Abstract
The β-chemokine receptor CCR5 is considered to be an attractive target for inhibition of macrophage-tropic (CCR5-using or R5) HIV-1 replication because individuals having a nonfunctional receptor (a homozygous 32-bp deletion in the CCR5 coding region) are apparently normal but resistant to infection with R5 HIV-1. In this study, we found that TAK-779, a nonpeptide compound with a small molecular weight (Mr 531.13), antagonized the binding of RANTES (regulated on activation, normal T cell expressed and secreted) to CCR5-expressing Chinese hamster ovary cells and blocked CCR5-mediated Ca2+ signaling at nanomolar concentrations. The inhibition of β-chemokine receptors by TAK-779 appeared to be specific to CCR5 because the compound antagonized CCR2b to a lesser extent but did not affect CCR1, CCR3, or CCR4. Consequently, TAK-779 displayed highly potent and selective inhibition of R5 HIV-1 replication without showing any cytotoxicity to the host cells. The compound inhibited the replication of R5 HIV-1 clinical isolates as well as a laboratory strain at a concentration of 1.6–3.7 nM in peripheral blood mononuclear cells, though it was totally inactive against T-cell line-tropic (CXCR4-using or X4) HIV-1.
Progress of combination chemotherapy with HIV-1 reverse transcriptase and protease inhibitors has achieved long-sustained suppression of viral replication in HIV-1-infected individuals (1, 2). However, considering high cost and low compliance of long-term combination chemotherapy (3), it seems still mandatory to discover novel anti-HIV-1 agents with different mechanism of action. The β-chemokine receptor CCR5 has been shown to act as a major coreceptor for fusion and entry of macrophage-tropic (CCR5-using or R5) HIV-1 into the host cells (4–8). R5 strains are predominant during the asymptomatic stages of HIV-1 infection whereas T-cell line-tropic (CXCR4-using or X4) strains become prevalent, concomitant with the decline of CD4+ T cells, in the symptomatic stages (9–11). A 32-bp deletion in the CCR5 coding region (CCR5Δ32) generates a nonfunctional receptor, and CCR5Δ32 homozygous individuals are apparently normal but resistant to infection with R5 HIV-1 (12, 13). Furthermore, a recent study suggests that most of the non-syncytium-inducing clinical isolates use only CCR5 for their infection (11, 14, 15). Thus, CCR5 is considered to be an attractive target for inhibition of R5 HIV-1 replication. Although the natural ligands for CCR5 [regulated on activation, normal T cell expressed and secreted (RANTES), macrophage inflammatory protein 1α, and macrophage inflammatory protein 1β] and their modifications (Met-RANTES and aminooxypentane-RANTES) are known to block R5 HIV-1 infection (16–18), nonpeptide CCR5 antagonists have not been identified. In this study, we found TAK-779 (Fig. 1), a small molecular weight (Mr 531.13) nonpeptide compound, to be a potent CCR5-specific antagonist. The compound inhibited R5 HIV-1 replication at concentrations <10 nM, although it was totally inactive against X4 HIV-1.
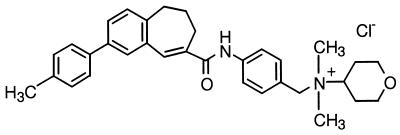
Figure 1.
Chemical structure of TAK-779, N,N-dimethyl-N-[4-[[[2-(4-methylphenyl)-6,7-dihydro-5H-benzocyclohepten-8-yl]carbonyl]amino]benzyl]tetrahydro-2H-pyran-4-aminium chloride.
MATERIALS AND METHODS
Compounds.
Synthesis and purification of TAK-779, N,N-dimethyl-N-[4-[[[2-(4-methylphenyl)-6,7-dihydro-5H-benzocyclohepten-8-yl]carbonyl]amino]benzyl]tetrahydro-2H-pyran-4-aminium chloride (Fig. 1) was carried out in Takeda Chemical Industries (Osaka) and is described elsewhere (M.S., unpublished work). The CXCR4 antagonist AMD3100 (19, 20) was kindly provided by E. De Clercq (Katholieke Universitiet Leuven, Leuven, Belgium). TAK-779 was dissolved in dimethyl sulfoxide at 20 mM to exclude any antiviral or cytotoxic effect of dimethyl sulfoxide. AMD3100 was dissolved in distilled water.
Cells and Viruses.
The Chinese hamster ovary cells (CHO-K1) and Jurkat cells were purchased from the American Type Culture Collection. The cells were cultured in Ham’s F-12 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% FCS and 50 μg/ml gentamycin (Life Technologies). MAGI-CCR5 cells, a HeLa-CD4 line that expresses CCR5 and has an integrated copy of the HIV-1 long terminal repeat-driven β-d-galactosidase reporter gene (21), were obtained through J. Overbaugh of the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases (Bethesda, MD). Four R5 HIV-1 strains (Ba-L, KK, HHA, and CTV) and three X4 HIV-1 strains (IIIB, SW, and MZ) were used in this study. KK, HHA, CTV, SW, and MZ are clinical isolates from infected patients in Japan. Except for IIIB, all strains were propagated in phytohemagglutinin-stimulated peripheral blood mononuclear cells (PBMCs) obtained from healthy donors. The IIIB strain was propagated in MT-4 cells (22). The virus stocks were determined for their p24 antigen levels and were stored at −80°C until use.
DNA Construct and Transfection.
CCR5 cDNA (23, 24) was amplified by PCR from a human spleen cDNA library (Toyobo, Osaka) and was subcloned into the pcDNA3.1 expression vector with human cytomegalovirus promoter (Funakoshi, Tokyo), using the upstream BamHI and downstream XbaI sites. Other β-chemokine receptor DNAs also were cloned by PCR from human cDNA libraries or a human genomic library. Transfection was performed with the cells (8 × 106 cells per 800 μl) by electroporation, using Gene Pulser (Bio-Rad) at 250 mV and 960 μF. Stable transfectants were selected in the presence of 500 μg/ml geneticin (Life Technologies).
Binding Assays.
CHO-K1 and CCR5-expressing CHO (CHO/CCR5) cells (5 × 104 cells per 100 μl) were cultured in a microtiter tray. After a 24-h incubation at 37°C, culture medium was replaced with the binding buffer [Ham’s F-12 medium containing 20 mM Hepes and 0.5% BSA (pH 7.2)]. Binding reactions were performed at room temperature for 40 min in the presence of [125I]-RANTES (specific activity: 2,000 Ci/mmol; Amersham Pharmacia) and various concentrations of the test compound. The binding reaction was terminated by washing out the free ligand with cold PBS, and the cell-associated radioactivity was counted by Top-count scintillation counter (Packard Japan, Tokyo). Binding assays for other receptors, CCR1, CCR2b, CCR3, CCR4, and CXCR4, were carried out in a similar way.
Ca2+ Mobilization Assays.
CHO/CCR5 cells (5 × 106 cells/ml) were suspended in the assay buffer (5 mM KCl/147 mM NaCl/0.22 mM KH2PO4/1.1 mM Na2HPO4/5.5 mM glucose/0.3 mM MgSO4/1 mM MgCl2/10 mM Hepes, pH 7.4). The cells were loaded with 5 μM Fura-PE3AM (Teflabs, Austin, TX) at 37°C for 30 min, were washed twice with the assay buffer containing 1 mM CaCl2, and were resuspended at 1 × 107 cells/ml in the same buffer. At 150 sec after exposure to TAK-779, 20 nM RANTES was added, and relative increase of cytoplasmic Ca2+ levels was monitored by F-2000 fluorescence spectrometer (Hitachi, Tokyo). Calibration was carried out with 50 μM ionomycin (Calbiochem) for total Ca2+ release and with 5 mM EGTA for Ca2+ chelating.
Antiviral Assays.
The anti-HIV-1 activities of the test compounds were based on the inhibition of virus-induced infectious focus formation in MAGI-CCR5 cells and the reduction of p24 antigen production in PBMCs, as described (25, 26). In brief, MAGI-CCR5 cells (1 × 104 cells per well) were cultured in a microtiter tray. After a 24-h incubation at 37°C, the culture supernatants were replaced with fresh culture media containing the virus (≈300 focus forming units per well) and various concentrations of the test compounds. After a 2-day incubation, the cells were fixed and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactosidase. The number of infected (blue) cells was counted microscopically. For the PBMC assays, phytohemagglutinin-stimulated PBMCs (2.5 × 105 cells per 500 μl) were infected with HIV-1 in the presence of various concentrations of the test compounds. The amounts of the virus used for infection were, depending on the replicability of each strain, generally 1–10 ng of p24 per 2.5 × 105 cells. After an overnight incubation at 37°C, the cells were washed extensively to remove unadsorbed viral particles and were incubated further with culture media containing the same concentrations of the compounds as those used during viral adsorption. On day 6 after viral infection, the culture supernatants were collected and determined for their p24 antigen levels with a sandwich ELISA kit (Cellular Products). The cytotoxicities of the compounds were evaluated in parallel with their antiviral activities. They were based on the viability and proliferation of mock-infected cells, as determined by the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method (27).
RESULTS
TAK-779 Inhibits Ligand Binding to CCR5.
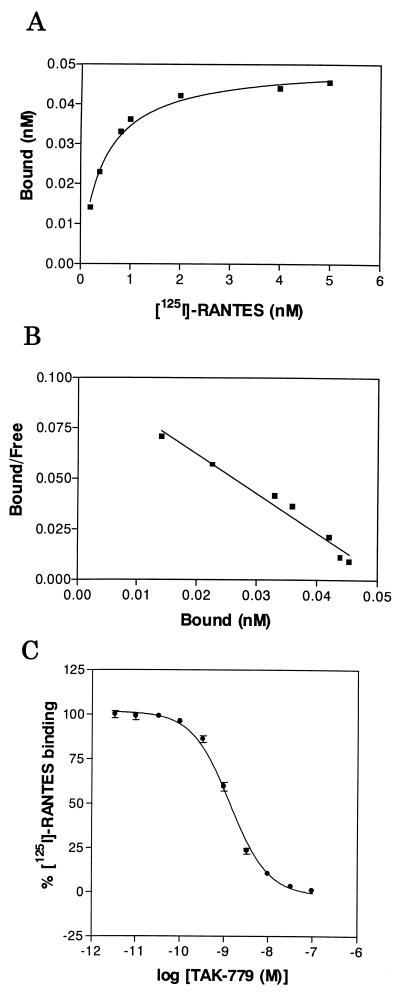
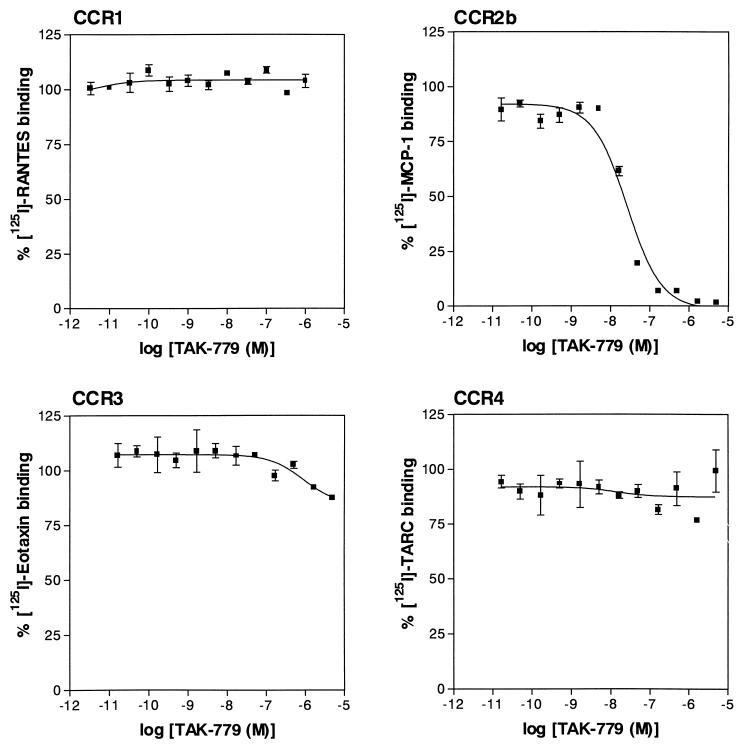
To identify CCR5 antagonists, we have established CHO/CCR5 cells stably expressing CCR5 on their surface and have found that [125I]-RANTES binds to the cells with a high affinity (Kd = 0.45 nM) (Fig. 2 A and B). A lead compound was discovered from the chemical library of Takeda Chemical Industries by high throughput screening based on receptor binding assay using [125I]-RANTES and CHO/CCR5 cells. Through the optimization of the lead compound, we have found TAK-779 as an extremely potent antagonist of CCR5, which completely inhibited the binding of [125I]-RANTES to CHO/CCR5 cells at a concentration of 100 nM (Fig. 2C). Its 50% inhibitory concentration (IC50) for the binding was 1.4 nM. The compound also blocked the binding of macrophage inflammatory protein 1α and macrophage inflammatory protein 1β to the cells with an IC50 of 1.0 nM (data not shown). From competitive binding experiments, the Ki value of TAK-779 was estimated to be 1.1 nM. To determine whether the inhibitory effect of TAK-779 on the chemokine binding is specific to CCR5, the activity of TAK-779 was examined in CHO cells stably expressing either CCR1, CCR2b, CCR3, or CCR4. The compound had no effect on the binding of [125I]-RANTES, [125I]-eotaxin, and [125I]-thymus- and activation-regulated chemokine to CCR1, CCR3, and CCR4, respectively (Fig. 3). Although TAK-779 inhibited the binding of [125I]-monocyte chemotactic protein 1 to CCR2b in CHO/CCR2b cells, its IC50 for CCR2b (27 nM) was ≈20-fold higher than that for CCR5, indicating that TAK-779 preferentially inhibits CCR5. Furthermore, we also examined the inhibitory effect on the binding of [125I]-stromal cell-derived factor 1α to CXCR4 in Jurkat cells (28). However, TAK-779 did not show any inhibition of the binding (data not shown).
Figure 2.
Characterization of CHO/CCR5 cells and inhibitory effect of TAK-779 on [125I]-RANTES binding to the cells. (A) Equilibrium binding of [125I]-RANTES to CHO/CCR5 cells. The cells were incubated in the presence of various concentrations of 100 pM [125I]-RANTES, as described in Materials and Methods. Specific binding was estimated by subtracting the binding of [125I]-RANTES to CHO-K1 cells (nonspecific binding) from the total binding to CHO/CCR5 cells. Data represent means obtained from measurement in triplicate wells. (B) Scatchard analysis for the binding of [125I]-RANTES to CHO/CCR5 cells. The Kd value was calculated by the prism program (Takara, Osaka). (C) Inhibitory effect of TAK-779 on the binding of [125I]-RANTES to CHO/CCR5 cells. The cells were incubated with [125I]-RANTES in the presence of various concentrations of the compound. The percent binding was calculated by an equation of 100 × [(binding with inhibitor − nonspecific binding)/(binding without inhibitor − nonspecific binding)]. The Ki value was calculated by the Cheng-Prusoff derivation (35). Data represent means ± SEM obtained from measurement in six wells from two separate experiments.
Figure 3.
Inhibitory effects of TAK-779 on chemokine binding to CCR1-, CCR2b-, CCR3-, or CCR4-expressing CHO cells. CHO/CCR1, CHO/CCR2b, CHO/CCR3, and CHO/CCR4 cells were incubated with [125I]-RANTES, [125I]-monocyte chemotactic protein 1, [125I]-eotaxin, and [125I]-thymus- and activation-regulated chemokine, respectively, in the presence of various concentrations of the compound. The percent binding was calculated by the same equation described in the legend of Fig. 2. Data represent means ± SEM obtained from measurements in triplicate wells.
TAK-779 Inhibits CCR5-Mediated Ca2+ Signaling.
In chemokine-induced Ca2+ mobilization experiments, RANTES clearly increased an intracellular Ca2+ level in CHO/CCR5 cells at a concentration of 20 nM (Fig. 4A). Addition of 10 nM TAK-779 did not affect the Ca2+ level but completely abrogated the RANTES-induced increase of intracellular Ca2+ level in CHO/CCR5 cells, and the effect was dose-dependent (Fig. 4A). In contrast, the compound did not affect the RANTES-induced Ca2+ mobilization in CHO/CCR1 cells even at a concentration of 100 nM (Fig. 4B). These results indicate that TAK-779 selectively blocks the CCR5-mediated Ca2+-signaling.
Figure 4.
Inhibitory effects of TAK-779 on RANTES-induced Ca2+ mobilization in CHO/CCR5 and CHO/CCR1 cells. (A) FuraPE3-AM-loaded CHO/CCR5 cells were incubated in the absence or presence (10, 1, and 0.1 nM) of TAK-779. Changes in the intracellular Ca2+ level in response to 20 nM RANTES were determined by a fluorescence spectrometer. (B) CHO/CCR1 cells were treated with 100 nM TAK-779 and then were stimulated with 20 nM RANTES.
TAK-779 Is a Potent and Selective Inhibitor of R5 HIV-1.
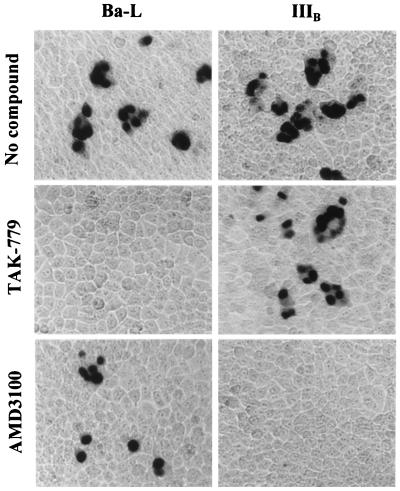
We evaluated the inhibitory effect of TAK-779 on HIV-1 replication in MAGI-CCR5 cells, which express endogenous CXCR4. As shown in Fig. 5, TAK-779 completely inhibited R5 HIV-1 (Ba-L strain) replication in MAGI-CCR5 cells at a concentration of 32 nM. Its 50% and 90% effective concentrations (EC50 and EC90) were 1.2 and 5.7 nM, respectively. However, TAK-779 did not affect X4 HIV-1 (IIIB strain) replication at concentrations up to 20 μM. The 50% cytotoxic concentration (CC50) of TAK-779 for MAGI-CCR5 cells was 51 μM (data not shown). Thus, the selectivity index (ratio of CC50 to EC50) of TAK-779 was 42,500, indicating that the compound is an extremely potent and selective inhibitor of R5 HIV-1 replication. Furthermore, TAK-779 was totally inactive against other X4 HIV-1 (MN and HE strains) or HIV-2 (ROD and EHO strains) in MAGI-CCR5 cells (data not shown). In marked contrast, the specific CXCR4 inhibitor AMD3100 (19, 20) completely inhibited the replication of IIIB strain at 32 nM, although it was not inhibitory to the replication of Ba-L strain at concentrations up to 100 μM (Fig. 5).
Figure 5.
Inhibitory effects of TAK-779 and AMD3100 on the replication of R5 (Ba-L) and X4 (IIIB) HIV-1 in MAGI-CCR5 cells. The assay methods are described in Materials and Methods. The nuclei of infected cells were stained blue. The concentrations of the compounds were 0.032 μM TAK-779 and 100 μM AMD3100 for Ba-L strain and 20 μM TAK-779 and 0.032 μM AMD3100 for IIIB strain. (×100.)
To confirm the anti-HIV-1 activity of TAK-779, the compound was examined for its inhibitory effects on the replication of R5 HIV-1 clinical isolates in PBMCs. As shown in Table 1, TAK-779 proved to be a highly potent inhibitor of the R5 HIV-1 clinical isolates KK, HHA, and CTV as well as the laboratory strain Ba-L. The EC50 and EC90 values for these isolates were 1.6–3.7 nM and 7.5–27 nM, respectively. TAK-779 did not reduce the viability and proliferation of mock-infected PBMCs at concentrations up to 20 μM (Table 1), indicating that TAK-779 also had outstanding selectivity indices for inhibition of R5 HIV-1 replication in PBMCs. The activity of TAK-779 against R5 HIV-1 replication also was demonstrated by a p24 accumulation experiment in culture supernatants of PBMCs infected with the Ba-L strain, where the p24 antigen levels increased with increasing the culture periods, such as <0.01, 0.21, and 9.8 ng/ml on days 2, 4, and 6 after virus infection, respectively. TAK-779 (1–100 μM) could reduce the antigen levels in a dose-dependent fashion at any time point (data not shown). On the other hand, TAK-779 failed to inhibit X4 HIV-1 strains, including two clinical isolates (SW and MZ), even at a concentration of 20 μM (Table 1).
Table 1.
Anti-HIV-1 activity of TAK-779 in PBMCs
| Virus | Tropism | EC50, μM | EC90, μM | CC50, μM |
|---|---|---|---|---|
| Ba-L | R5 | 0.0037 ± 0.0006 | 0.0128 ± 0.0019 | >20 |
| KK | R5 | 0.0016 ± 0.0005 | 0.0208 ± 0.0151 | |
| HHA | R5 | 0.0032 ± 0.0006 | 0.0075 ± 0.0017 | |
| CTV | R5 | 0.0035 ± 0.0015 | 0.0270 ± 0.0048 | |
| IIIB | X4 | >20 | ||
| SW | X4 | >20 | ||
| MZ | X4 | >20 |
EC50 and EC90 were defined as the concentrations of TAK-779 required to reduce p24 antigen production in culture supernatants of HIV-1-infected PBMCs by 50 and 90%, respectively. CC50 was defined as the concentration of TAK-779 required to reduce the viability and proliferation of mock-infected PBMCs by 50%. KK, HHA, and CTV strains are R5 HIV-1 clinical isolates whereas SW and MZ strains are X4 HIV-1 clinical isolates. All data represent means ± SEM of at least three separate experiments.
DISCUSSION
The inhibition of chemokine receptors by TAK-779 appears to be specific to CCR5 because it was highly inhibitory to the binding of [125I]-RANTES to CHO/CCR5 cells and, to a less extent, to the binding of [125I]-monocyte chemotactic protein-1 to CCR2b (Fig. 3). Furthermore, chemokine binding to neither CCR1, CCR3, CCR4, nor CXCR4 was inhibited by the compound. The inhibitory effect of TAK-779 was shown to be selective for CCR5 and not for RANTES because the compound had no effect on [125I]-RANTES binding to CCR1 (Fig. 3) and RANTES-induced Ca2+ mobilization in CHO/CCR1 cells (Fig. 4). The specificity of TAK-779 to CCR5 seems extremely important from a chemotherapeutic viewpoint because nonspecific inhibition of β-chemokine receptors may generate serious side effects associated with chemokine dysregulation (29). Although some HIV-1 isolates can use CCR3 as an alternative coreceptor for their entry into the host cells, in particular, macrophages and microglia (30, 31), the contribution of CCR3 to HIV-1 infection and pathogenesis in vivo has not fully been elucidated.
Unlike RANTES and aminooxypentane-RANTES (32), TAK-779 did not induce the internalization of CCR5 (experiments and data not shown). However, TAK-779 could suppress the binding of the anti-CCR5 mA 45531.111 (R & D Systems) to CHO/CCR5 cells (experiments and data not shown), suggesting the direct interaction between the compound and CCR5. Because the monoclonal antibody was directed to the second extracellular loop of seven-transmembrane receptor CCR5, TAK-779 may have interaction with this extracellular loop. Alternatively, TAK-779 may induce a conformational change of the second extracellular loop after binding to a different part of CCR5. Of interest, TAK-779 did not inhibit the binding of another anti-CCR5 mAb, 2D7 (PharMingen), to CHO/CCR5 cells (experiments and data not shown), although 2D7 also recognizes the second extracellular loop of CCR5 (33). The exact site on CCR5 molecule targeted by the compound remains to be determined.
Another important issue to be considered is the emergence of drug-resistance. It is unlikely that frequent mutations occur in the second extracellular loop of CCR5. However, HIV-1 may be able to acquire the resistance to TAK-779 by amino acid mutations of the viral envelope protein gp120. In fact, X4 HIV-1 resistant to stromal cell-derived factor 1α (natural ligand for CXCR4) and AMD3100 recently has been reported (34). The resistant virus had multiple mutations in gp120 but did not switch chemokine receptor usage. In conclusion, TAK-779 seems to be a promising agent for treatment and prophylaxis of HIV-1 infection. Studies of its toxicology and pharmacokinetics in animals are in progress, aiming at starting its phase I clinical trials in the near future.
Acknowledgments
We thank Dr. A. Nagaoka for his helpful discussions and T. Ito, K. Kuroshima, H. Imoto, M. Seto, and T. Oda for their excellent technical assistance. Thanks are also due to Dr. D. G. Cork for proofreading the manuscript. The R5 HIV-1 clinical isolates HHA and CTV were kindly provided by Dr. S. Matsushita (Kumamoto University, Kumamoto, Japan). This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture.
ABBREVIATIONS
- PBMC
peripheral blood mononuclear cell
- CC50
50% cytotoxic concentration
- CHO
Chinese hamster ovary
References
- 1.Havlir D V, Richman D D. Ann Intern Med. 1996;124:984–994. doi: 10.7326/0003-4819-124-11-199606010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter C C J, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S G, Richman D D, Saag M S, Schooley R T, et al. J Am Med Assoc. 1998;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 3.Deeks S G, Smith M, Holodniy M, Kahn J O. J Am Med Assoc. 1997;277:145–153. [PubMed] [Google Scholar]
- 4.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 5.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 6.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 7.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 8.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 9.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richman D D, Bozzette S A. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 11.Conner R I, Sheridan K E, Caradini D, Choe S, Landau N L. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 13.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C-M, Saragosti S, Lapouméroulie C, Cognaux J, Forceille C, et al. Nature (London) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 14.Bjorndal A, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyo E M. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, et al. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 16.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 17.Proudfoot A E I, Power C A, Hoogewerf A J, Montjovent M O, Borlat F, Offord R E, Wells T N C. J Biol Chem. 1996;271:2599–2603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- 18.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 19.Schols D, Struyf S, Van Damme J, Esté J A, Henson G, De Clercq E. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donzella G A, Schols D, Lin S W, Esté J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, et al. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 21.Chackerian B, Long E M, Luciw P A, Overbaugh J. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyoshi I, Taguchi H, Kubonishi I, Yoshimoto S, Ohtsuki Y, Shiraishi Y, Akagi T. Gann Monogr. 1982;28:219–228. [Google Scholar]
- 23.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 24.Raport C J, Gosling J, Schweickart V L, Gray P W, Charo I F. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 25.Kimpton J, Emerman M. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba M, De Clercq E, Tanaka H, Ubasawa M, Takashima H, Sekiya K, Nitta I, Umezu K, Nakashima H, Mori S, et al. Proc Natl Acad Sci USA. 1991;88:2356–2360. doi: 10.1073/pnas.88.6.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 28.Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass L F, Orsini M J, Taub D, Horuk R. J Immunol. 1998;160:877–883. [PubMed] [Google Scholar]
- 29.Luster A D. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 30.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofman W, Newman W, et al. Nature (London) 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 31.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuna A, Bock P, Che M, Limoges J, Gendelman H E, Mackay C R. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack M, Luckow B, Nelson P J, Cihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, et al. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, et al. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schols D, Esté J A, Cabrera C, De Clercq E. J Virol. 1998;72:4032–4037. doi: 10.1128/jvi.72.5.4032-4037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y-C, Prusoff H R. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]