Abstract
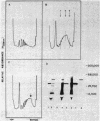
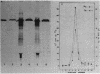
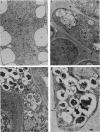
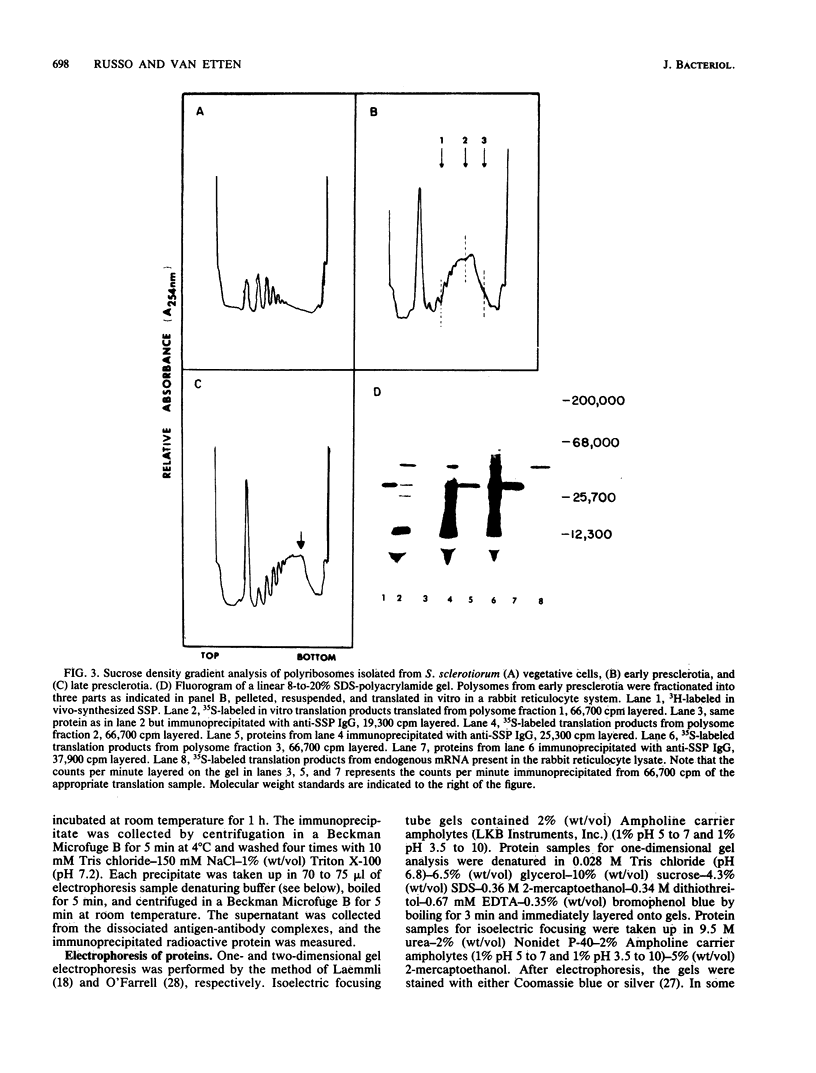
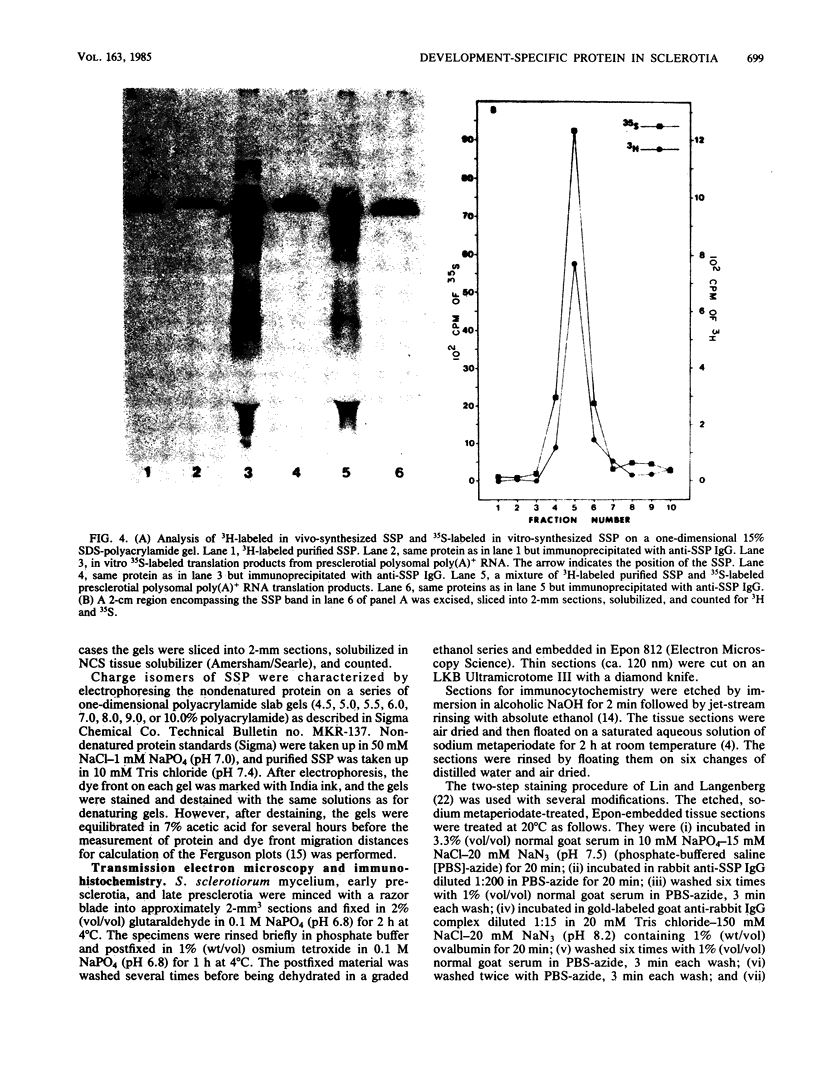
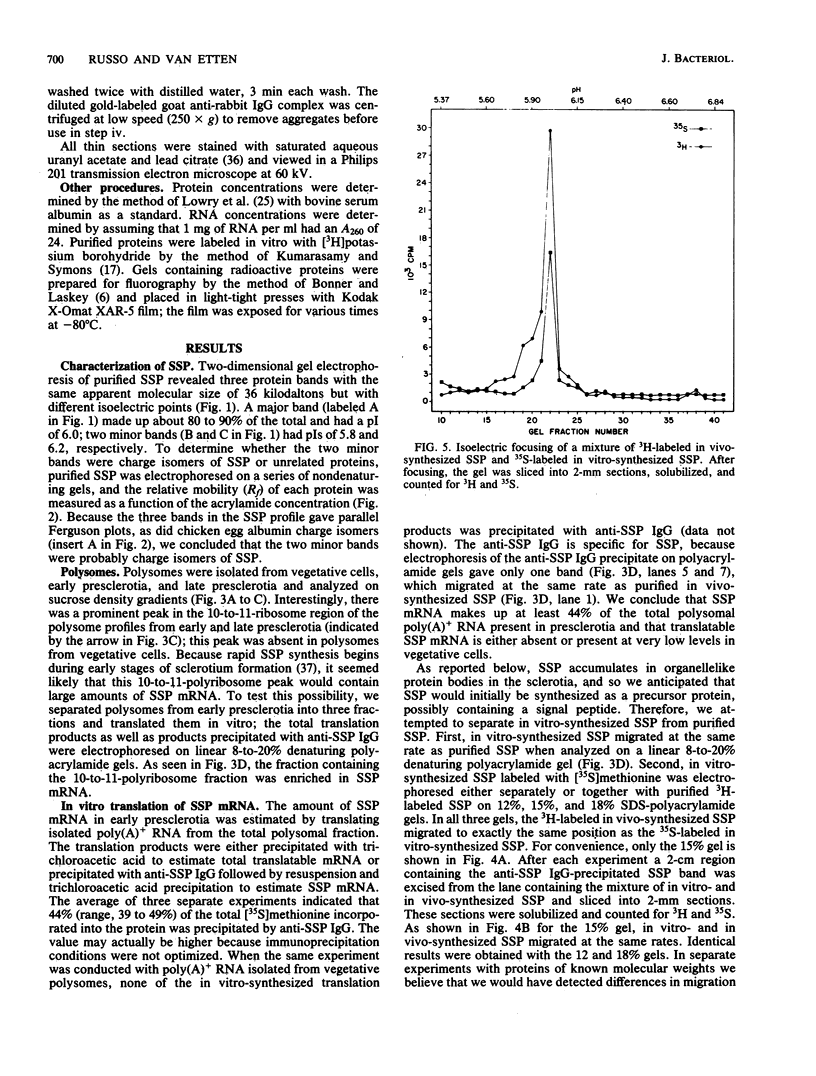
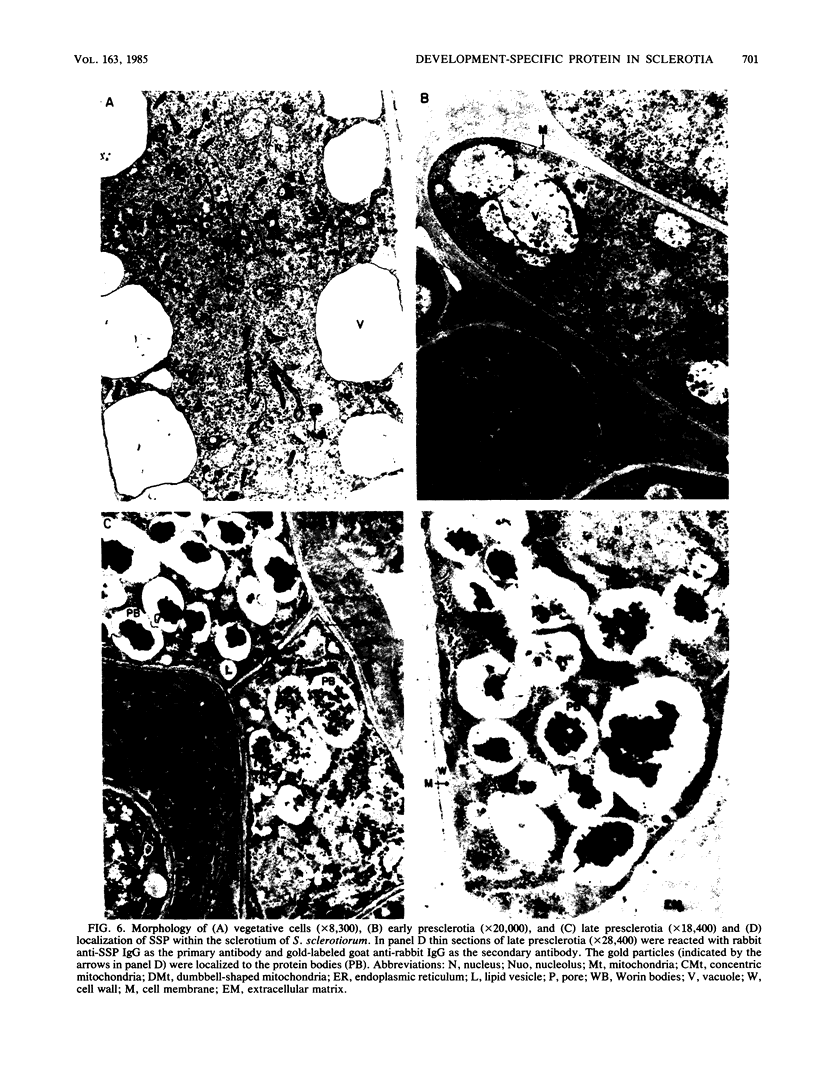
A development-specific protein (SSP) makes up about 35 to 40% of the total protein in sclerotia of the fungus Sclerotinia sclerotiorum. The protein consists of three charge isomers, with one isomer making up 80 to 90% of the total. In vitro translation of poly(A)+ RNA isolated from cells in early stages of sclerotia formation revealed that 44% of the amino acids incorporated was into SSP. In vivo- and in vitro-synthesized forms of SSP migrated at identical rates on both isoelectric focusing and denaturing polyacrylamide gels, indicating that SSP was not synthesized as a larger precursor. This was significant because SSP accumulated in membrane-bound, organellelike structures which resemble protein bodies found in seeds of many higher plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. Ovalbumin utilizes an NH2-terminal signal sequence. J Biol Chem. 1982 Apr 25;257(8):4578–4582. [PubMed] [Google Scholar]
- Erlandsen S. L., Parsons J. A., Rodning C. B. Technical parameters of immunostaining of osmicated tissue in epoxy sections. J Histochem Cytochem. 1979 Sep;27(9):1286–1289. doi: 10.1177/27.9.90080. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Kumarasamy R., Symons R. H. The tritium labeling of small amounts of protein for analysis by electrophoresis on sodium dodecyl sulfate--polyacrylamide slab gels. Anal Biochem. 1979 Jun;95(2):359–363. doi: 10.1016/0003-2697(79)90739-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Jones R. A., Tsai C. Y. Isolation and in vitro translation of zein messenger ribonucleic acid. Biochemistry. 1976 Dec 14;15(25):5506–5511. doi: 10.1021/bi00670a014. [DOI] [PubMed] [Google Scholar]
- Lin N. S., Langenberg W. G. Immunohistochemical localization of barley stripe mosaic virions in infected wheat cells. J Ultrastruct Res. 1983 Jul;84(1):16–23. doi: 10.1016/s0022-5320(83)90082-5. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Meek R. L., Walsh K. A., Palmiter R. D. The signal sequence of ovalbumin is located near the NH2 terminus. J Biol Chem. 1982 Oct 25;257(20):12245–12251. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Petersen G. R., Dahlberg K. R., Van Etten J. L. Biosynthesis and degradation of storage protein in spores of the fungus Botryodiplodia theobromae. J Bacteriol. 1983 Aug;155(2):601–606. doi: 10.1128/jb.155.2.601-606.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Burstein Y., Zemell R., Ziv E., Kantor F., Papermaster D. S. Messenger RNA of opsin from bovine retina: isolation and partial sequence of the in vitro translation product. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2654–2658. doi: 10.1073/pnas.76.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Freer S. N., McCune B. K. Presence of a major (storage?) protein in dormant spores of the fungus Botryodiplodia theobromae. J Bacteriol. 1979 May;138(2):650–652. doi: 10.1128/jb.138.2.650-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]