Abstract
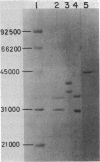
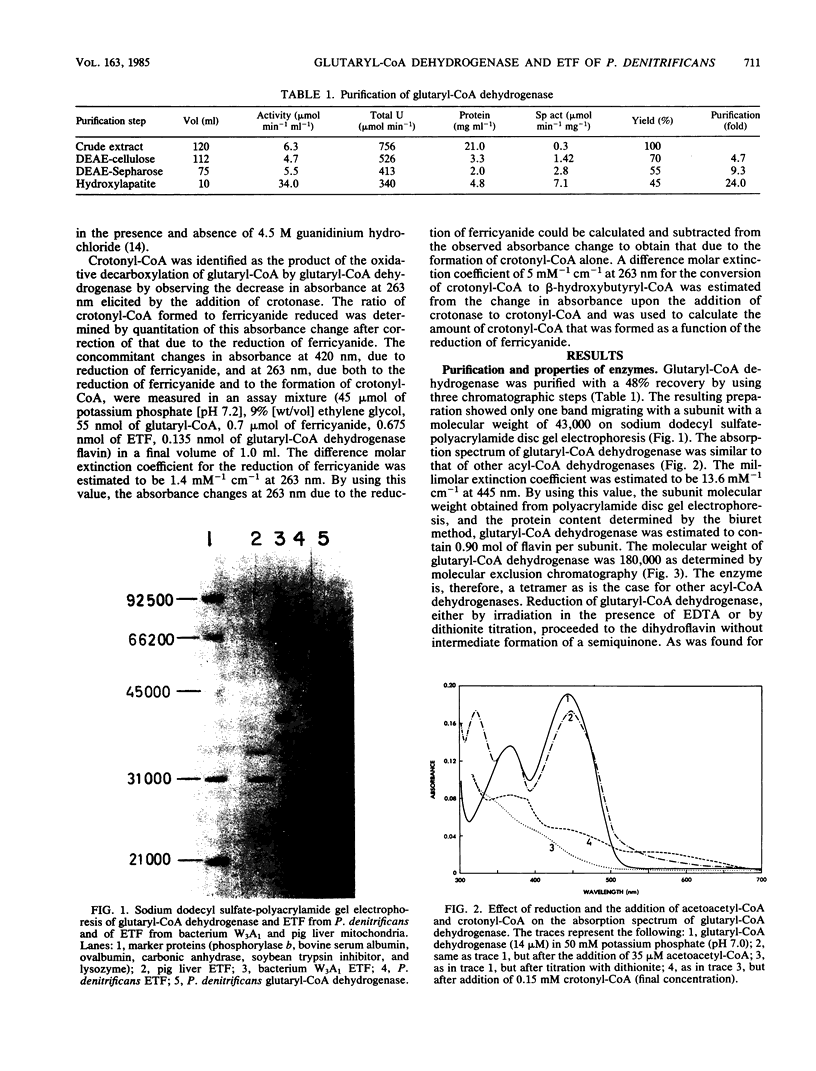
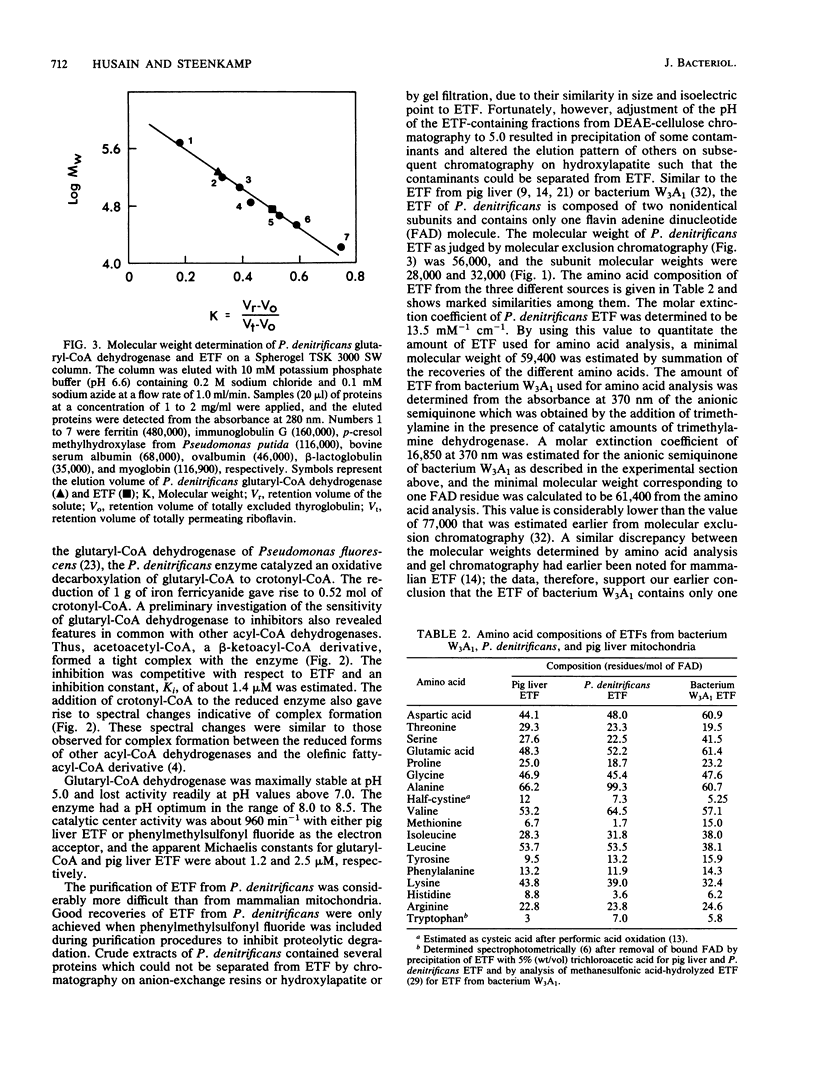
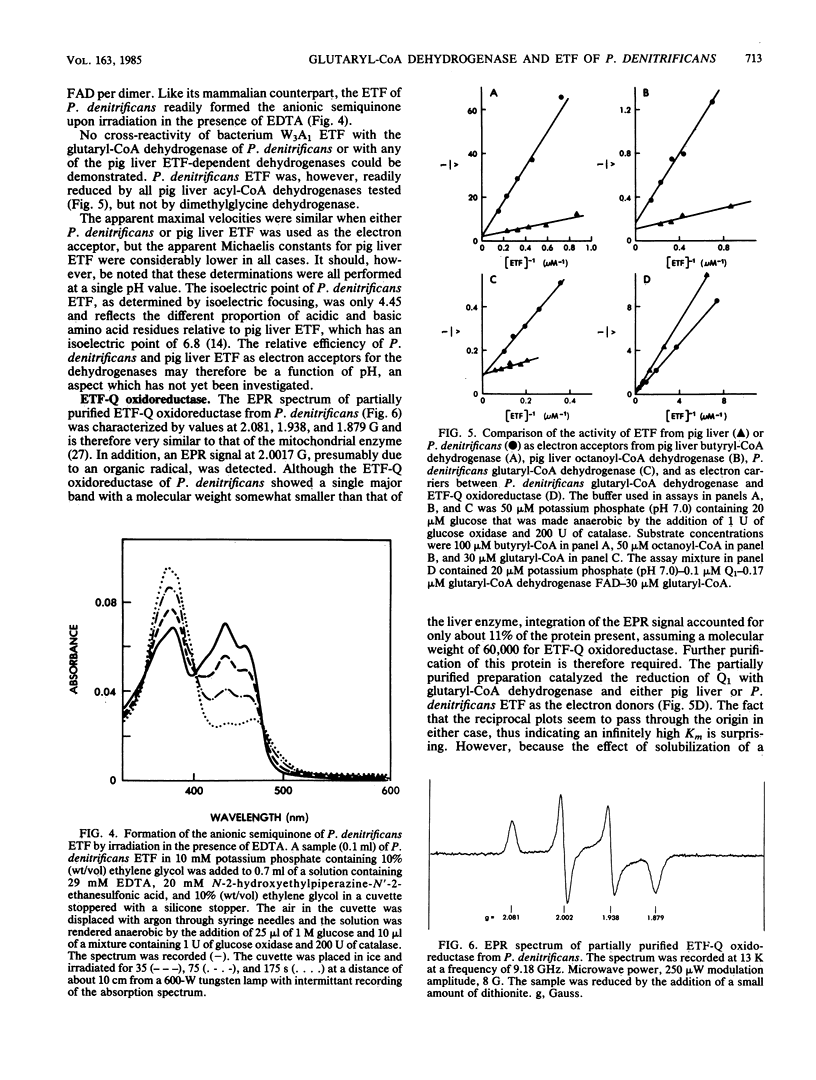
Glutaryl-coenzyme A (CoA) dehydrogenase and the electron transfer flavoprotein (ETF) of Paracoccus denitrificans were purified to homogeneity from cells grown with glutaric acid as the carbon source. Glutaryl-CoA dehydrogenase had a molecular weight of 180,000 and was made up of four identical subunits with molecular weights of about 43,000 each of which contained one flavin adenine dinucleotide molecule. The enzyme catalyzed an oxidative decarboxylation of glutaryl-CoA to crotonyl-CoA, was maximally stable at pH 5.0, and lost activity readily at pH values above 7.0. The enzyme had a pH optimum in the range of 8.0 to 8.5, a catalytic center activity of about 960 min-1, and apparent Michaelis constants for glutaryl-CoA and pig liver ETF of about 1.2 and 2.5 microM, respectively. P. denitrificans ETF had a visible spectrum identical to that of pig liver ETF and was made up of two subunits, only one of which contained a flavin adenine dinucleotide molecule. The isoelectric point of P. denitrificans ETF was 4.45 compared with 6.8 for pig liver ETF. P. denitrificans ETF accepted electrons not only from P. denitrificans glutaryl-CoA dehydrogenase, but also from the pig liver butyryl-CoA and octanoyl-CoA dehydrogenases. The apparent Vmax was of similar magnitude with either pig liver or P. denitrificans ETF as an electron acceptor for these dehydrogenases. P. denitrificans glutaryl-CoA dehydrogenase and ETF were used to assay for the reduction of ubiquinone 1 by ETF-Q oxidoreductase in cholate extracts of P. denitrificans membranes. The ETF-Q oxidoreductase from P. denitrificans could accept electrons from either the bacterial or the pig liver ETF. In either case, the apparent Km for ETF was infinitely high. P. denitrificans ETF-Q oxidoreductase was purified from contaminating paramagnets, and the resultant preparation had electron paramagnetic resonance signals at 2.081, 1.938, and 1.879 G, similar to those of the mitochondrial enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Albracht S. P., van Verseveld H. W., Hagen W. R., Kalkman M. L. A comparison of the respiratory chain in particles from Paracoccus denitrificans and bovine heart mitochondria by EPR spectroscopy. Biochim Biophys Acta. 1980 Dec 3;593(2):173–186. doi: 10.1016/0005-2728(80)90055-9. [DOI] [PubMed] [Google Scholar]
- Bensasson R., Land E. J. Optical and kinetic properties of semireduced plastoquinone and ubiquinone: electron acceptors in photosynthesis. Biochim Biophys Acta. 1973 Oct 19;325(1):175–181. doi: 10.1016/0005-2728(73)90163-1. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Massey V. The purification and properties of butyryl-coenzyme A dehydrogenase from Peptostreptococcus elsdenii. Biochem J. 1971 Dec;125(3):879–887. doi: 10.1042/bj1250879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRISELL W. R., MACKENZIE C. G. Separation and purification of sarcosine dehydrogenase and dimethylglycine dehydrogenase. J Biol Chem. 1962 Jan;237:94–98. [PubMed] [Google Scholar]
- Gorelick R. J., Mizzer J. P., Thorpe C. Purification and properties of electron-transferring flavoprotein from pig kidney. Biochemistry. 1982 Dec 21;21(26):6936–6942. doi: 10.1021/bi00269a049. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M., Steenkamp D. J. Electron transfer flavoprotein from pig liver mitochondria. A simple purification and re-evaluation of some of the molecular properties. Biochem J. 1983 Feb 1;209(2):541–545. doi: 10.1042/bj2090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Dabrowski C., Tanaka K. Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria. Identification of a new 2-methyl branched chain acyl-CoA dehydrogenase. J Biol Chem. 1983 Jan 25;258(2):1066–1076. [PubMed] [Google Scholar]
- Ikeda Y., Tanaka K. Purification and characterization of 2-methyl-branched chain acyl coenzyme A dehydrogenase, an enzyme involved in the isoleucine and valine metabolism, from rat liver mitochondria. J Biol Chem. 1983 Aug 10;258(15):9477–9487. [PubMed] [Google Scholar]
- John P., Whatley F. R. Paracoccus denitrificans and the evolutionary origin of the mitochondrion. Nature. 1975 Apr 10;254(5500):495–498. doi: 10.1038/254495a0. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. The bioenergetics of Paracoccus denitrificans. Biochim Biophys Acta. 1977 Oct 5;463(2):129–153. doi: 10.1016/0304-4173(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Schatz G. A two-subunit cytochrome c oxidase (cytochrome aa3) from Paracoccus dentrificans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):196–200. doi: 10.1073/pnas.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig B., Suda K., Cerletti N. Cytochrome c1 from Paracoccus denitrificans. Eur J Biochem. 1983 Dec 15;137(3):597–602. doi: 10.1111/j.1432-1033.1983.tb07867.x. [DOI] [PubMed] [Google Scholar]
- McKean M. C., Beckmann J. D., Frerman F. E. Subunit structure of electron transfer flavoprotein. J Biol Chem. 1983 Feb 10;258(3):1866–1870. [PubMed] [Google Scholar]
- McKean M. C., Frerman F. E., Mielke D. M. General acyl-CoA dehydrogenase from pig liver. Kinetic and binding studies. J Biol Chem. 1979 Apr 25;254(8):2730–2735. [PubMed] [Google Scholar]
- NUMA S., ISHIMURA Y., NAKAZAWA T., OKAZAKI T., HAYAISHI O. ENZYMIC STUDIES ON THE METABOLISM OF GLUTARATE IN PSEUDOMONAS. J Biol Chem. 1964 Nov;239:3915–3926. [PubMed] [Google Scholar]
- Owens J. D., Keddie R. M. The nitrogen nutrition of soil and herbage coryneform bacteria. J Appl Bacteriol. 1969 Sep;32(3):338–347. doi: 10.1111/j.1365-2672.1969.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Püttner I., Solioz M., Carafoli E., Ludwig B. Dicyclohexylcarbodiimide does not inhibit proton pumping by cytochrome c oxidase of Paracoccus denitrificans. Eur J Biochem. 1983 Jul 15;134(1):33–37. doi: 10.1111/j.1432-1033.1983.tb07527.x. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Gibson Q. H. Turnover of cytochrome c oxidase from Paracoccus denitrificans. J Biol Chem. 1983 Feb 10;258(3):1504–1507. [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. A new iron-sulfur flavoprotein of the respiratory chain. A component of the fatty acid beta oxidation pathway. J Biol Chem. 1977 Dec 10;252(23):8440–8445. [PubMed] [Google Scholar]
- Scholes P. B., Smith L. Composition and properties of the membrane-bound respiratory chain system of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):363–375. doi: 10.1016/0005-2728(68)90081-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Solioz M., Carafoli E., Ludwig B. The cytochrome c oxidase of Paracoccus denitrificans pumps protons in a reconstituted system. J Biol Chem. 1982 Feb 25;257(4):1579–1582. [PubMed] [Google Scholar]
- Steenkamp D. J., Gallup M. The natural flavorprotein electron acceptor of trimethylamine dehydrogenase. J Biol Chem. 1978 Jun 25;253(12):4086–4089. [PubMed] [Google Scholar]
- Steffens G. C., Buse G., Oppliger W., Ludwig B. Sequence homology of bacterial and mitochondrial cytochrome c oxidases. Partial sequence data of cytochrome c oxidase from Paracoccus denitrificans. Biochem Biophys Res Commun. 1983 Oct 14;116(1):335–340. doi: 10.1016/0006-291x(83)90419-9. [DOI] [PubMed] [Google Scholar]
- Thorpe C. Acyl-CoA dehydrogenase from pig kidney. Methods Enzymol. 1981;71(Pt 100):366–374. doi: 10.1016/0076-6879(81)71046-2. [DOI] [PubMed] [Google Scholar]
- Timkovich R., Dickerson R. E., Margoliash E. Amino acid sequence of Paracoccus denitrificans cytochrome c550. J Biol Chem. 1976 Apr 25;251(8):2197–2206. [PubMed] [Google Scholar]
- Timkovich R., Dickerson R. E. The structure of Paracoccus denitrificans cytochrome c550. J Biol Chem. 1976 Jul 10;251(13):4033–4046. doi: 10.2210/pdb155c/pdb. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Wagner C. Identification of folate binding protein of mitochondria as dimethylglycine dehydrogenase. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4484–4488. doi: 10.1073/pnas.77.8.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Verseveld H. W., Krab K., Stouthamer A. H. Proton pump coupled to cytochrome c oxidase in Paracoccus denitrificans. Biochim Biophys Acta. 1981 May 13;635(3):525–534. doi: 10.1016/0005-2728(81)90111-0. [DOI] [PubMed] [Google Scholar]