the RecA protease activity of the lambda recA mutant lysogens was measured by expression of beta-galactosidase from dinD::lac. One class of recA mutants had constitutive protease activity and was designated Prtc; in these cells the RecA protein was always in the protease form without the usual need for DNA damage to activate it. Some Prtc mutants were recombinase negative and were designated Prtc Rec-. Another class of 65 recA mutants isolated as being protease defective were all also recombinase defective. Unlike the original temperature-dependent Prtc Rec+ mutant (recA441), the new Prtc Rec+ mutants showed constitutive protease activity at any growth temperature, with some having considerably greater activity than the recA441 strain. Study of these strong Prtc Rec+ mutants revealed a new SOS phenomenon, increased permeability to drugs. Use of this new SOS phenomenon as an index of protease strength clearly distinguished 5 Prtc mutants as the strongest among 150. These five strongest Prtc mutants showed the greatest increase in spontaneous mutation frequency and were not inhibited by cytidine plus guanosine, which inhibited the constitutive protease activity of the recA441 strain and of all the other new Prtc mutants. Strong Prtc Rec+ mutants were more UV resistant than recA+ strains and showed indications of having RecA proteins whose specific activity of recombinase function was higher than that of wild-type RecA. A Prt+ Rec- mutant with an anomalous response to effectors is described.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach J. I., Howard-Flanders P. Identification of wild-type or mutant alleles of bacterial genes cloned on a bacteriophage lambda vector: isolation of uvrC(am) and other mutants. J Bacteriol. 1981 May;146(2):713–717. doi: 10.1128/jb.146.2.713-717.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Kenyon C. J., Walker G. C. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5749–5753. doi: 10.1073/pnas.78.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M., Herrera G., Collado P., Rebollo J. E., Botella L. M. Influence of RecA protein on induced mutagenesis. Biochimie. 1982 Aug-Sep;64(8-9):633–636. doi: 10.1016/s0300-9084(82)80102-8. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. recA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3433–3437. doi: 10.1073/pnas.78.6.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. Function of nucleoside triphosphate and polynucleotide in Escherichia coli recA protein-directed cleavage of phage lambda repressor. J Biol Chem. 1981 Aug 10;256(15):8039–8044. [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari R., Huisman O. Novel mechanism of cell division inhibition associated with the SOS response in Escherichia coli. J Bacteriol. 1983 Oct;156(1):243–250. doi: 10.1128/jb.156.1.243-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Froehlich B., Epstein W. Escherichia coli mutants in which transcription is dependent on recA function. J Bacteriol. 1981 Sep;147(3):1117–1120. doi: 10.1128/jb.147.3.1117-1120.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Glassberg J., Meyer R. R., Kornberg A. Mutant single-strand binding protein of Escherichia coli: genetic and physiological characterization. J Bacteriol. 1979 Oct;140(1):14–19. doi: 10.1128/jb.140.1.14-19.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., Aoki K. H., Ujita E. L., McEntee K. Identification of the amino acid substitutions in two mutant forms of the recA protein from Escherichia coli: recA441 and recA629. J Biol Chem. 1984 Sep 25;259(18):11279–11283. [PubMed] [Google Scholar]
- Krueger J. H., Elledge S. J., Walker G. C. Isolation and characterization of Tn5 insertion mutations in the lexA gene of Escherichia coli. J Bacteriol. 1983 Mar;153(3):1368–1378. doi: 10.1128/jb.153.3.1368-1378.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman H. B., Witkin E. M. DNA degradation, UV sensitivity and SOS-mediated mutagenesis in strains of Escherichia coli deficient in single-strand DNA binding protein: effects of mutations and treatments that alter levels of Exonuclease V or recA protein. Mol Gen Genet. 1983;190(1):92–100. doi: 10.1007/BF00330329. [DOI] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M. tif-1 mutation alters polynucleotide recognition by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6061–6065. doi: 10.1073/pnas.78.10.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand P., Blanco M., Devoret R. Characterization of lexB mutations in Escherichia coli K-12. J Bacteriol. 1977 Aug;131(2):572–582. doi: 10.1128/jb.131.2.572-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. Isolation and characterization of mutants of lambda recA which synthesize a hyperactive recA protein. Virology. 1979 Oct 30;98(2):484–488. doi: 10.1016/0042-6822(79)90574-9. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R., Imanaka T., Aiba S. Nucleotide sequence of the Bacillus stearothermophilus alpha-amylase gene. J Bacteriol. 1985 Jul;163(1):401–406. doi: 10.1128/jb.163.1.401-406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky E. M., Roberts J. W. Induction of SOS functions: regulation of proteolytic activity of E. coli RecA protein by interaction with DNA and nucleoside triphosphate. Cell. 1981 Jul;25(1):259–267. doi: 10.1016/0092-8674(81)90251-8. [DOI] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Williams J. G., Osber L., Radding C. M. Homologous pairing in genetic recombination. The pairing reaction catalyzed by Escherichia coli recA protein. J Biol Chem. 1981 Jul 25;256(14):7565–7572. [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. Isolation of protease-proficient, recombinase-deficient recA mutants of Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):688–695. doi: 10.1128/jb.163.2.688-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. tif-dependent induction of colicin E1, prophage lambda, and filamentation in Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1307–1317. doi: 10.1128/jb.143.3.1307-1317.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M., McCall J. O., Volkert M. R., Wermundsen I. E. Constitutive expression of SOS functions and modulation of mutagenesis resulting from resolution of genetic instability at or near the recA locus of Escherichia coli. Mol Gen Genet. 1982;185(1):43–50. doi: 10.1007/BF00333788. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


