Abstract
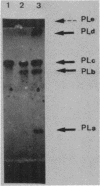
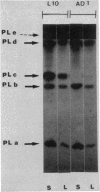
The pelC gene, which encodes one of the five major pectate lyase (PL) isoenzymes in Erwinia chrysanthemi 3937, designated PLc, was subcloned from a hybrid lambda phage into a pBR322 derivative and mutagenized with a mini-Mu-lacZ transposable element able to form fusions to the lacZ gene. One plasmid (pAD1) which had an inactivated pelC gene and a Lac+ phenotype was selected in Escherichia coli. This plasmid was introduced into Erwinia chrysanthemi, and the pelC::mini-Mu insertion was substituted for the chromosomal allele by homologous recombination. This strain lacks the PLc isoenzyme. This Erwinia chrysanthemi strain has a Lac+ phenotype that is inducible by polygalacturonate, as are the wild-type PL activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andro T., Chambost J. P., Kotoujansky A., Cattaneo J., Bertheau Y., Barras F., Van Gijsegem F., Coleno A. Mutants of Erwinia chrysanthemi defective in secretion of pectinase and cellulase. J Bacteriol. 1984 Dec;160(3):1199–1203. doi: 10.1128/jb.160.3.1199-1203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertheau Y., Madgidi-Hervan E., Kotoujansky A., Nguyen-The C., Andro T., Coleno A. Detection of depolymerase isoenzymes after electrophoresis or electrofocusing, or in titration curves. Anal Biochem. 1984 Jun;139(2):383–389. doi: 10.1016/0003-2697(84)90022-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J. In vivo formation of gene fusions encoding hybrid beta-galactosidase proteins in one step with a transposable Mu-lac transducing phage. Proc Natl Acad Sci U S A. 1984 Jan;81(2):535–539. doi: 10.1073/pnas.81.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Buchanan G. E., Behrens M. K., Starr M. P. Synthesis and excretion of polygalacturonic acid trans-eliminase in Erwinia, Yersinia, and Klebsiella species. Can J Microbiol. 1979 Jan;25(1):94–102. doi: 10.1139/m79-014. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Donor strains of the soft-rot bacterium Erwinia chrysanthemi and conjugational transfer of the pectolytic capacity. J Bacteriol. 1977 Dec;132(3):862–869. doi: 10.1128/jb.132.3.862-869.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetics of Erwinia species. Annu Rev Microbiol. 1980;34:645–676. doi: 10.1146/annurev.mi.34.100180.003241. [DOI] [PubMed] [Google Scholar]
- Collmer A., Bateman D. F. Impaired induction and self-catabolite repression of extracellular pectate lyase in Erwinia chrysanthemi mutants deficient in oligogalacturonide lyase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3920–3924. doi: 10.1073/pnas.78.6.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsegem F., Toussaint A., Schoonejans E. In vivo cloning of the pectate lyase and cellulase genes of Erwinia chrysanthemi. EMBO J. 1985 Mar;4(3):787–792. doi: 10.1002/j.1460-2075.1985.tb03698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Jezierska A., Braun-Breton C. lamB mutations in E. coli K12: growth of lambda host range mutants and effect of nonsense suppressors. Mol Gen Genet. 1976 May 7;145(2):207–213. doi: 10.1007/BF00269595. [DOI] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Lactose metabolism in Erwinia chrysanthemi. J Bacteriol. 1985 Apr;162(1):248–255. doi: 10.1128/jb.162.1.248-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoujansky A., Diolez A., Boccara M., Bertheau Y., Andro T., Coleno A. Molecular cloning of Erwinia chrysanthemi pectinase and cellulase structural genes. EMBO J. 1985 Mar;4(3):781–785. doi: 10.1002/j.1460-2075.1985.tb03697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Factors affecting the electrophoretic mobility of the major outer membrane proteins of Escherichia coli in polyacrylamide gels. Biochim Biophys Acta. 1979 Nov 23;581(1):163–178. doi: 10.1016/0005-2795(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Chatterjee A. K. The genus Erwinia: enterobacteria pathogenic to plants and animals. Annu Rev Microbiol. 1972;26:389–426. doi: 10.1146/annurev.mi.26.100172.002133. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]