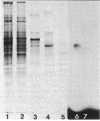
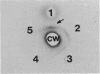
Abstract
One of the key components of the futile xylitol cycle of Lactobacillus casei Cl-16 is a phosphatase which dephosphorylates xylitol 5-phosphate to xylitol prior to the expulsion of the pentitol from cells. This enzyme has been partially purified and characterized. The phosphatase is active against a variety of four-, five-, and six-carbon sugars and sugar alcohols phosphorylated at the terminal 4, 5, and 6 positions, respectively, but exhibits little or no affinity for substrates phosphorylated at the C-1 position. The enzyme has an apparent molecular weight of 62,000 and a pH optimum between 5.5 and 6, and it requires a divalent cation (Mg2+) for maximal activity. A single protein band, exhibiting phosphatase activity, was excised from polyacrylamide gels and used to prepare antiphosphatase sera in rabbits. The antiserum was used to detect the enzyme on polyacrylamide gels and to determine the molecular weight of the monomer on sodium dodecyl sulfate-polyacrylamide gels. With a subunit molecular weight of 32,000, the native enzyme appears to be a dimer. Phosphatase activity and substrate specificity are regulated by some component associated with the cytoplasmic membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Maloney P. C. Characterization of phosphate:hexose 6-phosphate antiport in membrane vesicles of Streptococcus lactis. J Biol Chem. 1984 Oct 25;259(20):12576–12585. [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation and characterization of the galactose-phosphoenolpyruvate-dependent phosphotransferase system in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1204–1214. doi: 10.1128/jb.154.3.1204-1214.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Ghosh R., Ghosh A., Ghosh B. K. Properties of the membrane-bound alkaline phosphatase from glucose- and lactate-grown cells of Bacillus subtilis SB 15. J Biol Chem. 1977 Oct 10;252(19):6813–6822. [PubMed] [Google Scholar]
- Hausman S. Z., Thompson J., London J. Futile xylitol cycle in Lactobacillus casei. J Bacteriol. 1984 Oct;160(1):211–215. doi: 10.1128/jb.160.1.211-215.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horder M. Colorimetric determination of orthophosphate in the assay of inorganic pyrophosphatase activity. Anal Biochem. 1972 Sep;49(1):37–47. doi: 10.1016/0003-2697(72)90240-0. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- London J., Hausman S. Xylitol-mediated transient inhibition of ribitol utilization by Lactobacillus casei. J Bacteriol. 1982 May;150(2):657–661. doi: 10.1128/jb.150.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Kline K. Aldolase of lactic acid bacteria: a case history in the use of an enzyme as an evolutionary marker. Bacteriol Rev. 1973 Dec;37(4):453–478. doi: 10.1128/br.37.4.453-478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Intracellular hexose-6-phosphate:phosphohydrolase from Streptococcus lactis: purification, properties, and function. J Bacteriol. 1983 Oct;156(1):70–80. doi: 10.1128/jb.156.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-D-glucose uncouples energy production from growth. J Bacteriol. 1982 Sep;151(3):1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von HOFSTEN, PORATH J. Purification and some properties of an acid phosphatase from Escherichia coli. Biochim Biophys Acta. 1962 Oct 8;64:1–12. doi: 10.1016/0006-3002(62)90754-0. [DOI] [PubMed] [Google Scholar]