Abstract
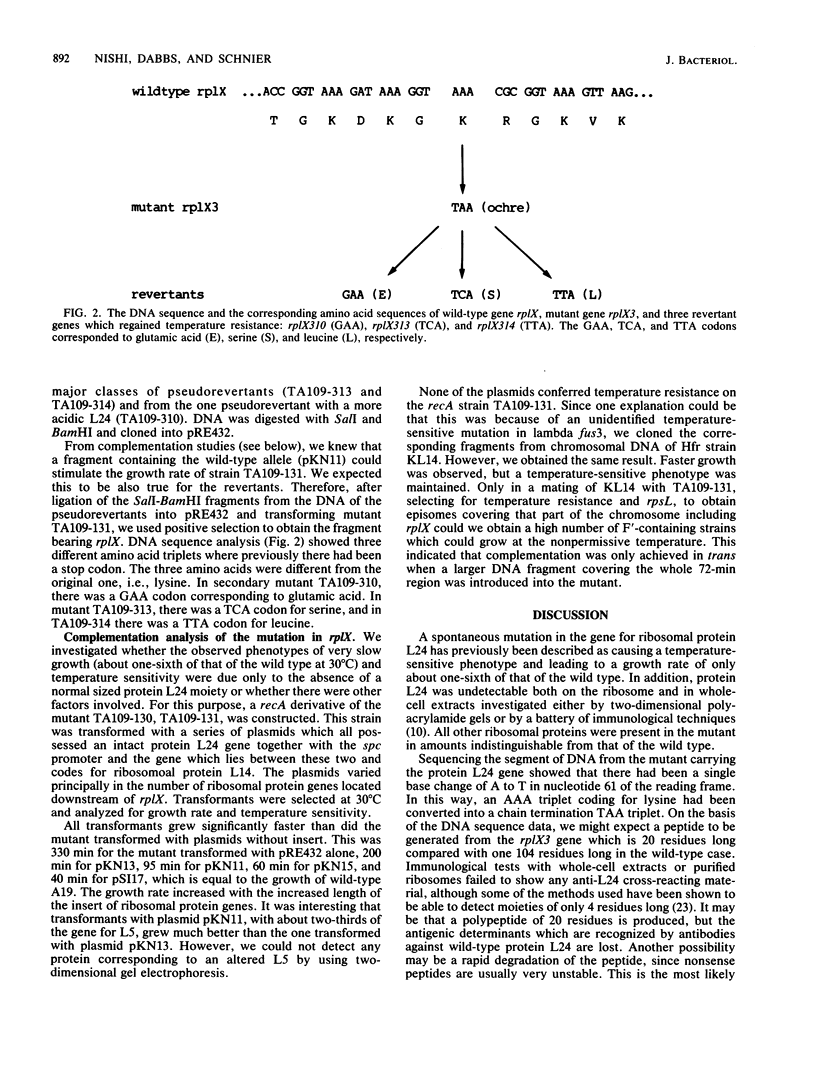
A mutation in Escherichia coli leads to the loss of ribosomal protein L24, severely impaired growth, and a temperature-sensitive phenotype. The mutation was shown to be in rplX, the gene for protein L24, and was due to the alteration of an AAA codon to a TAA stop codon at position 61 in rplX that resulted in a 20-amino acid peptide instead of the 104 amino acids of wild-type L24 protein. rplX genes from three temperature-resistant and fast growing pseudorevertants of the mutant were cloned and sequenced. They were found to have different base substitutions in the TAA codon, resulting in the reappearance of a full-sized protein L24 moiety. Complementation of the slow growth in trans could be achieved with several plasmids containing at least the spc promoter and intact L14 and L24 genes. Plasmids containing genes distal to rplX could further stimulate growth, and the wild type arose when the entire spc operon and the alpha operon were present. In all cases, protein L24 was expressed by the plasmids. Therefore, slow growth could be explained by polarity extending to the alpha operon. However, temperature sensitivity could not be complemented by any of the plasmids in trans, although we found that this phenotype was caused by the mutation in the rplX gene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böck A. Interconnection between assembly and synthesis of ribosomal proteins. Mol Gen Genet. 1981;184(1):62–67. doi: 10.1007/BF00271196. [DOI] [PubMed] [Google Scholar]
- Cabezón T., Herzog A., Petre J., Yaguchi M., Bollen A. Ribosomal assembly deficiency in an Escherichia coli thermosensitive mutant having an altered L24 ribosomal protein. J Mol Biol. 1977 Nov 5;116(3):361–374. doi: 10.1016/0022-2836(77)90075-4. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Kahmann R., Kamp D. Electron microscopic characterization of DNAs of non-defective deletion mutants of bacteriophage Mu. J Mol Biol. 1977 Jul 15;113(4):591–609. doi: 10.1016/0022-2836(77)90224-8. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. A spontaneous mutant of Escherichia coli with protein L24 lacking from the ribosome. Mol Gen Genet. 1982;187(3):453–458. doi: 10.1007/BF00332627. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R., Hasenbank R., Kastner B., Rak K. H., Wartusch B., Stöffler G. Immunological studies of Escherichia coli mutants lacking one or two ribosomal proteins. Mol Gen Genet. 1983;192(3):301–308. doi: 10.1007/BF00392166. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. Mutational alterations in 50 proteins of the Escherichia coli ribosome. Mol Gen Genet. 1978 Sep 20;165(1):73–78. doi: 10.1007/BF00270378. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. Selection for Escherichia coli mutants with proteins missing from the ribosome. J Bacteriol. 1979 Nov;140(2):734–737. doi: 10.1128/jb.140.2.734-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs E. R. The ribosomal components responsible for kasugamycin dependence, and its suppression, in a mutant of Escherichia coli. Mol Gen Genet. 1980 Jan;177(2):271–276. doi: 10.1007/BF00267438. [DOI] [PubMed] [Google Scholar]
- Hasenbank R., Guthrie C., Stöffler G., Wittmann H. G., Rosen L., Apirion D. Electrophoretic and immunological studies on ribosomal proteins of 100 Escherichia coli revertants from streptomycin dependence. Mol Gen Genet. 1973 Dec 14;127(1):1–18. doi: 10.1007/BF00267778. [DOI] [PubMed] [Google Scholar]
- Isono K., Kitakawa M. A new ribosomal protein locus in Escherichia coli: the gene for protein S6 maps at 97 min. Mol Gen Genet. 1977 Jun 8;153(2):115–120. doi: 10.1007/BF00264725. [DOI] [PubMed] [Google Scholar]
- Isono S., Isono K. Mutations affecting the structural genes and the genes coding for modifying enzymes for ribosomal proteins in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):15–20. doi: 10.1007/BF00270371. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Fallon A. M., Nomura M. Identification and organization of ribosomal protein genes of Escherichia coli carried by lambdafus2 transducing phage. J Biol Chem. 1977 Oct 25;252(20):7323–7336. [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny V., Nierhaus K. H. Initiator proteins for the assembly of the 50S subunit from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7238–7242. doi: 10.1073/pnas.79.23.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosset R., Vola C., Feunteun J., Monier R. A Thermosensitive mutant defective in ribosomal 30 S subunit assembly. FEBS Lett. 1971 Oct 15;18(1):127–129. doi: 10.1016/0014-5793(71)80426-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnier J., Isono K. Insertion of IS1 into the rpsE gene for ribosomal protein S5 causes cold-sensitivity in Escherichia coli. Mol Gen Genet. 1984;195(1-2):364–366. doi: 10.1007/BF00332774. [DOI] [PubMed] [Google Scholar]
- Sela M. Antigenicity: some molecular aspects. Science. 1969 Dec 12;166(3911):1365–1374. doi: 10.1126/science.166.3911.1365. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Wittmann H. G., Stöffler G. Altered S5 and S20 ribosomal proteins in revertants of an alanyl-tRNA synthetase mutant of Escherichia coli. Mol Gen Genet. 1974;134(3):225–236. doi: 10.1007/BF00267717. [DOI] [PubMed] [Google Scholar]



