Abstract
The centromere plays a critical role in the segregation of chromosomes during mitosis. In mammals, sister centromeres are resolved from one another in the G2 phase of the cell cycle. During prophase, chromosomes condense with sister centromeres oriented in a back to back configuration enabling only one chromatid to be captured by each half spindle. To study this process, we identified a centromere protein (CENP)-C–like protein, holocentric protein (HCP)-4, in Caenorhabditis elegans based on sequence identity, loss of function phenotype, and centromeric localization. HCP-4 is found in the cytoplasm during interphase, but is nuclear localized in mitosis, where it localizes specifically to the centromere. The localization of HCP-4 to the centromere is dependent on the centromeric histone HCP-3; in addition, HCP-3 and HCP-4 are both required for localization of a CENP-F–like protein, HCP-1, indicating an ordered assembly pathway. Loss of HCP-4 expression by RNA-mediated interference resulted in a failure to generate resolution of sister centromeres on chromosomes, suggesting that HCP-4 is required for sister centromere resolution. These chromosomes also failed to form a functional kinetochore. Thus, the CENP-C–like protein HCP-4 is essential for both resolution sister centromeres and attachment to the mitotic spindle.
Keywords: Caenorhabditis elegans, chromosome, mitosis, centromere, kinetochore
Introduction
The centromere is the site on the chromosome where the kinetochore is assembled. Before mitosis, the centromeric DNA must be replicated and centromere-specific proteins bound to generate sister centromeres capable of assembling functional kinetochore complexes. Staining of interphase nuclei with anticentromere antibodies revealed that the centromere is duplicated by interphase-early prophase (Brenner et al. 1981). During this process, the centromere becomes elongated and sister centromeres are formed adjacent to one another (He and Brinkley 1996). Distinct sister centromeres are found on opposite sides of the condensing chromosome in prophase (Jokelainen 1967; Brinkley and Stubblefield 1970; Roos 1973; Heneen 1975). This physical separation, or resolution, of sister centromeres has been suggested to be important for assembling sister kinetochores back to back (Nicklas 1971; Roos 1973, Roos 1976). Little is known about the proteins or mechanisms involved in these early steps of centromere resolution and kinetochore assembly.
Proteins localized to the centromere during interphase and early prophase are likely to play a critical role in centromere resolution and kinetochore assembly. Two such proteins are centromere protein (CENP)-A and CENP-C (Palmer et al. 1987; Saitoh et al. 1992). Homologues of CENP-A and CENP-C are present in many organisms, indicating a high degree of conservation of centromere structure in eukaryotes (Henikoff et al. 2000; Maney et al. 2000). CENP-A is a histone H3 variant present in centromeric chromatin (Palmer et al. 1991; Sullivan et al. 1994; Saitoh et al. 1997; Shelby et al. 1997; Meluh et al. 1998; Takahashi et al. 2000). CENP-A–like proteins may play an important role in marking the centromere because no aspects of kinetochore assembly are seen when CENP-A is absent (Howman et al. 2000). CENP-C also appears to be required for kinetochore assembly, although the specific role is not known (Tomkiel et al. 1994; Fukagawa and Brown 1997; Kalitsis et al. 1998; Fukagawa et al. 1999). Studies show that CENP-C–like proteins are closely associated with centromeric DNA and may bind DNA directly (Saitoh et al. 1992; Sugimoto et al. 1994; Meluh and Koshland 1995; Yang et al. 1996; Warburton et al. 1997). The observation that CENP-C and CENP-A colocalize and that CENP-C can bind DNA suggests that CENP-C may have some role in linking the kinetochore structure to the centromeric heterochromatin (Saitoh et al. 1992; Tomkiel et al. 1994; Warburton et al. 1997).
The nematode Caenorhabditis elegans provides a unique system to study centromere resolution and kinetochore assembly. The centromere of C. elegans is composed of many independent elements distributed throughout the chromosome (Buchwitz et al. 1999). These elements may be analogous to the unit repeats observed in vertebrate centromeres (Zinkowski et al. 1991). During chromosome condensation, these elements coalesce into a single aggregate of centromere units that form a line along the entire length of the chromosome. Later in prophase, two centromere lines are observed on opposite faces of the condensed chromosome and sister kinetochores are subsequently assembled adjacent to each (Moore et al. 1999). The phenomenon of a single centromere line early in prophase and two lines later in prophase is reminiscent of sister centromere resolution in mammals. Here we provide evidence that the one line stage contains two juxtaposed aggregates of centromere units, one from each sister chromatid, that are resolved from one another in prophase. We also show that a nematode CENP-C–like protein, holocentric protein (HCP)-4 is required for both kinetochore assembly and resolution of sister centromeres.
Materials and Methods
Sequencing
Four λZAPII yk cDNA clones, yk562a4, yk224a1, yk327d5, and yk247a5, were excised according to the manufacture's instructions (Stratagene) and sequenced using an automated ABI 373A DNA sequencer (ABI Biosystems). The 5′ and 3′ ends of the HCP-4 mRNA were obtained using 5′ and 3′ rapid amplification of cDNA ends with the oligos SL1, 5′-; 5R1HCP4, 5′-CGACGTATCACTCGAGCTCTTGG-3′; 5R2HCP-4, 5′-CGCTGTCGCTCTCACATTCGG-3′; CCPC5; and oligod T (Coen 1992). The complete mRNA sequence data is available from GenBank\EMBL\DDBJ under accession no. AF321299.
Antibody Production, Immunofluorescence, and Immunoblotting
A 2.2-kb BamHI fragment from yk562a4, encoding amino acid residues 1–833 of the HCP-4 protein, was cloned into the BamHI site of pET-28a by standard procedures. A 6× His-tagged HCP-4ΔB protein was purified following the manufactures instructions (QIAGEN). Mice containing the Robertsoninian translocation (Jackson ImmunoResearch Laboratories) were injected with the HCP-4ΔB protein mixed with complete Freud's adjuvant. All injections were intraperitoneal. Boosts were administered every 10 d and serum was obtained after 1 mo. In addition, rabbit polyclonal antibodies against HCP-4ΔB protein were produced in New Zealand white rabbits by R&R Research and Development. None of the preimmune sera reacted positively toward C. elegans embryos (data not shown). All sera gave identical results and could be competed by preincubation with HCP-4 protein (data not shown).
For immunofluorescence, Bristol strain N2 embryos were prepared as described previously (Moore et al. 1999). Primary antibodies were α–HCP-4 rabbit or mouse sera, an α–HCP-3 antibody (Buchwitz et al. 1999), mAb 6C4 ascites (Moore et al. 1999), a monoclonal antibody, mAb 414 (Davis and Blobel 1986) directed against nuclear pore proteins, and an anti–β-tubulin antibody YL 1/2 (Amersham Pharmacia Biotech).
For immunoblotting, embryo extracts were prepared as described previously (Frank and Roth 1998). Extracts were run on a 7.5% SDS-PAGE and transferred to Immobilon-P membrane according to the manufacture's instructions (Millipore). Membranes were blocked in 3% BSA in TBS (50 mM Tris-HCl, pH 8.0, 150 mM NaCl), incubated for 1 h at room temperature with 1:500 diluted primary antibody, washed in TBS, and detected using the biotin/avidin Vectastain system (Vector Laboratories).
RNAi
Double-stranded RNA for RNA-mediated interference (RNAi) was produced as described in Moore et al. 1999 using oligos 5′-TAATACGAC-TCACTATAGGGgaagccagaagatgctcca and 5′-cgtcgcccacttcttgcattctg for the sense strand and oligos 5′-gaagccagaagatgctcca and 5′-TAATAC-GACTCACTATAGGGcgtcgcccacttcttgcattctg for antisense strand synthesis. RNAi was performed by soaking L4 stage worms in a 5–10-μl drop of 2.5 mg/ml dsRNA plus 5 mM spermidine solution placed on a piece of parafilm in a humid chamber. Incubation was at room temperature overnight. Worms were released onto fresh plates and allowed to recover for 12–24 h before embryos were isolated.
Microscopy
Slides were examined either by a microscope (Axioscope; ZEISS) equipped with a camera (Sensys CCD; Photometric) or by three-dimensional multiple wavelength fluorescence microscopy using the Deltavision microscope (Applied Precision). When Deltavision was used, images were collected at the indicated wavelengths using 0.2-μm optical sections, and then stacks of optical sections were subsequently deconvolved and examined as either single sections or a projection of the entire stack (Carrington et al. 1995; Hiraoka et al. 1991). All images were first analyzed in Photoshop® (Adobe) then imported into Canvas (Deneba).
Microtubule Disruption
Embryos were isolated from gravid adults and washed twice in PBS containing 30 μg/ml nocodazole (Sigma-Aldrich). Coverslips were placed over the embryos and gentle pressure was applied to crack the eggshells. Slides were incubated at room temperature for 20, 30, and 45 min before freezing and fixation.
Results
Identification of a CENP-C–like Protein in C. elegans
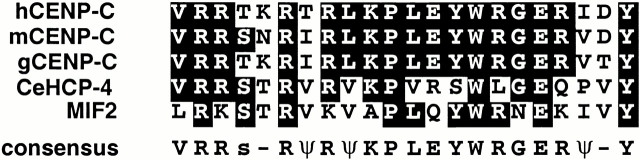
Because CENP-C–like proteins are associated with centromeric heterochromatin and are present when sister centromeres are resolved from one another, we wanted to study the function of CENP-C in C. elegans. To do this, we searched for a CENP-C–like protein in C. elegans. We identified the predicted ORF T03F1.9 as containing a short sequence of similarity to CENP-C–like proteins (Fig. 1). We named T03F1.9, HCP-4.
Figure 1.
Sequence and alignment of HCP-4 with CENP-C proteins. BLOCKMAKER and the Motif Alignment and Search Tool programs were used with human CENP-C (EMBL/GenBank/DDBJ accession no. M95724), mouse CENP-C (EMBL/GenBank/DDBJ accession no. U03113), chicken CENP-C (EMBL/GenBank/DDBJ accession no. AB004649), and MIF2 (EMBL/GenBank/DDBJ accession no. Z28089) to identify a region of similarity in HCP-4 (Bailey and Gribskov 1998; Henikoff and Henikoff 1991). Alignment was performed using the ClustalW multiple alignment program (Thompson et al. 1994) and displayed using Boxshade (http://www.ch.embnet.org/software/BOX_form.html). Identities are shaded black.
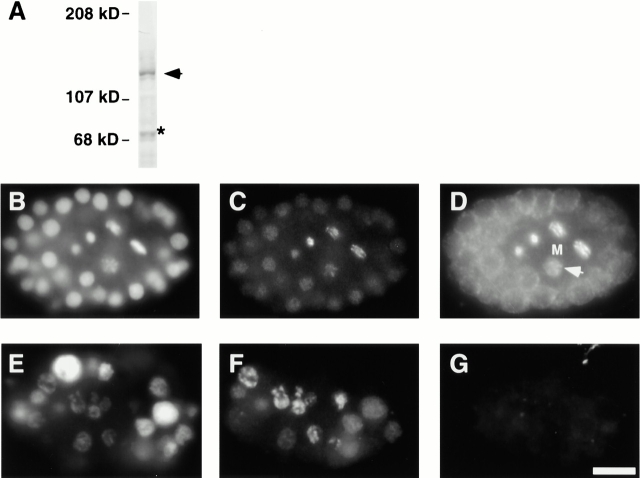
To study the localization of HCP-4, we raised antibodies directed against the predicted HCP-4 protein. Immunoblotting with the α–HCP-4 antisera detected a 136-kD band in embryo extracts (Fig. 2 A). The predicted size for HCP-4 is 97.3 kD. Sequencing of the HCP-4 mRNA did not reveal any additional sequence to account for the observed mobility in SDS-PAGE; in addition, bacterial-expressed HCP-4 protein migrated identically to endogenous protein, indicating that the altered mobility is intrinsic to the HCP-4 protein (data not shown). Similarly, CENP-C, which is predicted to migrate with an ORF of 107 kD, migrates with an ORF of 140 kD (Saitoh et al. 1992). We used the α–HCP-4 antibodies and the α–HCP-3 antibody to perform immunofluorescence of wild-type N2 embryos (Fig. 2, B–D). We observed that cells in interphase showed cytoplasmic staining that was reduced in mitotic cells (compare the four “M” cells to the surrounding interphase cells in Fig. 2 D). Furthermore, we observed that mitotic chromosomes stained positive for HCP-4. We verified that these staining patterns were dependent on HCP-4 expression by staining embryos in which HCP-4 expression had been reduced by RNAi (Fig. 2, E–G; Fire et al. 1998; Tabara et al. 1998).
Figure 2.
HCP-4 localization in C. elegans embryos. (A) Western blot of wild-type embryo extract (60 μg total protein) probed with α–HCP-4 antibody (diluted 1:500) shows that a single band of ∼136 kD is present. All other bands, including the band at 70 kD (*), were independent of the primary antibody. Wild-type embryos or hcp-4(RNAi) embryos were stained with DAPI (B and E), α–HCP-3 antibody (C and F), and α–HCP-4 antibody (D and G). Approximately equal staged embryos containing both mitotic (M) and interphase cells are shown. Exposure times were normalized relative to the HCP-3 staining. Arrow indicates prophase nucleus with nuclear staining. Bar, 5 μm.
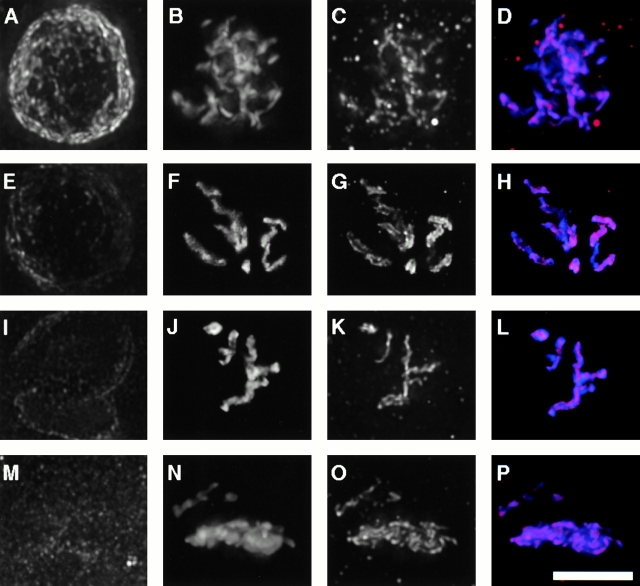
The localization of HCP-4 in mitotic cells suggested that HCP-4 might be a centromere protein (Fig. 2 D). To address this possibility, we used multiple wavelength fluorescence microscopy to perform a colocalization experiment between HCP-4 and the centromeric histone, HCP-3. HCP-3 is present during interphase as discrete dots or units and as a continuous line of reactivity along the axis of prophase chromosome (Fig. 3 A; Buchwitz et al. 1999). HCP-4 was also found to be present in a single line of reactivity along the axis of the chromosome (Fig. 3 B). HCP-4, like HCP-3, appeared as two lines of reactivity later in prophase (Fig. 3D and Fig. E). These two lines are oriented towards the two half spindles at metaphase and at anaphase (Fig. 3G and Fig. H, and Fig. J and Fig. K, respectively). When we merged the HCP-3 and HCP-4 images we observed that both colocalized nearly completely beginning in prophase and persisting through metaphase and anaphase (Fig. 3C, Fig. F, Fig. I, and Fig. L, yellow). This colocalization of HCP-4 and HCP-3 showed that HCP-4 was centromere localized during mitosis. During telophase as chromosome decondensation occurs, HCP-3 was again observed as discrete units throughout the nucleoplasm (Fig. 3 M). We observed HCP-4 staining also became punctate after mitosis and did not overlap with HCP-3 staining (Fig. 3N and Fig. O).
Figure 3.
Centromeric association of HCP-4 during mitosis. Two-cell C. elegans embryos at different stages of the mitotic cell cycle were fixed and stained with α-HCP3 antibody (A, D, G, J, and M, green), α-HCP4 (B, E, H, K, and N, red), and DAPI (blue). Colocalization of HCP-3 and HCP-4 was visualized in the merged images as yellow (C, F, I, L, and O). All images, except metaphase, are a projection of the Z-stack of the entire nucleus. (A–C) Early prophase; (D–F) late prophase; (G–I) metaphase; (J–L) anaphase; (M–O) telophase. Bars, 5 μm.
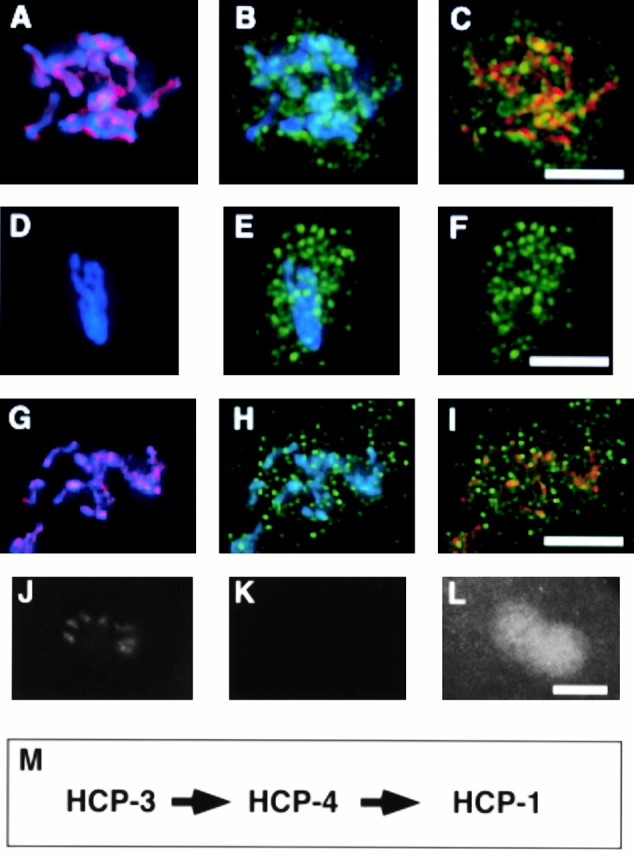
To test whether HCP-4, like CENP-C, has a role in chromosome segregation, we used RNAi to reduce expression of HCP-4. We observed in early hcp-4(RNAi) embryos that chromosomes failed to segregate at anaphase (Fig. 4A versus D). Later stage embryos showed many nuclei that contained variable amounts of DNA, including some with almost no detectable DNA, indicating, as previously observed for hcp-3(RNAi) embryos, that cell division continues despite an inability to properly segregate chromosomes (Fig. 4 G; Buchwitz et al. 1999). These results indicated that HCP-4 is necessary for chromosome segregation.
Figure 4.
HCP-4 is necessary for kinetochore function. One-cell wild-type (A–C) or hcp-4(RNAi) (D–F) embryos in anaphase were stained with an anti–β-tubulin antibody (B and E), and an α-HCP-1 antibody (C and F). DNA (A and D) was visualized with DAPI. The arrows in B show that the most intensely stained region of the spindle (excluding the centrosomes) is the kinetochore fiber. The kinetochore fiber not observed in hcp-4(RNAi) embryos is shown in E. (G) Late stage hcp-4(RNAi) embryo stained with DAPI, showing a nonuniform distribution of DNA. Bar, 5 μm.
To further characterize the hcp-4(RNAi) defect, we stained early hcp-4(RNAi) embryos with antibodies directed against tubulin. Given that defective segregation at the first mitotic division occurs, we looked for one-cell embryos and examined the localization of tubulin. At anaphase, we observed that chromosomes failed to orient perpendicularly to the mitotic spindle, indicating an apparent failure to attach to the spindle (Fig. 4 D). Furthermore, an examination of additional hcp-4(RNAi) embryos revealed that chromosomes failed to congress to the metaphase plate (data not shown). The mitotic spindle in hcp-4(RNAi) embryos was bipolar and appeared similar to the spindle in wild-type embryos (Fig. 4B versus E). However, the kinetochore fiber was absent from hcp-4(RNAi) spindles. From these observations, we conclude that the failure of hcp-4(RNAi) embryos to segregate chromosomes is due to a defect in the attachment of chromosomes to the spindle. Thus, HCP-4 shares several functional as well as sequence similarities to CENP-C.
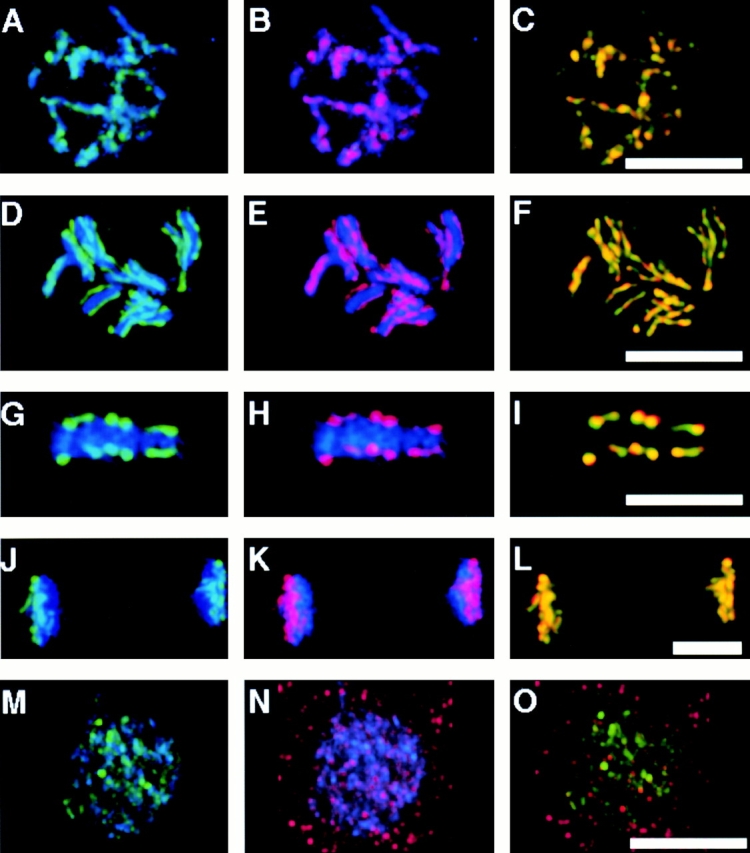
Assembly of the Kinetochore Is an Ordered Pathway
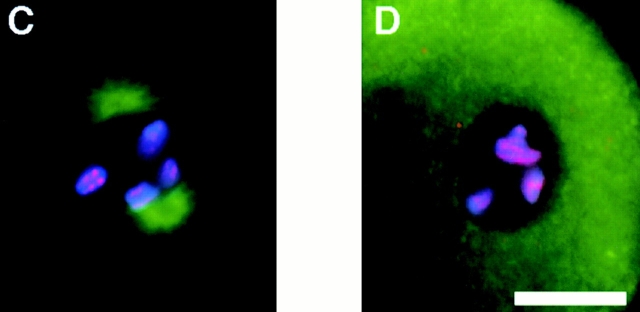
HCP-4 is the third protein that we have localized to the centromere. Because of differences in the timing of association of these proteins with the centromere, we wanted to determine if there is an ordered assembly pathway. We reduced the expression of HCP-3 by RNAi and observed that HCP-1 and HCP-4 were not localized to chromosomes (Fig. 5E and Fig. L, respectively). HCP-1 was present as dots distributed throughout the nucleus (Fig. 5 F) and the mitotic spindle (Fig. 4 F). HCP-4, although not chromosomally associated, was diffusely present in the nucleus (Fig. 5 L). Reduction of HCP-4 expression did not eliminate HCP-3 chromosome association, but did eliminate chromosomal association of HCP-1, which was again present as dots distributed throughout the nucleus (Fig. 5, G–I). These results, combined with the dynamic changes in localization during mitosis (Fig. 3), suggest that HCP-3 is necessary for HCP-4 centromere localization, which in turn is necessary for localization of the kinetochore protein, HCP-1 (Fig. 5 M).
Figure 5.
The centromere is assembled during prophase. Nuclei from wild-type (A–C), hcp-3(RNAi) (D–F), and hcp-4 (RNAi) (G–I) embryos were fixed and stained with DAPI (blue), α–HCP-3 pAb (red), and α–HCP-1 (green). A P0 hcp-3(RNAi) embryo, postpronuclei fusion, stained with DAPI (J), α–HCP-3 pAb (K), and α–HCP-4 pAb (L). (M) Diagram summarizing the order of assembly for the centromere–kinetochore complex. Bar, 5 μm.
HCP-4 Is Required for Sister Centromere Resolution
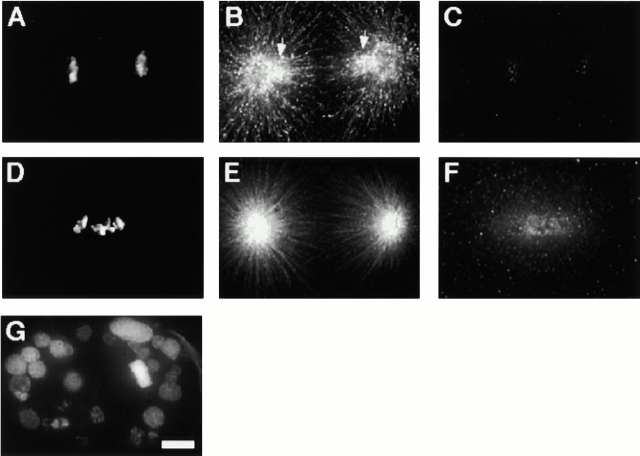
We were interested in whether HCP-4 was required at an early step in centromere maturation. An early aspect of centromere maturation is sister centromere resolution. In C. elegans, only a single centromere of α–HCP-3 staining is observed early in prophase; later in prophase two sister centromeres are observed on opposite sides of the chromosome (Buchwitz et al. 1999). Further examination of prophase nuclei showed chromosomes with two sister centromeres in various degrees of separation from one another (Fig. 6 A). Initiation of separation was sometimes seen as a “Y”-shaped intermediate (Fig. 6 B). This resolution of sister centromeres was not dependent on microtubules, because microtubules are likely to be absent from prophase nuclei (Fig. 6 C) and because resolution of sister centromeres occurred in the presence of the microtubule destabilizing drug nocodazole (Fig. 6 D).
Figure 6.
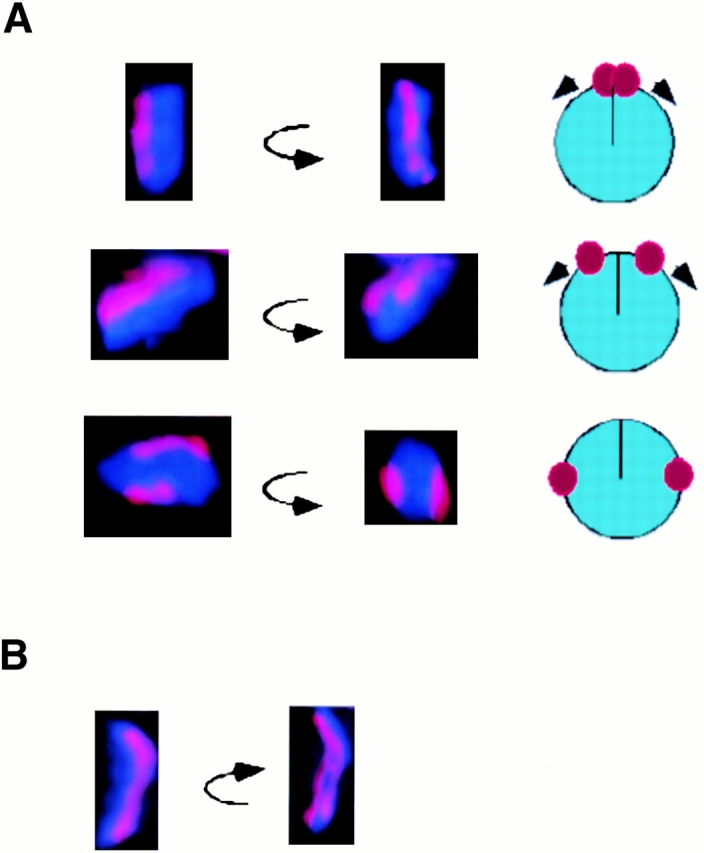
Sister centromere resolution. Individual chromosomes from prophase nuclei were stained with DAPI (blue), α–HCP-1 antibody (not shown), and α–HCP-3 antibody (red). Nuclei containing little or no HCP-1 staining were optically sectioned and a three-dimensional projection generated using the volume viewer function. The resulting images were rotated around the Y-axis. (A) Chromosomes showing different degrees of sister centromere separation. Cartoon of the Z-axis of each chromosome depicting the sister centromeres migration from a juxtaposed position to a maximally resolved orientation is shown adjacent to each image. (B) A single chromosome showing an intermediate “splitting” of sister centromeres. (C and D) Sister centromere resolution is a microtubule-independent process. (C) P1 blastomere from a wild-type embryo stained with DAPI (blue), antitubulin antibody (green), and α–HCP-3 antibody (red). (D) P1 blastomere, from an embryo incubated in the presence of 30 μg/ml nocodazole at room temperature for 30 min, stained as in C. The division time for a two-cell embryo at room temperature is ∼20–30 min, so this embryo had probably just finished cell division when treated with nocodazole. Furthermore, the centrosomes are duplicated (not shown) but have not migrated, indicating that loss of spindle microtubules occurred before centrosome migration, an event that occurs concurrent or before sister centromere resolution (C). Bar, 5 μM.
Having characterized wild-type, we next looked at sister centromere resolution in hcp-4(RNAi) embryos. Because reduction of expression of HCP-4 causes missegregation of chromosomes, we needed an independent marker for cell cycle position. We used mAb 414, which stains nuclear pore complexes with staining most intense during interphase and prophase, decreasing at prometaphase, and absent at anaphase (Lee et al. 2000). We examined two-cell hcp-4(RNAi) embryos stained with α–HCP-3 antibody and mAb 414. In 19 of 19 prometaphase nuclei examined, chromosomes had a single line of HCP-3 staining along the axis of each chromosome (Fig. 7, I–L). In wild-type at this stage, sister centromeres were present on opposite faces of the chromosome (Fig. 7, E–H). In five of these hcp-4(RNAi) embryos we could count the number of chromosomes in both nuclei and observed ∼9–12 chromosomes per nucleus. This is close to the wild-type number of 12 chromosomes, indicating that the observed chromosomes are likely paired sister chromatids.
Figure 7.
HCP-4 is required for sister centromere resolution. Nuclei from wild-type and hcp-4(RNAi) embryos stained with mAb 414 (A, E, I, and M), α–HCP-3 antibody (C, G, K, and O) and DAPI (B, F, J, and N). Merged DAPI (blue) and HCP-3 (red) are shown in (D, H, L, and P). (A–D) Wild-type prophase; (E–H) wild-type prometaphase; (I–L) hcp-4(RNAi) prometa-/metaphase; (M–P) hcp-4(RNAi) anaphase. The cell cycle position was inferred from mAb 414 staining (Lee et al. 2000). Bar, 5 μM.
Based on the observation in prophase that only one centromere can be seen in the hcp-4(RNAi) embryos, there are at least two possible events in centromere maturation where HCP-4 might function. One is during deposition of new centromere proteins to the new centromere and a second is during sister centromere resolution. To distinguish between these two, we observed nuclei in anaphase (n = 6), presumably after sister chromatids separated (Fig. 7, M–P). We reasoned that the number of DAPI-stained “chromosomes”, each a chromatid, and HCP-3–stained centromeres should each be 24. The actual number for each was between 20 and 23, indicating that sister chromatids had separated. Furthermore, the ratio of “chromosomes” to centromeres was near 1:1. We would expect to observe some chromosomes that lack a detectable centromere if centromere protein deposition was the event affected by loss of HCP-4. Instead we observe that every DAPI-stained “chromosome” contained HCP-3 staining. Another test of this hypothesis is to look at hcp-4(RNAi) embryos that have undergone several rounds of division and observe HCP-3 localization. We observed that HCP-3 staining remains abundant, although the relative intensity with respect to DAPI staining is highly variable (Fig. 2, E–F). These results together suggest that HCP-4 is likely to function in the resolution of sister centromeres.
Discussion
We identified a CENP-C–like protein, HCP-4, in C. elegans based on both sequence and functional similarities. HCP-4, like CENP-C, is required for proper assembly of the kinetochore, which further indicates that the kinetochore of holocentric chromosomes resemble those of monocentric chromosomes. The high degree of functional similarity between HCP-4 and CENP-C suggests that differences in localization between the two proteins may be due to the difference between holocentric and monocentric chromosome organization. Given this, we investigated the role of HCP-4 in holocentric chromosome segregation to further our knowledge of centromere function.
The elongated centromeres of C. elegans holocentric chromosomes enabled us to more closely examine the process of sister centromere resolution and to demonstrate that HCP-4 is required for this resolution. The model for the centromere cycle proposed by He and Brinkley 1996 suggests that the mammalian centromere is decondensed during DNA replication, with old centromere proteins being retained on only one of the two daughter chromatids. Later in the cell cycle centromere proteins are deposited on the other sister chromatid. After this centromere duplication, condensation would then generate the two closely juxtaposed sister centromeres. These sister centromeres are resolvable from one another in G2. Interestingly, sister centromere resolution appears to occur in prophase of C. elegans. The difference in the timing of centromere resolution in C. elegans and in mammals may be the result of overall differences in chromosome organization. The centromere of holocentric chromosomes is dispersed throughout the chromosome and this feature may require coordination of sister centromere resolution with chromosome condensation to establish the relationship between where the kinetochore will be assembled and where sister chromatid cohesion will be maintained. Because the centromere in mammals is localized to constitutive heterochromatin and does not undergo significant decondensation during interphase, sister centromere resolution in mammals can take place immediately after replication.
A relationship may also exist between chromosome organization and CENP-C–like protein localization to the centromere. We find that localization of HCP-4 to the centromere occurs only during mitosis. Although it is possible that HCP-4 and CENP-C localization are not different, with our antibodies simply unable to detect the HCP-4 at the centromere during interphase, the localization of CENP-C–like proteins may also be dependent on the state of condensation of the centromere. In mammals, the centromere remains relatively condensed and hence CENP-C is constitutively localized, whereas in C. elegans HCP-4 is only localized to the centromere during mitosis when the centromere is condensed. Condensation of the chromosome does not appear to be sufficient to localize HCP-4 because chromosomes are condensed, and yet HCP-4 is not localized to the centromere when the centromeric histone, HCP-3, was reduced in expression. These results suggest a model whereby chromosome condensation is required to bring HCP-3–containing chromatin together in a higher order chromatin structure that in turn is required for the recruitment of HCP-4 to the centromere.
Our results suggest an ordered pathway for the assembly and possibly disassembly of the centromere–kinetochore. We found that the CENP-A homologue HCP-3 is required for the deposition of the CENP-C–like protein HCP-4. This is in agreement with the previous results of mammalian cell studies (Howman et al. 2000). Both HCP-4 and HCP-3 are required for assembly of the CENP-F–like protein HCP-1. Likewise, CENP-C was shown to be required for localization of ZW10 to the kinetochore, which is then involved in recruiting the motor protein dynein (Starr et al. 1998; Fukagawa et al. 1999; Chan et al. 2000). In anaphase, HCP-1 gradually disappears from the kinetochore and is no longer detectable by telophase (Moore et al. 1999). We observed that in hcp-4(RNAi) embryos, HCP-1 did not disappear in anaphase. Because this HCP-1 was also not localized to the kinetochore, it is possible that HCP-4 may directly or indirectly influence the turnover of kinetochore-localized HCP-1.
An early aspect of centromere maturation that requires HCP-4 is sister centromere resolution. Sister centromere resolution is likely to be important in generating two distinct sister kinetochores on opposite faces of the chromosome. As microtubules from one pole begin to interact with one kinetochore, stearic inhibition makes it is less likely that productive interactions can be made by these same microtubules to the sister kinetochore (Nicklas 1971; Roos 1973, Roos 1976). Furthermore, interaction of one kinetochore with one pole may enhance the probability of capture of the opposing kinetochore by the other pole. Studying holocentric chromosomes of C. elegans provides an excellent system to continue to understand the mechanisms by which sister centromere resolution occurs.
Acknowledgments
The authors would like to thank Brian Buchwitz, Pamilla Padilla, Maxine Linial, James Priess, Dan Gottschling, Steve Henikoff, Rafal Ciosk, and Stephen Morrison for critical reading of the manuscript, and Yuji Kohara for yk cDNAs.
This work was supported by a National Institutes of Health grant (GM48435) to M.B. Roth.
Footnotes
Abbreviations used in this paper: CENP, centromere protein; HCP, holocentric protein; RNAi, RNA-mediated interference.
References
- Bailey T.L., Gribskov M. Combining evidence using p-valuesapplication to sequence homology searches. Bioinformatics. 1998;14:48–54. doi: 10.1093/bioinformatics/14.1.48. [DOI] [PubMed] [Google Scholar]
- Brenner S., Pepper D., Berns M.W., Tan E., Brinkley B.R. Kinetochore structure, duplication, and distribution in mammalian cellsanalysis by human autoantibodies from scleroderma patients. J. Cell Biol. 1981;91:95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B.R., Stubblefield E. Ultrastructure and interaction of the kinetchore and centriole in mitosis and meiosis. In: Prescott D.M., Goldstein L., McConkey E., editors. Advances in Cell Biology. Vol. 1. Appleton-Century-Crofts; New York: 1970. pp. 119–185. [Google Scholar]
- Buchwitz B.J., Ahmad K., Moore L.L., Roth M.B., Henikoff S. A histone-H3-like protein in C. elegans . Nature. 1999;401:547–548. doi: 10.1038/44062. [DOI] [PubMed] [Google Scholar]
- Carrington W.A., Lynch R.M., Moore E.D., Isenberg G., Fogarty K.E., Fay F.S. Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science. 1995;268:1483–1487. doi: 10.1126/science.7770772. [DOI] [PubMed] [Google Scholar]
- Chan G.K., Jablonski S.A., Starr D.A., Goldberg M.L., Yen T.J. Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nat. Cell Biol. 2000;2:944–947. doi: 10.1038/35046598. [DOI] [PubMed] [Google Scholar]
- Coen D.M. The polymerase chain reaction Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Stahl K. Short Protocols in Molecular Biology 2nd ed 1992. 15 John Wiley & Sons; New York, NY: 13–15.15. [Google Scholar]
- Davis L.I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986;45:699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans . Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Frank D.J., Roth M.B. ncl-1 is required for the regulation of cell size and ribosomal RNA synthesis in Caenorhabditis elegans . J. Cell Biol. 1998;140:1321–1329. doi: 10.1083/jcb.140.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa T., Brown W.R. Efficient conditional mutation of the vertebrate CENP-C gene. Hum. Mol. Genet. 1997;6:2301–2308. doi: 10.1093/hmg/6.13.2301. [DOI] [PubMed] [Google Scholar]
- Fukagawa T., Pendon C., Morris J., Brown W. CENP-C is necessary but not sufficient to induce formation of a functional centromere. EMBO J. 1999;18:4196–4209. doi: 10.1093/emboj/18.15.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Brinkley B.R. Structure and dynamic organization of centromeres/prekinetochores in the nucleus of mammalian cells. J. Cell Sci. 1996;109:2693–2704. doi: 10.1242/jcs.109.11.2693. [DOI] [PubMed] [Google Scholar]
- Heneen W.K. Ultrastructure of the prophase kinetochore in cultured cells of rat-kangaroo (Potorous tridactylis) Hereditas. 1975;79:209–220. [PubMed] [Google Scholar]
- Henikoff S., Henikoff J.G. Automated assembly of protein blocks for database searching. Nucleic Acids Res. 1991;19:6565–6572. doi: 10.1093/nar/19.23.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Ahmad K., Platero J.S., van Steensel B. Heterochromatic deposition of centromeric histone H3-like proteins. Proc. Natl. Acad. Sci. USA. 2000;97:716–721. doi: 10.1073/pnas.97.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y., Swedlow J.R., Paddy M.R., Agard D.A., Sedat J.W. Three-dimensional multiple-wavelength fluorescence microscopy for the structural analysis of biological phenomena. Semin. Cell Biol. 1991;2:153–165. [PubMed] [Google Scholar]
- Howman E.V., Fowler K.J., Newson A.J., Redward S., MacDonald A.C., Kalitsis P., Choo K.H. Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc. Natl. Acad. Sci. USA. 2000;97:1148–1153. doi: 10.1073/pnas.97.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokelainen P.T. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J. Ultrastruct. Res. 1967;19:19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- Kalitsis P., Fowler K.J., Earle E., Hill J., Choo K.H. Targeted disruption of mouse centromere protein C gene leads to mitotic disarray and early embryo death. Proc. Natl. Acad. Sci. USA. 1998;95:1136–1141. doi: 10.1073/pnas.95.3.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.K., Gruenbaum Y., Spann P., Liu J., Wilson K.L. C. elegans nuclear envelope proteins emerin, MAN1, laminin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol. Biol. Cell. 2000;11:3089–3099. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney T., Ginkel L.M., Hunter A.W., Wordeman L. The kinetochore of higher eukaryotesa molecular view. Int. Rev. Cytol. 2000;194:67–131. doi: 10.1016/s0074-7696(08)62395-5. [DOI] [PubMed] [Google Scholar]
- Meluh P.B., Koshland D. Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Mol. Biol. Cell. 1995;6:793–807. doi: 10.1091/mbc.6.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meluh P.B., Yang P., Glowczewski L., Koshland D., Smith M.M. Cse4p is a component of the core centromere of Saccharomyces cerevisiae . Cell. 1998;94:607–613. doi: 10.1016/s0092-8674(00)81602-5. [DOI] [PubMed] [Google Scholar]
- Moore L.L., Morrison M., Roth M.B. HCP-1, a protein involved in chromosome segregation, is localized to the centromere of mitotic chromosomes in Caenorhabditis elegans . J. Cell Biol. 1999;147:471–480. doi: 10.1083/jcb.147.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R.B. Mitosis. Adv. Cell Biol. 1971;2:225–297. doi: 10.1007/978-1-4615-9588-5_5. [DOI] [PubMed] [Google Scholar]
- Palmer D.K., O'Day K., Wener M.H., Andrews B.S., Margolis R.L. A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J. Cell Biol. 1987;104:805–815. doi: 10.1083/jcb.104.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D.K., O'Day K., Trong H.L., Charbonneau H., Margolis R.L. Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc. Natl. Acad. Sci. USA. 1991;88:3734–3738. doi: 10.1073/pnas.88.9.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos U.P. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma. 1973;41:195–220. doi: 10.1007/BF00319696. [DOI] [PubMed] [Google Scholar]
- Roos U.P. Light and electron microscopy of rat kangaroo cells in mitosis. III. Patterns of chromosome behavior during prometaphase. Chromosoma. 1976;54:363–385. doi: 10.1007/BF00292816. [DOI] [PubMed] [Google Scholar]
- Saitoh H., Tomkiel J., Cooke C.A., Ratrie H.D., Maurer M., Rothfield N.F., Earnshaw W.C. CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell. 1992;70:115–125. doi: 10.1016/0092-8674(92)90538-n. [DOI] [PubMed] [Google Scholar]
- Saitoh S., Takahashi K., Yanagida M. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 1997;90:131–143. doi: 10.1016/s0092-8674(00)80320-7. [DOI] [PubMed] [Google Scholar]
- Shelby R.D., Vafa O., Sullivan K.F. Assembly of CENP-A into centromeric chromatin requires a cooperative array of nucleosomal DNA contact sites. J. Cell Biol. 1997;136:501–513. doi: 10.1083/jcb.136.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D.A., Williams B.C., Hays T.S., Goldberg M.L. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Yata H., Muro Y., Himeno M. Human centromere protein C (CENP-C) is a DNA-binding protein which possesses a novel DNA-binding motif. J. Biochem. (Tokyo) 1994;116:877–881. doi: 10.1093/oxfordjournals.jbchem.a124610. [DOI] [PubMed] [Google Scholar]
- Sullivan K.F., Hechenberger M., Masri K. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J. Cell Biol. 1994;127:581–592. doi: 10.1083/jcb.127.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H., Grishok A., Mello C.C. RNAi in C. eleganssoaking in the genome sequence. Science. 1998;282:430–431. doi: 10.1126/science.282.5388.430. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Chen E.S., Yanagida M. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science. 2000;288:2215–2219. doi: 10.1126/science.288.5474.2215. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL Wimproving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkiel J., Cooke C.A., Saitoh H., Bernat R.L., Earnshaw W.C. CENP-C is required for maintaining proper kinetochore size and for a timely transition to anaphase. J. Cell Biol. 1994;125:531–545. doi: 10.1083/jcb.125.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton P.E., Cooke C.A., Bourassa S., Vafa O., Sullivan B.A., Stetten G., Gimelli G., Warburton D., Tyler-Smith C., Sullivan K.F., Poirier G.G., Earnshaw W.C. Immunolocalization of CENP-A suggests a distinct nucleosome structure at the inner kinetochore plate of active centromeres. Curr. Biol. 1997;7:901–904. doi: 10.1016/s0960-9822(06)00382-4. [DOI] [PubMed] [Google Scholar]
- Yang C.H., Tomkiel J., Saitoh H., Johnson D.H., Earnshaw W.C. Identification of overlapping DNA-binding and centromere-targeting domains in the human kinetochore protein CENP-C. Mol. Cell. Biol. 1996;16:3576–3586. doi: 10.1128/mcb.16.7.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkowski R.P., Meyne J., Brinkley B.R. The centromere–kinetochore complexa repeat subunit model. J. Cell Biol. 1991;113:1091–1110. doi: 10.1083/jcb.113.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]