Abstract
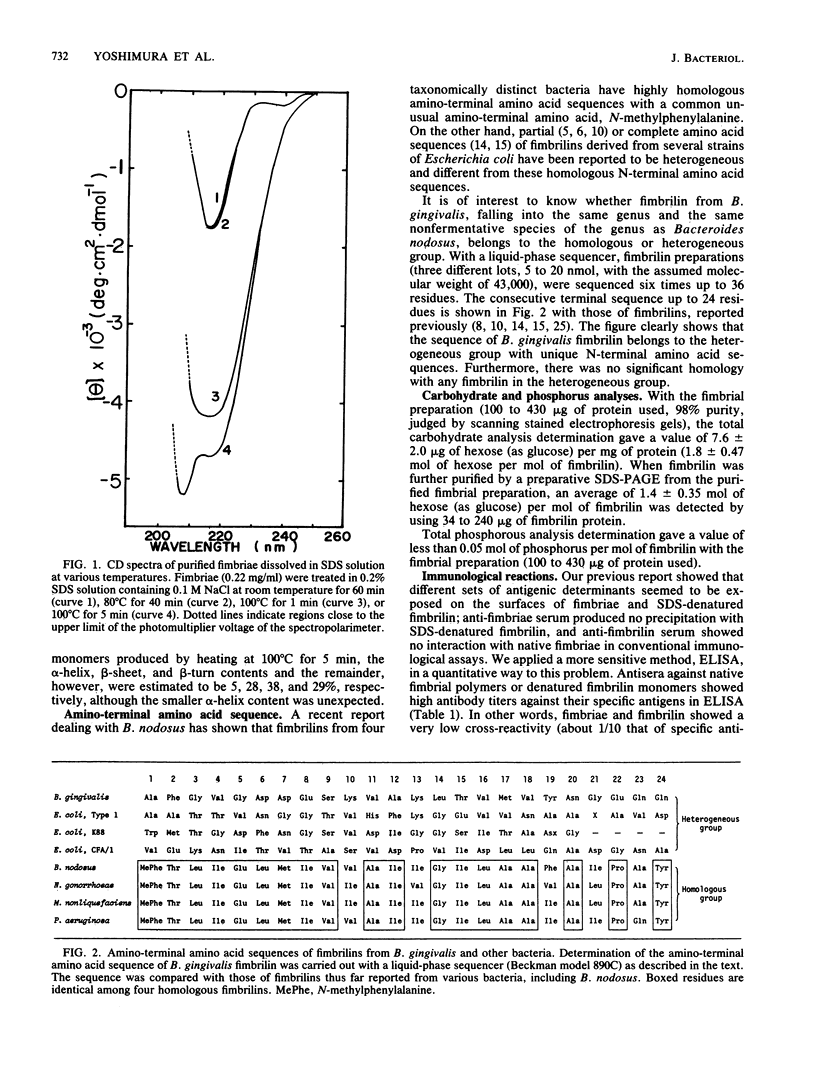
Circular dichroism spectra indicated the predominance of beta-sheet structure in Bacteroides gingivalis fimbriae regardless of the presence of sodium dodecyl sulfate. By using a computer program, the alpha-helix, beta-sheet, and beta-turn contents and the remainder were estimated to be 0, 55, 18, and 27%, respectively, judging from the circular dichroism spectra of the fimbriae. Heating for 5 min at 100 degrees C in sodium dodecyl sulfate was necessary to denature the fimbriae into their constituent protein (fimbrilin) monomers with a reduced content of beta-sheet structure. The amino-terminal amino acid sequence of the fimbrilin was different from partial or complete amino acid sequences of fimbrilins so far determined from Bacteroides nodosus, which falls into the same nonfermentative species of the genus Bacteroides as B. gingivalis, and from various other bacteria. Fimbrilin monomers had an isoelectric point of 6.0. Examination of antibodies against fimbriae and sodium dodecyl sulfate-denatured fimbrilin by enzyme-linked immunosorbent assay reinforced a previous notion (F. Yoshimura, K. Takahashi, Y. Nodasaka, and T. Suzuki, J. Bacteriol. 160:949-957, 1984) that different sets of antigenic determinants seemed to be exposed on their surfaces.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Greenfield N. J., Fasman G. D. Circular dichroism and optical rotatory dispersion of proteins and polypeptides. Methods Enzymol. 1973;27:675–735. doi: 10.1016/s0076-6879(73)27030-1. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Deal C. D., Kaplan S. Solubilization, isolation, and immunochemical characterization of the major outer membrane protein from Rhodopseudomonas sphaeroides. J Biol Chem. 1983 May 25;258(10):6524–6529. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Froholm L. O., Sletten K. Purification and N-terminal sequence of a fimbrial protein from Moraxella nonliquefaciens. FEBS Lett. 1977 Jan 15;73(1):29–32. doi: 10.1016/0014-5793(77)80008-2. [DOI] [PubMed] [Google Scholar]
- GILVARG C., BLOCH K. The utilization of acetic acid for amino acid synthesis in yeast. J Biol Chem. 1951 Nov;193(1):339–346. [PubMed] [Google Scholar]
- Hayashi K. A rapid determination of sodium dodecyl sulfate with methylene blue. Anal Biochem. 1975 Aug;67(2):503–506. doi: 10.1016/0003-2697(75)90324-3. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Klemm P. Primary structure of the CFA1 fimbrial protein from human enterotoxigenic Escherichia coli strains. Eur J Biochem. 1982 May 17;124(2):339–348. doi: 10.1111/j.1432-1033.1982.tb06597.x. [DOI] [PubMed] [Google Scholar]
- Klemm P. The complete amino-acid sequence of the K88 antigen, a fimbrial protein from Escherichia coli. Eur J Biochem. 1981 Jul;117(3):617–627. doi: 10.1111/j.1432-1033.1981.tb06382.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A., Brosi B. J., Buys J. Serodiagnosis of Trichinella spiralis infections in pigs by enzyme-linked immunosorbent assays. Bull World Health Organ. 1974;51(1):108–109. [PMC free article] [PubMed] [Google Scholar]
- Sastry P. A., Pearlstone J. R., Smillie L. B., Paranchych W. Amino acid sequence of pilin isolated from pseudomonas aeruginosa PAK. FEBS Lett. 1983 Jan 24;151(2):253–256. doi: 10.1016/0014-5793(83)80080-5. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Tarr G. E. A general procedure for the manual sequencing of small quantities of peptides. Anal Biochem. 1975 Feb;63(2):361–370. doi: 10.1016/0003-2697(75)90358-9. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Kay C. M., Paranchych W. Spectral properties of three quaternary arrangements of Pseudomonas pilin. Biochemistry. 1983 Jul 19;22(15):3640–3646. doi: 10.1021/bi00284a016. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Klemm P., Gaastra W. Purification, characterization, and partial covalent structure of Escherichia coli adhesive antigen K99. Infect Immun. 1981 Sep;33(3):877–883. doi: 10.1128/iai.33.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Roorda I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect Immun. 1982 May;36(2):751–758. doi: 10.1128/iai.36.2.751-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]