Abstract
The transition of cell–matrix adhesions from the initial punctate focal complexes into the mature elongated form, known as focal contacts, requires GTPase Rho activity. In particular, activation of myosin II–driven contractility by a Rho target known as Rho-associated kinase (ROCK) was shown to be essential for focal contact formation. To dissect the mechanism of Rho-dependent induction of focal contacts and to elucidate the role of cell contractility, we applied mechanical force to vinculin-containing dot-like adhesions at the cell edge using a micropipette. Local centripetal pulling led to local assembly and elongation of these structures and to their development into streak-like focal contacts, as revealed by the dynamics of green fluorescent protein–tagged vinculin or paxillin and interference reflection microscopy. Inhibition of Rho activity by C3 transferase suppressed this force-induced focal contact formation. However, constitutively active mutants of another Rho target, the formin homology protein mDia1 (Watanabe, N., T. Kato, A. Fujita, T. Ishizaki, and S. Narumiya. 1999. Nat. Cell Biol. 1:136–143), were sufficient to restore force-induced focal contact formation in C3 transferase-treated cells. Force-induced formation of the focal contacts still occurred in cells subjected to myosin II and ROCK inhibition. Thus, as long as mDia1 is active, external tension force bypasses the requirement for ROCK-mediated myosin II contractility in the induction of focal contacts. Our experiments show that integrin-containing focal complexes behave as individual mechanosensors exhibiting directional assembly in response to local force.
Keywords: adhesion-dependent signaling, cell contractility, GFP–vinculin, myosin II, Rho
Introduction
Cells adhere to the extracellular matrix (ECM) via integrin-mediated adhesions that link the ECM to the actin cytoskeleton. Receptors for ECM proteins, transmembrane integrin molecules, are associated via their cytoplasmic domains with a complex of proteins including vinculin, talin, paxillin, tensin, and many others (Jockusch et al. 1995; Burridge and Chrzanowska-Wodnicka 1996; Yamada and Geiger 1997; Small et al. 1998; Bershadsky and Geiger 1999; Critchley 2000), which are all involved in the dynamic association with actin filaments. In cultured cells, integrin-based molecular complexes form discrete morphological entities of several types. Small (0.5–1 μm) dot-like or point contacts (Bershadsky et al. 1985; Tawil et al. 1993) also known as focal complexes (Nobes and Hall 1995) are localized at the edges of lamellipodia. Elongated (3–10 μm in length) streak-like structures associated with actin- and myosin-containing filament bundles (stress fibers) are known as focal contacts or focal adhesions (Heath and Dunn 1978; Rottner et al. 1999; Zamir et al. 2000). An additional form of adhesion site, tensin-enriched fibrillar adhesions (Zamir et al. 1999, Zamir et al. 2000; Katz et al. 2000), is involved in the fibronectin fibrillogenesis (Pankov et al. 2000).
In addition to their function as adhesion sites, matrix adhesions participate in adhesion-dependent signaling (Yamada and Geiger 1997; Schoenwaelder and Burridge 1999). In particular, focal contacts, but not fibrillar adhesions (Zamir et al. 1999), contain high levels of tyrosine-phosphorylated proteins, a hallmark of signaling molecules. Among the components of focal contacts, several types of signaling molecules including tyrosine kinases, tyrosine phosphatases, and adaptor proteins have been identified (Yamada and Geiger 1997; Bershadsky and Geiger 1999; Schoenwaelder and Burridge 1999). Thus, focal contacts function as both adhesion and signal transduction organelles, informing cells about the state of the ECM.
Assembly and morphogenesis of matrix adhesions are in turn regulated by signals from small G-proteins of the Rho family. Activation of Rho is required for the formation of focal contacts and the associated stress fibers, whereas formation of punctate focal complexes depends on the activity of Rac (Nobes and Hall 1995; Rottner et al. 1999). It was observed that focal complexes appear first after cell–matrix interaction, and focal contacts evolve from them in a Rho-dependent manner (Clark et al. 1998; Rottner et al. 1999). Rho functions by triggering many target molecules that, in turn, initiate cascades of downstream events. Two of the immediate Rho targets, Rho-associated kinase (ROCK) (known also as ROK or Rho-kinase) and the formin homology protein mDia1 (a mammalian homologue of Drosophila Diaphanous protein) were shown to mediate the effects of Rho on matrix adhesions and the actin cytoskeleton (Watanabe et al. 1999). Inhibition of ROCK function by chemical inhibitors or dominant negative mutants prevents the formation of focal contacts (Amano et al. 1997; Uehata et al. 1997), but not of focal complexes (Rottner et al. 1999). Appropriately balanced ROCK and mDia1 activities are sufficient to induce stress fiber and focal contact formation indistinguishable from that induced by activated Rho (Watanabe et al. 1999).
Both ROCK and mDia1 have many downstream targets, and to understand the mechanics of focal contact assembly, it is necessary to elucidate which of these targets are involved in the process. In particular, ROCK was shown to stimulate myosin II–driven contractility in smooth muscle and nonmuscle cells by phosphorylating, and thereby inactivating, the myosin light chain phosphatase (Kimura et al. 1996; Kawano et al. 1999) and possibly by direct phosphorylation of the myosin light chain (Kureishi et al. 1997; Totsukawa et al. 2000). Cell contractility is a major factor controlling the formation of stress fibers, as was proposed by Burridge 1981. More recently, experiments with inhibitors affecting different pathways of myosin II regulation revealed a strong correlation between suppression of myosin II–driven contractility and impaired formation of stress fibers and their associated focal contacts (Volberg et al. 1994; Bershadsky et al. 1996; Chrzanowska-Wodnicka and Burridge 1996; Pelham and Wang 1997; Kaverina et al. 1999; Rottner et al. 1999). Overexpression of a natural inhibitor of myosin ATPase, caldesmon, blocks cell contractility and interferes with the transition of focal complexes into focal contacts (Helfman et al. 1999). Both chemical myosin II inhibitors (Chrzanowska-Wodnicka and Burridge 1996) and caldesmon overexpression (Helfman et al. 1999) prevent focal contact formation even if the cells express constitutively active Rho. These studies strongly suggest that Rho-ROCK–mediated increase of cell contractility is a necessary event in the pathway leading to the formation of focal contacts.
Contraction of the cells attached to the substrate leads to the development of tension forces applied to the adhesion sites (Harris et al. 1980; Lee et al. 1994; Galbraith and Sheetz 1997; Dembo and Wang 1999). It is possible that the tension itself affects the focal complexes, inducing their growth and transition into focal contacts. This suggestion is supported by experiments showing that solid substrates induce formation of large focal contacts more efficiently than flexible substrates of the same chemical composition (Pelham and Wang 1997). More recently it was shown that the physical state of the ECM can regulate protein composition of cell–matrix adhesions (Katz et al. 2000).
To determine whether local tension applied to the initial focal complex is in fact a link in the chain of events connecting Rho activation with the formation of focal contacts, we decided to mimic cell contractility–driven tension by local application of external pulling force. To this end we used micropipettes coated with adhesive ligands and a micromanipulation technique to apply controlled stresses to the cell surface. This approach demonstrated convincingly that activation of focal complex growth is a local phenomenon, and that individual focal contacts behave as mechanosensors responding to the application of force by directional elongation. The downstream target of Rho that is sufficient for the force-induced contact formation was shown to be mDia1. At the same time, the entire ROCK-activated pathway, including myosin II activation, appeared to be dispensable when focal contacts were induced by application of external force.
Thus, our experiments show that the probing of the external substrate by the cell can be divided into two phases: first, the ROCK-dependent creation of force by the contractile apparatus; and, second, the response at the level of a single focal contact. The second step does not depend on ROCK but appears to require mDia1. The focal contacts are individual mechanosensors whose elongation reveals the local balance between the force generated by the cell and ECM rigidity.
Materials and Methods
Cells and Transfections
SV-80 human fibroblasts and NIH 3T3 cells were maintained in DME supplemented with 10% bovine calf serum (Hyclone). Cells were transfected using the Ca2+-phosphate method with constructs encoding green fluorescent protein (GFP)–vinculin (Zamir et al. 1999), GFP–paxillin (Zamir et al. 2000), or GFP–actin (provided by G. Marriott, Max-Plank-Institute for Biochemistry, Martinsried, Germany) (Choidas et al. 1998). In some experiments, cells were cotransfected with GFP–vinculin and with caldesmon, epitope tagged with hemagglutinin (HA) (Helfman et al. 1999), with GFP–vinculin and HA-tagged botulinum ADP–ribosyltransferase C3 (C3 toxin), or with a combination of C3 toxin and constitutively active mutants of mDia1. The construct encoding C3, obtained from Dr. A. Hall (University College, London, UK), was subcloned into the pCGN-HA expression vector using the Xba I and BamH I sites. The plasmids pFL-mDia1ΔN1 and pFL-mDia1ΔN3 encoding FLAG-tagged constitutively active mutants of mDia1 were described previously (Watanabe et al. 1999). In these experiments, a constant total amount of DNA (10 μg) was used. The ratio between amounts of C3, mDia1ΔΝ1, and GFP–vinculin DNA was 1:2:7; the ratio between C3, mDia1ΔN3, and GFP–vinculin DNA was 1:4:4. In control experiments, mDia1 constructs were replaced by empty vectors.
5 h after transfection, the medium was changed and cells were incubated in serum-containing medium for 16 h; then, cells were replated in observation chambers (#1 coverslip–bottomed Petri dishes). In some experiments, the cells were plated directly on the observation chambers and transfected after plating. Micromanipulations were performed 36–60 h later; cells were incubated in serum-free medium 5–10 h before the experiments. When the role of substrate coating was studied, transfected cells were replated in serum-free medium on substrates coated with poly-l-lysine (P-1274; Sigma-Aldrich) or poly-l-lysine and fibronectin (F-1141; Sigma-Aldrich) as described (Bershadsky et al. 1996), and experiments were performed 1–3 h later.
Micromanipulations
Micromanipulation experiments were conducted in DME lacking sodium bicarbonate and containing 20 mM Hepes to maintain the pH at 7.2 throughout the experiment. Alternatively, we used CO2-independent medium (Life Technologies). Glass capillaries with outside diameter of 1 mm (B100-75-15; Sutter Instrument Co.) (or GC100T-10; Harvard Apparatus) were pulled with a micropipette puller (P-2000 or P-97; Sutter Instrument Co.). The elastic constant of the pipette tips was ∼60 nN/μm, as estimated by the vertical tip deflection induced by wires of known mass. The section of the pipette attached to the cell was ∼1 μm in diameter. For fibronectin coating, pipettes were incubated at 4°C in a 10 μg/ml fibronectin solution in PBS for 24 h. For poly-l-lysine coating, pipettes were incubated for 10 min at room temperature with a 0.1-mg/ml aqueous solution of poly-l-lysine followed by drying. Manipulations were performed with a Leitz micromanipulator or with a Sutter MP-285 micromanipulator (Sutter Instrument Co.).
Inhibitors
The drug concentrations used were 10 μM for Y-27632 (Welfide Corporation), 30 mM for 2,3-butanedione monoxime (BDM) (B-0753; Sigma-Aldrich) and 5 μM for latrunculin A (Molecular Probes, Inc.). Y-27632 was added 30 min, BDM, 15 min, and latrunculin A, 10 min, before force application. These concentrations and time intervals were sufficient to disrupt focal adhesions of SV-80 cells. The traction forces exerted by substrate-attached cells treated with inhibitors were assessed using the silicone rubber substratum method (Harris et al. 1980), modified as recently described (Burton et al. 1999; Helfman et al. 1999). Coexpression of HA-caldesmon or HA-C3 toxin with GFP–vinculin in transfected cells was confirmed by immunofluorescence staining using monoclonal anti-HA tag antibody (clone 16B12; Babco). Immunolabeling was performed as previously described (Helfman et al. 1999). For each treatment, our evaluation of focal contact growth was based on the results of at least three independent experiments. When an inhibitor interfered with the force-induced growth of focal contacts, as in the presence of latrunculin A or in C3 toxin–transfected cells, we elevated the force by increasing the pipette shift and confirmed that a larger force was also unable to induce focal contact growth.
Microscopy and Image Analysis
Cells were observed with an Axiovert microscope (ZEISS) using the DeltaVision acquisition system (Applied Precision) or with an IX70 microscope (Olympus) and a cooled couple-charged device camera (MicroMAX-1300YHX; Princeton Instruments and Roper Scientific). For living cell experiments, a ZEISS 100× Plan Apochromat objective (Ph3, 1.3 NA) or an Olympus 60× Plan Apochromat objective (Ph3, 1.4) were used. The temperature of the cell under observation was maintained at 37°C by temperature control of the oil-immersion objective, using a heating ring and a controller device (CT15; Minco), or with a water circulating heating unit. Images were processed with Priism (Applied Precision) or WinView software (Roper Scientific).
The total fluorescence intensities of focal contacts were measured using the water algorithm as described (Zamir et al. 1999), and the area, axial ratio, and intensity per pixel were measured by interactive segmentation followed by NIH Image analysis routines. To compare images of GFP fluorescence acquired at two different time points (before and after force application), a temporal fluorescence ratio imaging technique was used as described (Zamir et al. 2000). Figures were composed using Adobe® Photoshop™ software (Adobe Systems, Inc.). In some cases, a high-pass filtration was used to eliminate gradients of intensity in the background.
Online Supplemental Material
Supplemental Figure S1 (available at http://www.jcb.org/cgi/content/full/153/6/1175/DC1) shows formation of focal contacts in response to the application of force to the flexible substrate nearby the cell. GFP–vinculin transfected SV-80 cells were plated on a patterned flexible substrate coated with fibronectin (Balaban et al. 2001). Cells were incubated in serum-free medium 10 h before the experiment. GFP–fluorescence images and phase–contrast images of a cell on the dotted pattern are shown. Distortion of the pattern corresponds to the substrate deformation in the proximity of the cell edge after application of the force by micropipette. Photographs were taken 20 s, 2 min, and 6 min after the substrate deformation. Centripetal tension developed at the opposite cell edge induces focal contact formation and growth. In this set-up, development of tension force is not accompanied by any compression on the cell.
Supplemental Figure S2 (available at http://www.jcb.org/cgi/content/full/153/6/1175/DC1) shows that application of external force at 4°C does not induce focal contact formation. Serum-starved GFP–vinculin-transfected SV-80 fibroblasts were cooled to 4°C at the microscope stage using a water-circulating cooling device mounted on the objective. GFP–vinculin fluorescence before pipette shift, the phase image taken immediately after the shift, and the fluorescence image taken 5 min later are shown. No formation of focal contacts was observed in these experiments.
Results
Mechanical Force Induces ECM-dependent Formation of Focal Contacts in Serum-starved Fibroblasts
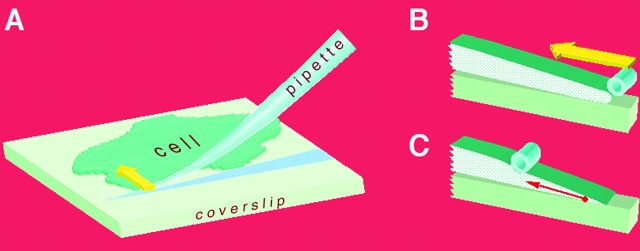
We first examined whether application of mechanical force induces focal contact growth. To apply a force able to locally affect focal complexes at the cell edge, we used micropipette manipulation. The tip of the pipette was coated with an adhesive ligand such as fibronectin or poly-l-lysine. This flexible tip was pressed against the coverslip in the proximity of a cell and shifted towards the cell edge (Fig. 1 A). This motion was performed in a few seconds and extended to several micrometers from the cell edge. This procedure permitted mechanical stress to be induced: the pipette was attached to the upper surface of the cell and its inward displacement resulted in a local mechanical force that was transmitted to the focal complexes (Fig. 1, B–C). For our experiments, we used human SV-80 fibroblasts or NIH 3T3 mouse fibroblasts that were transiently transfected with a GFP–vinculin or GFP–paxillin constructs. Observations of GFP fluorescence together with interference reflection microscopy (IRM) made it possible to follow focal contact dynamics.
Figure 1.
Schemes depicting the method of application of external force. The pipette, covered either with poly-l-lysine or fibronectin, was bent against the coverslip and moved along the chosen lamellipodium as indicated by the yellow arrow (A). The cross sections through the lamellipodium are shown before (B) and after (C) pipette shift. Tension force applied to a focal complex at the cell edge is represented by the red arrow (C).
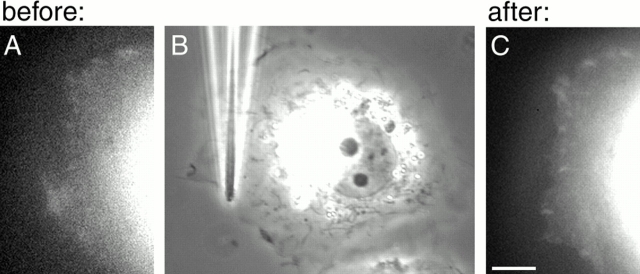
In agreement with previous studies (Ridley and Hall 1992; Bershadsky et al. 1996; Chrzanowska-Wodnicka and Burridge 1996; Helfman et al. 1999), serum deprivation for 5–10 h prevented development of focal contacts in both NIH 3T3 and SV-80 cells so that GFP–vinculin was localized primarily in punctate focal complexes at the cell edge (Fig. 2A and A′). The rapid stress produced by the pipette shift (Fig. 2, compare D with B) induced, after ∼1 min, growth of the focal complexes leading within several minutes to the appearance of streak-like focal contacts (Fig. 2C and Fig. C′). Local force caused a local response: growth was observed only in the focal complexes located at the cell edge in the vicinity of the pipette tip, and the direction of focal adhesion growth corresponded to the direction of the applied stress (Fig. 2E and Fig. E′). Focal complexes and focal contacts in the same cell that were not directly affected by the pulling did not change (Fig. 2E and Fig. F). The observed decrease in the fluorescence of some of these structures (Fig. 2 F) was not significant, since this decrease was close to the decrease induced by photobleaching during the observation period (the estimated ratio between average fluorescence within the cell body after and before pulling was 0.7). Force-induced increase in area (Fig. 2 G) and elongation (Fig. 2 H) of the focal contacts were accompanied by the recruitment of new vinculin molecules, since the fluorescence per unit area of each focal contact remained constant (Fig. 2 I). This experiment was performed on 18 cells (including also NIH 3T3 fibroblasts) and always gave similar results.
Figure 2.
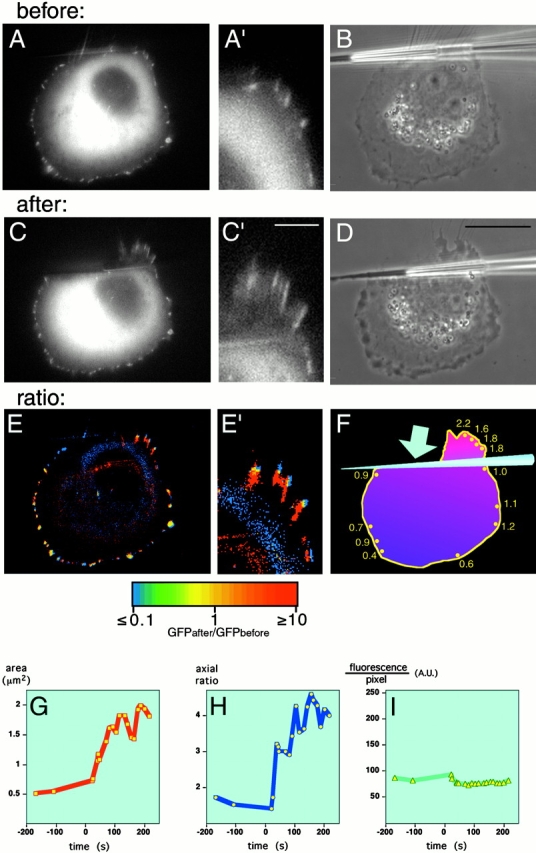
Local formation of focal contacts in response to the application of external force. GFP–vinculin-transfected SV-80 cells incubated in serum-free medium are shown before (A–B) and after (C–D) application of pulling force produced by micropipette shift. (A, A′, C, and C′) GFP fluorescence showing the distribution of vinculin; (B and D) phase image of the same cell. The photographs were taken 2 min before pipette shift (A–B), immediately after the shift (D), and 3 min 37 s after the shift (C and C′). A′ and C′ represent higher magnifications of upper right parts of images A and C, respectively. (E) Image representing pixel-to-pixel ratio between the intensity of the GFP fluorescence 5 min after the pulling and 20 s before pulling. E′ represents high magnification of the upper right part of the image E. The values of the ratio between intensities of after and before images are represented by pseudocolors, as indicated in the spectrum scale shown below E and E′. Thus, red indicates newly formed parts of focal contacts; yellow, stationary parts; and blue, the parts that disappeared. (F) Ratio between the total intensity of individual focal contacts after and before pulling. For each focal contact, the average of the three maximal total intensity values during the 4 min after the pulling was divided by the average of the three maximal values registered during last 5 min before pulling. Positions of the adhesions are indicated by the yellow dots; and the ratio values, by the yellow numbers nearby. The pipette tip is represented by a blue cone; and the direction of the pulling force, by the blue arrow. (G–I) Typical graphs illustrating individual focal adhesion growth. Changes in the focal adhesion area (G), focal adhesion axial ratio (H), and the fluorescence per pixel of the focal contact (I) are shown. Bars: (A, B, C, D, and E; shown in D) 20 μm; (A′, C′, E′; shown in C′) 5 μm.
Our method of force application might also produce, in addition to the centripetal tension force, some pressure on the upper cell surface (Fig. 1 C). To exclude the contribution of this pressure component, we developed another set-up. We plated cells on a flexible-patterned substrate (prepared as in Balaban et al. 2001), and imposed the force by pushing away the pipette tip pressed to the substrate near the cell edge (Supplemental Figure S1, available at http://www.jcb.org/cgi/content/full/153/6/1175/DC1). This procedure was similar to the procedure of local substrate deformation described by Lo et al. 2000. In our case, such deformation caused some displacement of the affected cell in the direction of substrate pushing and apparent development of the centripetal tension at the opposite cell edge. Obviously, this tension was not accompanied by any pressure force. We observed that focal contacts that experienced tension of this sort increased in size similarly to the contacts manipulated by our standard procedure (Supplemental Figure S1, available at http://www.jcb.org/cgi/content/full/153/6/1175/DC1). Thus, a hypothetical pressure contribution seems to be not essential for the focal contact growth induced by our standard micromanipulation technique.
Force-induced changes of adhesion sites visualized in live cells by expression of GFP–paxillin were similar to those visualized by GFP–vinculin fluorescence. Incubation of cells in serum-free medium greatly reduced the size and intensity of GFP–paxillin spots at the cell edge (Fig. 3 A), whereas the pipette shift (Fig. 3 B) induced formation of typical focal contacts elongated in the direction of pulling (Fig. 3 C).
Figure 3.
Force-induced focal contact formation as revealed by GFP–paxillin fluorescence. (A and C) GFP–paxillin distribution at the leading edge of serum-starved SV-80 cell 2 min before (A) and 1.5 min after (C) development of the pulling force. (B) Phase image showing the pipette location after the shift. Bar, 10 μm.
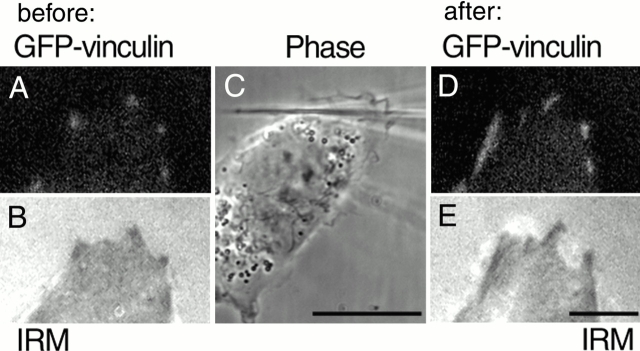
To demonstrate that the recruitment of GFP–vinculin corresponded to the real growth of focal adhesions, we visualized the process with IRM. The dark areas in the IRM images, indicating regions of closest apposition between the lower cell surface and the substrate (Abercrombie and Dunn 1975; Izzard and Lochner 1976), overlapped with the bright areas in the GFP–vinculin fluorescence, both in the case of initial dot-like focal complexes (Fig. 4A and Fig. B) and in the case of elongated focal contacts (Fig. 4D and Fig. E) formed after pipette pulling (Fig. 4 C). Experiments with cells transfected with GFP–actin revealed GFP fluorescence overlapping with the dark areas in the IRM images of focal contacts formed in response to applied stress (not shown). Thus, force-induced formation of focal contacts is accompanied by the recruitment of vinculin, paxillin, and actin, and the elongation of the corresponding dark areas in the IRM images. These results indicate that focal contacts induced by external force have the same structural characteristics as those produced by cells during spreading and locomotion.
Figure 4.
Force-induced recruitment of GFP–vinculin is accompanied by growth of focal contacts as revealed by IRM observations. GFP–vinculin (A and D) and IRM (B and E) images essentially overlap each other both 7 min before (A and B) and 9 min after (D and E) pulling. Phase image after pulling is shown in the middle (C). Bars: (C) 20 μm; (A, B, D, and E; shown in E) 5 μm.
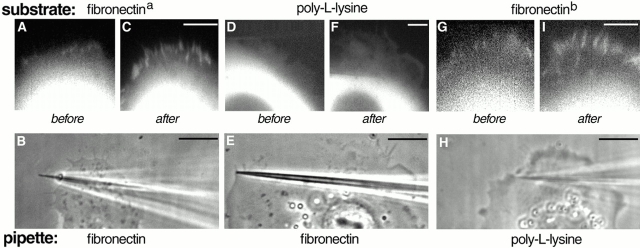
To examine the ECM dependence of force-induced focal contact formation, we compared responses to pipette pulling of cells plated on fibronectin versus poly-l-lysine–coated substrates in serum-free medium. The assay was performed 1–3 h after seeding, before substantial deposition of ECM proteins by the cells plated on poly-l-lysine occurred. Cells plated on the fibronectin-coated coverslips (Bershadsky et al. 1996) responded to the local force by developing focal adhesions as described above (Fig. 5, A–C and G–I). In contrast, cells plated in serum-free medium on coverslips coated with poly-l-lysine did not form focal contacts after the application of force (Fig. 5, D–F). Thus, engagement of integrin receptors with the corresponding ECM proteins on the substrate is an essential requirement for the induction of focal contact formation by external force. In contrast, the nature of the adhesive material used to coat the pipette tip did not affect its capacity to stimulate focal adhesion formation. Indeed, force applied with a poly-l-lysine–coated pipette was as efficient as a fibronectin coated pipette in the induction of focal adhesions (Fig. 5, compare I with C).
Figure 5.
Covering of the substrate with fibronectin is essential for force-induced growth of focal contacts. GFP–vinculin-transfected SV-80 cells plated on poly-l-lysine in serum-free medium do not respond to pulling with a fibronectin-coated pipette by formation of focal contacts (D–F), whereas cells plated on substrate precoated with poly-l-lysine and then coated with fibronectin (fibronectina) produce normal focal contacts (A–C). Under standard conditions (fibronectinb), cells were plated in serum-containing medium and then serum starved. Pulling of these cells with a poly-l-lysine–coated pipette still produces growth of focal contacts (G–I). The positions of pipette immediately after shift are indicated in B, E, and H. Images labeled before and after were taken 1–10 min before and 3–7 min after pipette shift. Bars: (B, E, and H) 10 μm; (C, F, and I) 5 μm. In each before–after pair, the pictures were taken with the same magnification.
External Force–induced Focal Contact Formation Depends on Actin Filament Integrity, but Not on Myosin II–driven Contractility
Since the contractile actomyosin system was shown to be essential for the formation of focal contacts (Volberg et al. 1994; Bershadsky et al. 1996; Chrzanowska-Wodnicka and Burridge 1996; Pelham and Wang 1997; Helfman et al. 1999; Rottner et al. 1999), we examined the requirement for actin filament integrity and actin–myosin interactions in our assay. To disrupt actin filaments, we used latrunculin A, which binds monomeric actin and prevents its polymerization (Ayscough 1998). After latrunculin A treatment, preexisting focal contacts were completely disrupted, leaving only scattered focal complexes intact. Tension applied to latrunculin A–treated cells did not induce growth of focal contacts (Fig. 6, A–B′). This result indicates that integrity of actin filaments is necessary for focal contact induction by external force. Incubation of cells at 4°C, a temperature at which actin polymerization is greatly inhibited (Kane 1976), also interfered with the force-induced growth of focal contacts (Supplemental Figure S2, available at http://www.jcb.org/cgi/content/full/153/6/1175/DC1).
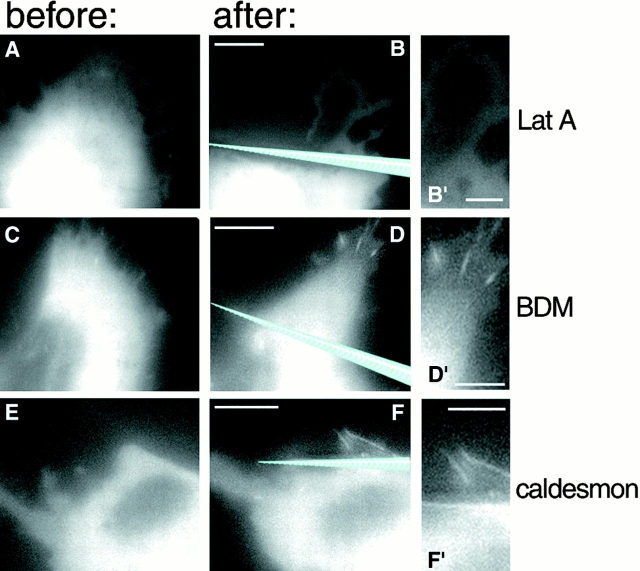
Figure 6.
Function of actin cytoskeleton in the force-induced growth of focal contacts. To probe the role of actin cytoskeleton integrity, SV-80 cells were pretreated with 5 μM latrunculin A (Lat A) (A–B′); to block actomyosin-driven contractility, the cells were pretreated with 30 mM BDM (C–D′) or cotransfected with nonmuscle caldesmon (E–F′). Positions of the pipette tips, immediately after shift, are indicated by blue drawings. B′, D′ and F′ represent enlarged parts of B, D, and F, respectively. Images labeled before and after were taken 1–8 min before and 3–7 min after the pipette shift, respectively. Bars: (B, D, and F) 20 μm; (B′, D′, F′) 5 μm. Note that latrunculin A prevented formation of focal adhesions, whereas neither BDM nor caldesmon transfection interfered with this process.
In contrast, force-induced formation of focal contacts did not appear to require actin–myosin-mediated cell contraction. Indeed, the application of force led to focal contact formation even in the presence of BDM, a potent inhibitor of myosin ATPase (Higuchi and Takemori 1989; Cramer and Mitchison 1995) (Fig. 6, C–D′). Treatment of SV-80 cells with the same concentration of BDM rapidly but reversibly blocked their contractility, as revealed by observations of wrinkling of flexible substrate (Fig. 7, A–E). Another method for blocking actin–myosin contractility was transfection of cells with a construct encoding the regulatory protein caldesmon, a physiological inhibitor of actin-activated myosin ATPase (Helfman et al. 1999). The inhibitory effect of caldesmon transfection on focal contact formation and contractility of SV-80 cells was extensively documented in our previous studies (Helfman et al. 1999). After caldesmon overexpression, application of a local force still induced the formation of elongated focal contacts despite a severely altered cell morphology (Fig. 6, E–F′). Thus, the application of an external force can replace actomyosin contractility during the process of focal contact formation, as long as actin polymerization and actin filament integrity are not disturbed.
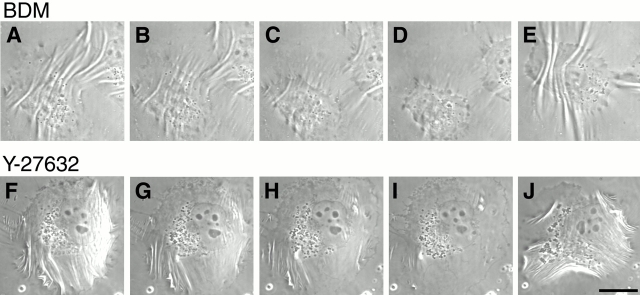
Figure 7.
Reversible suppression of cell contractility by BDM and Y-27632. SV-80 cells growing on the silicon rubber substrate and producing wrinkles (A and F) were treated with either 30 mM actomyosin ATPase inhibitor BDM (top) or 10 μM ROCK inhibitor Y-27632 (bottom). Images of BDM-treated cells were taken at 2.5 (B), 5 (C), and 9.5 min (D) after drug addition; the drug was washed off after 30 min incubation, and the last image (E) was taken at 45 min after washing. Images of the cell treated with Y-27632 were captured at 2.5 (G), 6 (H), and 9.5 min (I) after drug addition; the drug was washed off after 30 min incubation, and the image (J) was taken 50 min later. Bar, 20 μm.
The Functions of Rho Signaling in Focal Contact Formation
It has been shown that activation of Rho signaling is necessary for the formation of focal adhesions induced by a variety of external factors (Ridley and Hall 1992; Hotchin and Hall 1995). Two downstream targets of Rho, namely ROCK and the formin homology protein mDia1, are able to replace Rho in the process of focal contact induction in HeLa cells (Watanabe et al. 1999). We therefore studied the roles of Rho, ROCK, and mDia1 in the development of focal adhesions induced by externally applied mechanical force.
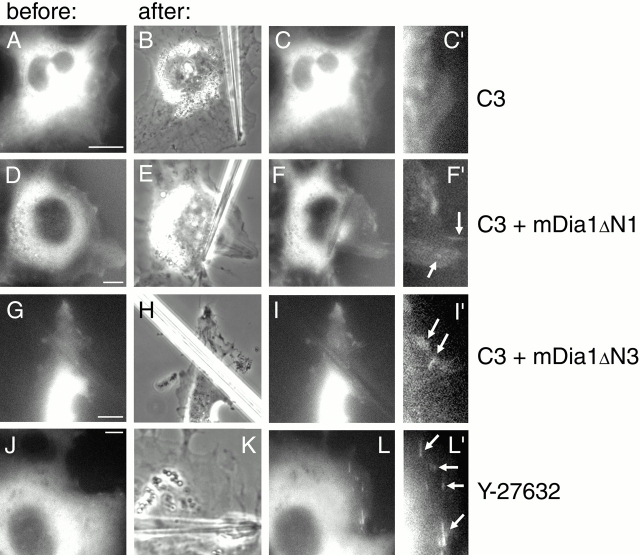
Transfection of cells with a construct encoding C3 toxin, a potent and specific inhibitor of Rho (Aktories and Hall 1989), blocked force-induced focal contact formation (Fig. 8, A–C′). To investigate the role of ROCK, we used a specific inhibitor of its activity, Y-27632 (Uehata et al. 1997). This compound was shown previously to inhibit the formation of focal contacts in serum-containing medium (Uehata et al. 1997); we have confirmed it for SV-80 cells (Riveline, D., and A.D. Bershadsky, unpublished results). Using the substrate wrinkling assay, we observed, as expected, that Y-27632 also rapidly and reversibly blocked cell contractility (Fig. 7, F–J). However, the same concentration of the inhibitor did not block force-induced focal contact growth (Fig. 8, J–L′). Thus, application of external force bypasses the requirement for ROCK activity for the formation of focal contacts.
Figure 8.
Involvement of Rho, ROCK, and mDia1 in the force-induced formation of focal contacts. SV-80 cells were cotransfected with GFP–vinculin and C3 toxin (A–C′) or with GFP–vinculin, C3 toxin, and constitutively active mutants of mDia1, mDia1ΔN1 (D–F′) or mDia1ΔN3 (G–I′). Alternatively, the cells transfected with GFP–vinculin were treated with 10 μM ROCK inhibitor Y-27632 before application of force (J–L′). GFP–vinculin distributions in transfected cells several minutes before pipette shift are shown (A, D, G, and J). Positions of the pipettes, immediately after shift, are shown in phase images (B, E, H, and K). GFP–vinculin distributions in the cells 3–7 min after pipette shift are shown (C, F, I, and L). C′, F′, I′, and L′ represent enlarged parts of cells shown in C, F, I, and L, respectively. Newly formed focal contacts are indicated by arrows. Bars, 10 μm.
The activity of Rho in our assay can be replaced by constitutively active mutants of mDia1. Co-transfection of cells with C3 and constitutively active mDia1 mutants abolished the inhibitory effect of C3 toxin and permitted formation of elongated focal contacts after application of pulling force (Fig. 8F, Fig. F′, I, and I′). Five cells transfected with C3 together with mDia1ΔN1 were analyzed, and all demonstrated pulling-induced focal contact formation (one example is shown in Fig. 8, D–F′). Of four cells cotransfected with C3 and mDia1ΔN3, three cells also demonstrated focal contact growth upon pulling (Fig. 8, G–I′). Cells transfected with the same concentrations of C3 and empty vector instead of mDia1 demonstrated no focal contacts after pulling, similar to the situation when the cells were transfected with the C3 plasmid only (Fig. 8, A–C′). Thus, formation of the focal contacts induced by external force does not depend on ROCK, but requires mDia1 activity.
Discussion
In this study, we introduced a new approach for the micromanipulation of cultured cells enabling us to locally and selectively affect the precursors of focal adhesions at the cell edge. Previously, application of mechanical force with micropipettes coated with adhesive ligands were used to stimulate axonal growth (Bray 1984; Zheng et al. 1991) to measure growth cone adhesion to the substratum (Zheng et al. 1994), and to explore mechanical characteristics of the cytoskeleton (Heidemann et al. 1999). Our purpose was to mimic, using micropipette manipulation, the forces that the cell itself generates at the regions where initial focal complexes are formed. Micropipette pulling was performed close to the cell edge on a thin lamella, consisting mainly of the actin cortex coupled to the plasma membrane. Thus, the force applied by the micropipette is transmitted to the focal complexes and developing focal contacts by stretching a cortical sheet.
To estimate the magnitude of this force, we assumed that the two-dimensional shear modulus of the cell cortex is 2 × 10−3 Pa·m, as was recently measured for NIH fibroblasts (Bausch et al. 1998). With a Poisson ratio ∼0.5, this corresponds to a two-dimensional Young modulus of 6 × 10−3 J/m2. In our experiments, the micropipette was applied to a region of the cortex with both lateral lengths of ∼10 μm, and its displacement was of a similar order of magnitude (Fig. 2B and Fig. D). A scaling approach that circumvents model-dependent calculations by focusing on the relevant physical dimensions predicts that the force exerted is on the order of 6 × 10−3 J/m2 × 10 μm = 60 nN. This force is transmitted to the focal complexes at the cell edge. Although some focal complexes may be uprooted, the tension concentrated on the remaining adhesion sites typically corresponds to 10 nN per growing focal contact. Thus, the estimation of the force experienced by a focal contact in our micropipette manipulation yields the same order of magnitude as the experimentally measured forces normally applied by the cells at their adhesion sites. Estimates of such forces have included 3 nN per single adhesive contact of chick embryo fibroblast, as estimated using special micromachined device (Galbraith and Sheetz 1997), 6 nN per μm2 of the advancing edge of 3T3 cell, as revealed by the elastic substrate method (Dembo and Wang 1999), and 5.5 nN per μm2 of focal contact area, as measured using elastic micropatterned substrate (Balaban et al. 2001).
We have shown, using pipette manipulation, that the local application of centripetal force induces a centripetal growth of the focal complexes under stress: new proteins are recruited, and the complexes elongate and become indistinguishable from normal focal contacts. This experimental system allowed us to further dissect the Rho-dependent pathway responsible for the formation of the focal contacts (Fig. 9). Rho function in the natural process of focal contact–stress fiber formation can be replaced by a combination of constitutively active ROCK and mDia1 (Watanabe et al. 1999). This study shows that, upon application of external force, mDia1 alone is sufficient for the focal contact assembly, whereas ROCK activity is not required. Moreover, ROCK-activated myosin-driven cell contractility is also not essential for focal contact formation. Thus, the segment of the Rho-dependent pathway, from activation of ROCK to the gain of myosin light chain phosphorylation, can be bypassed by application of mechanical load to the initial focal complexes.
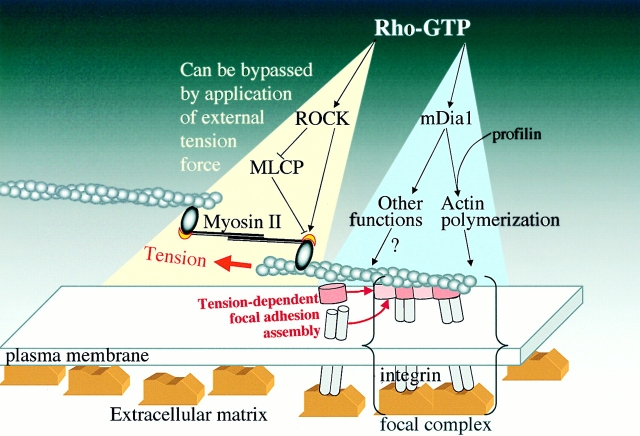
Figure 9.
Scheme depicting involvement of tension force generation in the process of ROCK- and mDia1-dependent formation of focal contacts. Rho induces formation of the focal contacts by activation of two essential pathways, ROCK dependent and mDia1 dependent. The main function of the ROCK-dependent pathway is to activate myosin II–driven cell contractility due to direct or indirect (via myosin light chain phosphatase [MLCP]) effects on the phosphorylation of myosin light chain (yellow crescent). This pathway can be bypassed if the tension force is applied externally. mDia1-dependent pathway includes activation of actin polymerization via interactions with profilin, or possibly other effects on the organization of actin network and microtubules. If mDia1 is active, application of tension force to the focal complexes triggers the transition of these structures into focal contacts and growth of the focal contacts.
It is worth noting that, in addition to its role in regulation of contractility (Kimura et al. 1996; Kawano et al. 1999), ROCK was shown to have other targets related to the regulation of the actin cytoskeleton. Among these targets are ERM proteins (Fukata et al. 1998; Matsui et al. 1998), adducin (Kimura et al. 1998; Fukata et al. 1999), LIM kinase (Maekawa et al. 1999), and the Na-H exchanger NHE1 (Tominaga et al. 1998). In principle, these targets could also mediate the ROCK function in the focal contact formation, but since we have shown that ROCK can be fully replaced by the mechanical force, these targets are nonessential. The minimal function of ROCK, which is necessary for the assembly of focal contacts under normal conditions, is to create force via activation of myosin II. Functions of ROCK mediated by other targets might be involved in fine tuning of the focal contacts and in the organization of the system of stress fibers associated with them.
Function(s) of another Rho target, the formin homology protein, mDia1, which allowed induction of focal adhesions by external force when Rho activity was blocked, are less clear. One possibility is that mDia1, in cooperation with profilin, stimulates assembly of the actin filaments (Watanabe et al. 1997). As shown in our experiments with latrunculin A, the assembly of actin filaments is necessary for the focal contact formation even when external force is applied. A minimal level of actin polymerization is obviously required for the transmission of mechanical force through the cell cytoplasm stretched by micropipette, however intermediate filaments may also be involved in the force transmission (Helmke et al. 2000, Helmke et al. 2001). Moreover, polymerization of actin could participate in the growth of focal contact per se, since filamentous actin may be an essential component of these structures. On the other hand, a novel function of mDia1, also related to the stress fiber–focal adhesion formation, was recently discovered. This function is associated with FH2 domain of the mDia1 molecule, which is not involved in the profilin-dependent regulation of actin polymerization (Ishizaki et al. 2001). It is possible that this function (related to the microtubule-dependent delivery of some components to primordial adhesions) is also necessary for the force-dependent generation of focal contacts.
External force-induced formation of the focal contacts, as well as formation of the focal contacts under normal conditions, requires a specific interaction with the ECM, since contact formation was observed for cells plated on fibronectin, but not on a poly-l-lysine–coated substrate. At the same time, the nature of pipette coating was not critical, and similar results were obtained by pulling with pipettes coated with either fibronectin or poly-l-lysine. This suggests that the stress is not sensed at the point of contact with the pipette or through generalized membrane distortion, but rather via transmission of forces through the cytoskeleton to the cell's basal focal adhesions where mechanosensing takes place.
The idea of integrin involvement in the mechanosensing process was proposed originally by Ingber (Ingber 1991, Ingber 1997). It is currently recognized that physical force applied to molecular complexes associated with integrin receptors can modulate integrin-mediated signaling. Manifestations of integrin signaling, such as tyrosine phosphorylation of specific proteins (Glogauer et al. 1997; Li et al. 1997; Schmidt et al. 1998), increase in free intracellular calcium (Nebe et al. 1995; Pommerenke et al. 1996; Glogauer et al. 1997) and in cAMP level (Meyer et al. 2000), and activation of MAP and Jun kinases (Li et al. 1997; MacKenna et al. 1998) can be efficiently stimulated upon application of external forces to the integrin receptors by stretching the substrate (MacKenna et al. 1998) by fluid shear stress (Li et al. 1997) or via attached magnetic beads (Nebe et al. 1995; Pommerenke et al. 1996; Glogauer et al. 1997; Schmidt et al. 1998; Meyer et al. 2000). Moreover, in the endothelial cells subjected to shear stress, formation and growth of focal contacts aligned in the direction of flow was observed (Davies et al. 1994). These authors suggested that components of integrin-based adhesion complex may be mechanically responsive elements coupled to the cytoskeleton. However, in these studies, the effects of locally applied forces were not considered.
In this study, we have demonstrated that local application of the mechanical force leads primarily to a local effect: further assembly of the integrin-containing molecular complex. This result confirms and extends previous observations of the effects of local force on the integrin receptor complexes. In particular, in experiments of Choquet et al. 1997, a centripetally moving bead attached to the cell surface via integrin was trapped by laser tweezers, thereby inducing a mechanical force applied to the integrin receptor complex. This trapping immediately led to an increase of the force exerted on the bead by the cell (reinforcement), a process that can be explained by the force-dependent recruitment of new components into a primary adhesion complex. This is analogous to our results demon-strating force-induced recruitment of vinculin and paxillin into focal contacts. Observations of Maniotis et al. 1997 have shown that application of tension to surface integrins using fibronectin-coated pipette might result in repositioning and apparent elongation of the individual stress fibers along the direction of tension. More recently, Chicurel et al. 1998 showed that mechanical tension applied to integrin clusters via magnetic beads might even induce local recruitment of ribosomes and mRNA to the focal adhesion-like molecular complexes.
It is premature to speculate on the molecular mechanism within the focal complex that reacts to the application of force. A single molecule or a molecular complex, that could reveal new binding sites in response to stretching, is an attractive possibility. An example of such a molecule is fibronectin. Supramolecular fibronectin assembly can be induced by application of cell-generated mechanical force, unmasking an epitope involved in the assembly process (Zhong et al. 1998; Krammer et al. 1999; Shaub 1999). Such a mechanism might be important in the fibrillogenesis driven by translocation of tensin-rich fibrillar adhesions (Pankov et al. 2000).
Mature focal contacts are dynamic structures (Smilenov et al. 1999; Zamir et al. 2000) thought to maintain their size due to an assembly–disassembly equilibrium. Increase of cell contractility leads to an increase in the focal contact size, whereas blocking of contractility induces rapid disassembly of the focal contacts. Since, as we have shown, the tension-dependent regulation of the focal contact assembly is local, a correlation should exist between local tension applied to individual focal contacts in the cell and the size of this contact. Such a correlation was, in fact, observed recently in our experiments (Balaban et al. 2001).
In summary, Rho-GTP activates two pathways, one ROCK dependent and the other mDia1 dependent, that are both essential for the normal assembly of the focal contacts and subsequent downstream signaling. Activation of ROCK is required for maintenance of myosin II–driven contractility, which in turn is necessary for the generation of tension applied by the cell to focal complexes. This tension is a major factor locally regulating the growth of these complexes and their conversion into focal contacts. External force application allows the bypass of the ROCK-dependent pathway. On the contrary, the mDia1-dependent pathway is essential for induction of focal contact assembly by either cellular or external forces. Thus, we have shown that focal complexes and focal contacts respond to the tension and behave as miniature mechanosensors, and this feature of matrix adhesions plays an important role in the reaction of cells to their microenvironment.
Supplemental Material
Acknowledgments
We thank L. Romer for critical reading of the manuscript and E. Moses, M. Shtutman, H. Delanoë, and M. Vallade for stimulating discussions. We would like to thank the reviewers for their stimulating comments. We acknowledge help of I. Grosheva, M. Lev Ran, M. Shtutman, G. Tzur, and A. Carminati. We are grateful to G. Marriott for GFP–actin construct, to A. Hall for C3 botulinum toxin construct, and to Welfide Corporation for Y-27632.
This work was supported in part by grants from the Minerva Foundation, Israel Science Foundation, Israel Ministry of Science, and Crown Endowment Fund to A.D. Bershadsky, and Région Rhône-Alpes and the French National Center for Scientific Research (CNRS) to D. Riveline. A.D. Bershadsky also acknowledges support from Yad Abraham Center for Cancer Diagnostics and Therapy. D. Riveline acknowledges support from the Scientific Department of the French Embassy in Israel. U.S. Schwarz acknowledges support from the Minerva Foundation. B. Geiger holds the E. Neter Chair in Cell and Tumor Biology. A.D. Bershadsky holds the Joseph Moss Chair of Biomedical Research.
Footnotes
The online version of this article contains supplemental material.
Abbreviations used in this paper: BDM, 2,3-butanedione monoxime; C3 toxin, botulinum ADP–ribosyltransferase C3; ECM, extracellular matrix; GFP, green fluorescent protein; HA, hemagglutinin; IRM, interference reflection microscopy; ROCK, Rho-associated kinase.
References
- Abercrombie M., Dunn G.A. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp. Cell Res. 1975;92:57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- Aktories K., Hall A. Botulinum ADP-ribosyltransferase C3a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol. Sci. 1989;10:415–418. doi: 10.1016/0165-6147(89)90191-0. [DOI] [PubMed] [Google Scholar]
- Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho- kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Ayscough K. Use of latrunculin-A, an actin monomer-binding drug. Methods Enzymol. 1998;298:18–25. doi: 10.1016/s0076-6879(98)98004-1. [DOI] [PubMed] [Google Scholar]
- Balaban N.Q., Schwarz U.S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L., Geiger B. Force and focal adhesion assemblya close relationship studied using elastic micro-patterned substrates. Nat. Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Bausch A.R., Ziemann F., Boulbitch A.A., Jacobson K., Sackmann E. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophys. J. 1998;75:2038–2049. doi: 10.1016/S0006-3495(98)77646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A., Geiger B. Cytoskeleton-associated anchor and signal transduction proteins. Introduction. In: Kreis T., Vale R., editors. Guidebook to the extracellular matrix, anchor, and adhesion proteins. Oxford University Press; New York: 1999. pp. 3–11. [Google Scholar]
- Bershadsky A.D., Tint I.S., Neyfakh A.A., Jr., Vasiliev J.M. Focal contacts of normal and RSV-transformed quail cells. Hypothesis of the transformation-induced deficient maturation of focal contacts. Exp. Cell Res. 1985;158:433–444. doi: 10.1016/0014-4827(85)90467-7. [DOI] [PubMed] [Google Scholar]
- Bershadsky A., Chausovsky A., Becker E., Lyubimova A., Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Bray D. Axonal growth in response to experimentally applied mechanical tension. Dev. Biol. 1984;102:379–389. doi: 10.1016/0012-1606(84)90202-1. [DOI] [PubMed] [Google Scholar]
- Burridge K. Are stress fibres contractile? Nature. 1981;294:691–692. doi: 10.1038/294691a0. [DOI] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burton K., Park J.H., Taylor D.L. Keratocytes generate traction forces in two phases. Mol. Biol. Cell. 1999;10:3745–3769. doi: 10.1091/mbc.10.11.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel M.E., Singer R.H., Meyer C.J., Ingber D.E. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature. 1998;392:730–733. doi: 10.1038/33719. [DOI] [PubMed] [Google Scholar]
- Choidas A., Jungbluth A., Sechi A., Murphy J., Ullrich A., Marriott G. The suitability and application of a GFP-actin fusion protein for long-term imaging of the organization and dynamics of the cytoskeleton in mammalian cells. Eur. J. Cell Biol. 1998;77:81–90. doi: 10.1016/S0171-9335(98)80075-7. [DOI] [PubMed] [Google Scholar]
- Choquet D., Felsenfeld D.P., Sheetz M.P. Extracellular matrix rigidity causes strengthening of integrin–cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E.A., King W.G., Brugge J.S., Symons M., Hynes R.O. Integrin-mediated signals regulated by members of the Rho family of GTPases. J. Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer L.P., Mitchison T.J. Myosin is involved in postmitotic cell spreading. J. Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley D.R. Focal adhesions—the cytoskeletal connection. Curr. Opin. Cell Biol. 2000;12:133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- Davies P.F., Robotewskyj A., Griem M.L. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J. Clin. Invest. 1994;93:2031–2038. doi: 10.1172/JCI117197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M., Wang Y.L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y., Kimura K., Oshiro N., Saya H., Matsuura Y., Kaibuchi K. Association of the myosin-binding subunit of myosin phosphatase and moesindual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J. Cell Biol. 1998;141:409–418. doi: 10.1083/jcb.141.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y., Oshiro N., Kinoshita N., Kawano Y., Matsuoka Y., Bennett V., Matsuura Y., Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J. Cell Biol. 1999;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith C.G., Sheetz M.P. A micromachined device provides a new bend on fibroblast traction forces. Proc. Natl. Acad. Sci. USA. 1997;94:9114–9118. doi: 10.1073/pnas.94.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glogauer M., Arora P., Yao G., Sokholov I., Ferrier J., McCulloch C.A. Calcium ions and tyrosine phosphorylation interact coordinately with actin to regulate cytoprotective responses to stretching. J. Cell Sci. 1997;110:11–21. doi: 10.1242/jcs.110.1.11. [DOI] [PubMed] [Google Scholar]
- Harris A.K., Wild P., Stopak D. Silicone rubber substrataa new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Heath J.P., Dunn G.A. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J. Cell Sci. 1978;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Heidemann S.R., Kaech S., Buxbaum R.E., Matus A. Direct observations of the mechanical behaviors of the cytoskeleton in living fibroblasts. J. Cell Biol. 1999;145:109–122. doi: 10.1083/jcb.145.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman D.M., Levy E.T., Berthier C., Shtutman M., Riveline D., Grosheva I., Lachish-Zalait A., Elbaum M., Bershadsky A.D. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol. Biol. Cell. 1999;10:3097–3112. doi: 10.1091/mbc.10.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke B.P., Goldman R.D., Davies P.F. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ. Res. 2000;86:745–752. doi: 10.1161/01.res.86.7.745. [DOI] [PubMed] [Google Scholar]
- Helmke B.P., Thakker D.B., Goldman R.D., Davies P.F. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys. J. 2001;80:184–194. doi: 10.1016/S0006-3495(01)76006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J. Biochem. 1989;105:638–643. doi: 10.1093/oxfordjournals.jbchem.a122717. [DOI] [PubMed] [Google Scholar]
- Hotchin N.A., Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J. Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D.E. Integrins as mechanochemical transducers. Curr. Opin. Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Ingber D.E. Tensegritythe architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ishizaki T., Morishima Y., Okamoto M., Furuyashiki T., Kato T., Narumiya S. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 2001;3:8–14. doi: 10.1038/35050598. [DOI] [PubMed] [Google Scholar]
- Izzard C.S., Lochner L.R. Cell-to-substrate contacts in living fibroblastsan interference reflexion study with an evaluation of the technique. J. Cell Sci. 1976;21:129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Jockusch B.M., Bubeck P., Giehl K., Kroemker M., Moschner J., Rothkegel M., Rudiger M., Schluter K., Stanke G., Winkler J. The molecular architecture of focal adhesions. Annu. Rev. Cell Dev. Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Kane R.E. Actin polymerization and interaction with other proteins in temperature-induced gelation of sea urchin egg extracts. J. Cell Biol. 1976;71:704–714. doi: 10.1083/jcb.71.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B.Z., Zamir E., Bershadsky A., Kam Z., Yamada K.M., Geiger B. Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol. Biol. Cell. 2000;11:1047–1060. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Small J.V. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Fukata Y., Oshiro N., Amano M., Nakamura T., Ito M., Matsumura F., Inagaki M., Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kimura K., Fukata Y., Matsuoka Y., Bennett V., Matsuura Y., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of the association of adducin with actin filaments by Rho- associated kinase (Rho-kinase) and myosin phosphatase. J. Biol. Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- Krammer A., Lu H., Isralewitz B., Schulten K., Vogel V. Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proc. Natl. Acad. Sci. USA. 1999;96:1351–1356. doi: 10.1073/pnas.96.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureishi Y., Kobayashi S., Amano M., Kimura K., Kanaide H., Nakano T., Kaibuchi K., Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- Lee J., Leonard M., Oliver T., Ishihara A., Jacobson K. Traction forces generated by locomoting keratocytes. J. Cell Biol. 1994;127:1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Kim M., Hu Y.L., Jalali S., Schlaepfer D.D., Hunter T., Chien S., Shyy J.Y. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J. Biol. Chem. 1997;272:30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- Lo C.-M., Wang H.-B., Dembo M., Wang Y.-l. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenna D.A., Dolfi F., Vuori K., Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix- specific in rat cardiac fibroblasts. J. Clin. Invest. 1998;101:301–310. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A., Obinata T., Ohashi K., Mizuno K., Narumiya S. Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Maniotis A.J., Chen C.S., Ingber D.E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Maeda M., Doi Y., Yonemura S., Amano M., Kaibuchi K., Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J. Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C.J., Alenghat F.J., Rim P., Fong J.H., Fabry B., Ingber D.E. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat. Cell Biol. 2000;2:666–668. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- Nebe B., Rychly J., Knopp A., Bohn W. Mechanical induction of β 1-integrin-mediated calcium signaling in a hepatocyte cell line. Exp. Cell Res. 1995;218:479–484. doi: 10.1006/excr.1995.1181. [DOI] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Pankov R., Cukierman E., Katz B.Z., Matsumoto K., Lin D.C., Lin S., Hahn C., Yamada K.M. Integrin dynamics and matrix assemblytensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham R.J., Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommerenke H., Schreiber E., Durr F., Nebe B., Hahnel C., Moller W., Rychly J. Stimulation of integrin receptors using a magnetic drag force device induces an intracellular free calcium response. Eur. J. Cell Biol. 1996;70:157–164. [PubMed] [Google Scholar]
- Ridley A.J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rottner K., Hall A., Small J.V. Interplay between rac and rho in the control of substrate contact dynamics. Curr. Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Pommerenke H., Durr F., Nebe B., Rychly J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J. Biol. Chem. 1998;273:5081–5085. doi: 10.1074/jbc.273.9.5081. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Shaub A. Unravelling the extracellular matrix. Nat. Cell Biol. 1999;1:E173–E175. doi: 10.1038/15608. [DOI] [PubMed] [Google Scholar]
- Small J.V., Rottner K., Kaverina I., Anderson K.I. Assembling an actin cytoskeleton for cell attachment and movement. Biochim. Biophys. Acta. 1998;1404:271–281. doi: 10.1016/s0167-4889(98)00080-9. [DOI] [PubMed] [Google Scholar]
- Smilenov L.B., Mikhailov A., Pelham R.J., Marcantonio E.E., Gundersen G.G. Focal adhesion motility revealed in stationary fibroblasts. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- Tawil N., Wilson P., Carbonetto S. Integrins in point contacts mediate cell spreadingfactors that regulate integrin accumulation in point contacts vs. focal contacts. J. Cell Biol. 1993;120:261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T., Ishizaki T., Narumiya S., Barber D.L. p160ROCK mediates RhoA activation of Na-H exchange. EMBO J. 1998;17:4712–4722. doi: 10.1093/emboj/17.16.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G., Yamakita Y., Yamashiro S., Hartshorne D. J., Sasaki Y., Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Volberg T., Geiger B., Citi S., Bershadsky A.D. Effect of protein kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. Cell Motil. Cytoskeleton. 1994;29:321–338. doi: 10.1002/cm.970290405. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Madaule P., Reid T., Ishizaki T., Watanabe G., Kakizuka A., Saito Y., Nakao K., Jockusch B.M., Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Kato T., Fujita A., Ishizaki T., Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- Yamada K.M., Geiger B. Molecular interactions in cell adhesion complexes. Curr. Opin. Cell Biol. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- Zamir E., Katz B.Z., Aota S., Yamada K.M., Geiger B., Kam Z. Molecular diversity of cell-matrix adhesions. J. Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zamir E., Katz M., Posen Y., Erez N., Yamada K.M., Katz B.Z., Lin S., Lin D.C., Bershadsky A., Kam Z., Geiger B. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- Zheng J., Lamoureux P., Santiago V., Dennerll T., Buxbaum R.E., Heidemann S.R. Tensile regulation of axonal elongation and initiation. J. Neurosci. 1991;11:1117–1125. doi: 10.1523/JNEUROSCI.11-04-01117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Buxbaum R.E., Heidemann S.R. Measurements of growth cone adhesion to culture surfaces by micromanipulation. J. Cell Biol. 1994;127:2049–2060. doi: 10.1083/jcb.127.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C., Chrzanowska-Wodnicka M., Brown J., Shaub A., Belkin A.M., Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.