Abstract
We previously demonstrated that bone morphogenetic proteins (BMPs) induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1. Transcription factors Smads mediate transforming growth factor-β signaling and the ATF/CREB family transcription factor ATF-2 has recently been shown to act as a common target of the Smad and the TAK1 pathways. We here examined the role of Smads and ATF-2 in cardiomyocyte differentiation of P19CL6, a clonal derivative of murine P19 cells. Although P19CL6 efficiently differentiates into cardiomyocytes when treated with dimethyl sulfoxide, P19CL6noggin, a P19CL6 cell line constitutively overexpressing the BMP antagonist noggin, did not differentiate into cardiomyocytes. Cooverexpression of Smad1, a ligand-specific Smad, and Smad4, a common Smad, restored the ability of P19CL6noggin to differentiate into cardiomyocytes, whereas stable overexpression of Smad6, an inhibitory Smad, completely blocked differentiation of P19CL6, suggesting that the Smad pathway is necessary for cardiomyocyte differentiation. ATF-2 stimulated the βMHC promoter activity by the synergistic manner with Smad1/4 and TAK1 and promoted terminal cardiomyocyte differentiation of P19CL6noggin, whereas overexpression of the dominant negative form of ATF-2 reduced the promoter activities of several cardiac-specific genes and inhibited differentiation of P19CL6. These results suggest that Smads, TAK1, and their common target ATF-2 cooperatively play a critical role in cardiomyocyte differentiation.
Keywords: bone morphogenetic protein, Smad, ATF-2, cardiomyocyte differentiation, P19CL6
Introduction
The heart forms through multiple developmental steps such as determination of the cardiac field in the mesoderm, differentiation of cardiac precursor cells into cardiomyocytes, and morphogenesis of the chambered heart (Nascone and Mercola 1995; Olson and Srivastava 1996). Many classical embryonic studies have implicated the mechanisms of how and where these steps take place in the developing embryos. The vertebrate heart arises from paired mesodermal primordia that migrate to the anterior ventral midline, where they fuse and undergo terminal differentiation (Rosenquist and Dehaan 1966; Han et al. 1992). In Xenopus embryos, the cardiac field is located in the dorsal mesoderm lateral to the Spemann organizer and is specified before the end of gastrulation. In this relatively early step of cardiac development, inductive signals from the adjacent deep endoderm and the organizer region have been shown to play an important role in determination of the cardiac field. It has been reported that the presence of deep dorsoanterior endoderm markedly enhances the heart formation in explants of heart primordia and that the presence of both the endoderm and the organizer is necessary and sufficient to induce beating heart tissue in ventral mesoderm explants (Nascone and Mercola 1995). In chicks, the anterior endoderm also has been shown to induce differentiation of nonprecardiac mesodermal cells into heart tissue (Schultheiss et al. 1995). These observations suggest that the endoderm-derived signals play a vital role both in specification of the cardiac field and in differentiation of determined cardiac precursor cells. However, little is known about the molecular mechanisms that regulate these inductive events during the formation of the heart.
Recently, decapentaplegic (dpp) and bone morphogenetic proteins (BMPs), which are the members of the TGF-β superfamily, have been demonstrated to be important candidates that regulate expression of some cardiac-enriched transcription factors such as tinman, Csx/Nkx-2.5, and GATA-4 and induce cardiomyocyte differentiation. In Drosophila, it has been reported that expression of tinman is restricted to the dorsal part of the mesoderm by the ectodermally expressed dpp, which is most closely related to vertebrate BMP-2 or BMP-4 (Frasch 1995). Subsequently, experiments using chick embryos have demonstrated that expression of BMP-2/BMP-4 is detected in the ectoderm or the endoderm adjacent to the precardiac mesoderm and that ectopic expression of BMP-2 induces differentiation of nonprecardiac mesodermal cells into beating cardiomyocytes (Schultheiss et al. 1997), suggesting that BMPs play a pivotal role in induction of vertebrate cardiac development. Furthermore, gene targeting experiments have shown that normal cardiac development is impaired both in BMP-2 and BMP-4 knockout mice (Winnier et al. 1995; Zhang and Bradley 1996). These results indicate that BMPs are required for normal cardiac development.
However, the precise molecular mechanisms by which BMPs regulate cardiogenesis have been largely unknown because of the complexity in the in vivo situation. From this viewpoint, we used P19CL6, an in vitro system of cardiomyocyte differentiation to dissect the cardiogenic pathways mediated by BMPs. P19CL6 is a clonal derivative isolated from murine P19 embryonic carcinoma cells by limiting dilution method (Habara-Ohkubo 1996). Unlike P19 cells, whose utility is limited because of their multipotential properties, this CL6 subline efficiently differentiates into beating cardiomyocytes with adherent conditions when treated with 1% DMSO. As almost all cells differentiate into cardiomyocytes with expression of cardiac-specific genes, P19CL6 is thought to be a useful in vitro model to study cardiomyocyte differentiation (Habara-Ohkubo 1996).
By using the P19CL6 in vitro system, we previously demonstrated that BMPs are indispensable for cardiomyocyte differentiation and that BMPs induce cardiomyocyte differentiation through TAK1, a member of the mitogen-activated protein kinase kinase kinase (MAPKKK) family that has been demonstrated to be involved in TGF-β signaling (Monzen et al. 1999). As well as TAK1, the Smad proteins have been identified and characterized as important mediators of TGF-β signal transduction pathways (Heldin et al. 1997; Attisano and Wrana 1998; Massagué 1998). Among the members of Smads, Smad1, Smad5, and Smad8 transduce signals from BMPs specifically, whereas Smad4 is a general partner of ligand-specific Smads. After ligand stimulation and phosphorylation of ligand-specific Smads by the receptors, Smad4 forms heterooligomers with ligand-specific Smads, which in turn translocate into the nucleus and activate transcriptional responses. Recent studies in Drosophila have shown that Dpp-induced tinman gene expression is positively regulated by the Smad4 homologue Medea (Xu et al. 1998), suggesting that the Smads-mediated signal transduction pathway is also involved in the BMP-induced differentiation into cardiomyocytes. Therefore, we investigated the role of Smads in cardiomyocyte differentiation of P19CL6 in this study.
ATF-2 is a member of the ATF/CREB family transcription factors, all of which contain a DNA binding domain, b-ZIP, consisting of a cluster of basic amino acids and leucine zipper structures (Maekawa et al. 1989; Busch and Sassone-Corsi 1990). They form dimers through their leucine zipper regions and bind to cAMP response element (CRE). ATF-2 is phosphorylated and stimulated by stress-activated protein kinases (SAPKs) such as c-Jun NH2-terminal kinases (JNKs) and p38 at Thr-69, Thr-71, and Ser-90 which lie close to the NH2-terminal transcriptional activation domain (Gupta et al. 1995) and binds to CRE with high affinity as a homodimer or a heterodimer with c-Jun (Macgregor et al. 1990; Hai and Curran 1991). Recently, ATF-2 has been reported to bind directly to heterooligomers of Smads and be phosphorylated by TGF-β signaling via TAK1 and p38, indicating that ATF-2 is a common nuclear target of the Smad and the TAK1 pathways in TGF-β signaling (Hanafusa et al. 1999; Sano et al. 1999). The actions caused by both pathways are shown to be responsible for the synergistic stimulation of ATF-2 transactivating capacity (Sano et al. 1999). From this point of view, we have examined the involvement of ATF-2 in BMP-induced cardiomyocyte differentiation in this study.
Here we demonstrate several lines of evidence which suggest that Smads, TAK1, and their common downstream target ATF-2 cooperatively play a critical role in differentiation of P19CL6 cells into cardiomyocytes.
Materials and Methods
Plasmids
Expression plasmids constructed by inserting FLAG-tagged murine Smad1, Smad4, and Smad6 into pcDNA3 vectors were described previously (Imamura et al. 1997). Expression plasmids encoding the TAK1 mutants (Yamaguchi et al. 1995), murine GATA-4 (Arceri et al. 1993), and murine MEF2C cDNA (Lin et al. 1997) were provided by H. Shibuya (Okazaki, Japan), D.B. Wilson (St. Louis, MO), and E.N. Olson (Dallas, TX), respectively. Expression plasmids containing human wild-type ATF-2 cDNA and the ATF-2 mutants (ATF-2Ala and ATF-2Δ107) were described previously (Matsuda et al. 1991; Sano et al. 1999). Plasmids containing luciferase gene driven by βMHC, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and skeletal α-actin promoters were also described previously (Takano et al. 1998; Shiojima et al. 1999). Expression plasmids encoding green fluorescent protein (GFP) was commercially purchased (pEGFP-1; CLONTECH Laboratories, Inc.).
Cell Culture and Differentiation
P19CL6 cells were cultured essentially as described previously (Habara-Ohkubo 1996). In brief, the cells were grown in a 100-mm tissue culture grade dish under adherent conditions with α-minimal essential medium (GIBCO BRL), supplemented with 10% FBS (JRH Bioscience), penicillin (100 U/ml), and streptomycin (100 μg/ml) (growth medium), and were maintained in a 5% CO2 atmosphere at 37°C. To induce differentiation under adherent conditions, P19CL6 cells were plated at a density of 3.7 × 105 cells in a 60-mm tissue culture grade dish with the growth medium containing 1% DMSO (differentiation medium). The medium was changed every 2 d. Days of differentiation were numbered consecutively after the first day of DMSO treatment as day 0. Natural bovine BMP cocktail (Sangi) which contains almost all types of bone-derived BMPs including BMP-2 and BMP-4 was added into the differentiation medium at a final concentration of 100 ng/ml.
Stable Transformants
Establishment of P19CL6noggin was described previously (Monzen et al. 1999). To isolate the permanent cell line P19CL6Smad6, P19CL6 cells were transfected with pcDNA3-Smad6 (Imamura et al., 1997) by the lipofection method (Tfx™ Reagents; Promega). Stable transformants were selected with 400 μg/ml of neomycin (G418), and six independent cell lines were cloned.
Transfection and Reporter Assay
To examine the differentiation ability of P19CL6 and P19CL6noggin cells, the cells were transfected with expression vectors containing Smad1, Smad4, Smad6, the TAK1 mutants, and the ATF-2 mutants on day 3 of differentiation according to the lipofection method as recommended (Promega). The morphological features and the beating ability of the cells were observed accompanied by immunostaining with MF20 on day 12 or day 14 as described below. To test the promoter activities, the cells were transfected simultaneously with effector and reporter plasmids on day 5 by the calcium phosphate method and then the cell lysates were extracted on day 7. The luciferase activities were measured as described previously (Takano et al. 1998).
Immunofluorescence
Immunostaining with MF20, a monoclonal antibody against sarcomeric MHC, was performed as described previously (Bader et al. 1982) using anti–mouse immunoglobulin G conjugated with tetramethyl rhodamine isothiocyanate as the secondary antibody. MF20-positive areas were measured on day 14 by directly tracing the stained areas on a photograph. Immunostaining with anti-FLAG antibody was performed as described previously (Zhu et al. 1999) using fluorescein isothiocyanate–conjugated goat anti–mouse immunoglobulin G as the secondary antibody.
RNA Analysis
Total RNA was extracted by the acid guanidine method (RNAzol B™; Biotecx Laboratories, Inc.), and Northern blot analysis was performed as described previously (Monzen et al. 1999) with 10 μg of total RNA from each sample for Smad6, GATA-4, MEF2C, MHC, MLC2v, and ATF-2. The following cDNA fragments were used as probes: the EcoRI fragment of pcDNA3 containing murine Smad6 cDNA (Imamura et al. 1997), the EcoRI fragment of pMT2 containing murine GATA-4 cDNA (Arceri et al. 1993), the EcoRI fragment of pcDNA1 containing murine MEF2C cDNA (Lin et al. 1997), the PstI fragment of pMHC25 containing rat skeletal muscle MHC cDNA (Takano et al. 1998), the EcoRI fragment of pCRII containing PCR product obtained by using oligonucleotide primers specific for MLC2v (Lyons et al. 1995), and the XbaI/HincII fragment of pact-ATF-2 containing human ATF-2 cDNA (Sano et al. 1999). For the analysis of Csx/Nkx-2.5 mRNA, reverse transcription (RT)-PCR was performed as described previously (Monzen et al. 1999).
Detection of Phosphorylated ATF-2 Protein
To examine the phosphorylation of endogenous ATF-2, whole cell extracts from the P19CL6 cells on day 0 and day 6 of differentiation were prepared as described previously (Shiojima et al. 1999). The cell extracts were analyzed by SDS-PAGE followed by Western blotting. The phosphorylation of ATF-2 was examined using Phosphoplus ATF-2 (New England Biolabs, Inc.), which detects phosphorylation of Thr-71 specifically. The protocols of SDS-PAGE and Western blot analysis were described previously (Zhu et al. 1999).
Results
Cooverexpression of Smad1 and Smad4 Restored the Ability of P19CL6noggin Cells to Differentiate into Cardiomyocytes
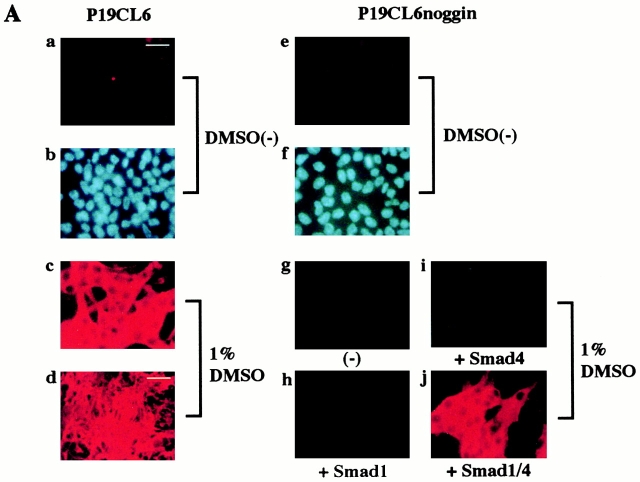
To elucidate the precise molecular mechanisms by which BMP signaling regulates cardiomyocyte differentiation, we used the P19CL6 in vitro system. When cultured in growth medium without DMSO, P19CL6 cells grew well and did not differentiate into any specific cells including cardiomyocytes (Fig. 1 A, a and b). When 1% DMSO was added to the medium, P19CL6 efficiently differentiated into mononucleated, spontaneously contracting cardiomyocytes, immunostained by antisarcomeric MHC antibody MF20 (Fig. 1 A, c and d). As described previously (Habara-Ohkubo 1996; Monzen et al. 1999), spontaneous beating was first observed on a limited area on day 10 (10 d after the initiation of DMSO treatment), and subsequently, the majority of cells started to beat synchronously. To test the requirement of BMPs for cardiomyocyte differentiation, we previously established the permanent cell line named P19CL6noggin, which constitutively overexpresses the BMP antagonist noggin (Monzen et al. 1999). In contrast to parental P19CL6 cells, P19CL6noggin cells did not differentiate into cardiomyocytes even when they were treated with 1% DMSO (Fig. 1 A, g), suggesting that BMPs are indispensable for cardiomyocyte differentiation. To elucidate whether Smads may mediate BMP-induced cardiomyocyte differentiation of P19CL6, P19CL6noggin cells were transfected with expression plasmids containing Smad1 or Smad4 on day 3 by the lipofection method and the differentiation ability was assessed around day 14. Smad1 is one of ligand-specific Smads and transduces signals from BMPs specifically, whereas Smad4 is a general partner of ligand-specific Smads. Unlike the control P19CL6noggin cells, the cells transfected simultaneously with Smad1 and Smad4 partially differentiated into beating cardiomyocytes positive for MF20 by the treatment with DMSO (Fig. 1 A, j). On the other hand, the cells transfected with Smad1 alone or Smad4 alone did not differentiate into cardiomyocytes (Fig. 1 A, h and i). The same results were obtained with at least three independent cell lines.
Figure 1.

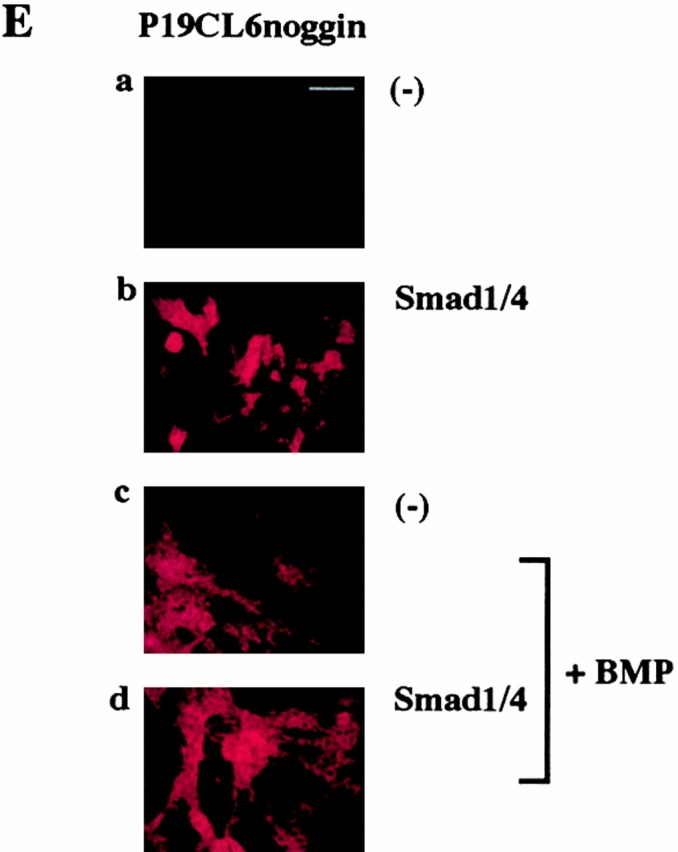
Simultaneous overexpression of Smad1 and Smad4 restored the ability of P19CL6noggin cells to differentiate into cardiomyocytes. (A) P19CL6 cells (a–d) and P19CL6noggin cells (e–j) were cultured in growth medium (a, b, e, and f) or in differentiation medium containing 1% DMSO (c, d, g–j). Both parental P19CL6 cells (a and b) and P19CL6noggin cells (e and f) grew well and remained undifferentiated in growth medium. Parental P19CL6 cells differentiated into beating cardiomyocytes when cultured in the medium containing 1% DMSO. On day 14, most P19CL6 cells had differentiated into mononucleated contracting cardiomyocytes (c and d). On the other hand, P19CL6noggin cells did not differentiate into beating cardiomyocytes even after treatment with DMSO (g). The P19CL6noggin cells transfected with Smad1 alone (h) or Smad4 alone (i) did not differentiate into cardiomyocytes, whereas cooverexpression of Smad1 and Smad4 induced differentiation of P19CL6noggin cells into cardiomyocytes in the presence of DMSO (j). The cells were stained with antisarcomeric myosin heavy chain antibody (MF20) (a, c–e, g–j) or Hoechst dye (b and f). Bars, 250 μm (d) and 50 μm (others). (B) The localization of exogenous Smad1 in P19CL6noggin cells. In the presence or the absence of BMP (100 ng/ml), P19CL6noggin cells were transfected with expression vectors containing FLAG-tagged Smad1 and those containing Smad4, and immunostained by anti-FLAG antibody 12 h later. (a, c, and e) Hoechst dye; (b, d, and f) immunostaining with anti-FLAG antibody. Arrows, a FLAG-positive cell; arrowheads, a FLAG-negative cell. Bar, 20 μm. (C) The correlation between overexpression of Smad1/4 and the P19CL6 differentiation. P19CL6noggin cells were transfected with expression plasmids encoding Smad1 and Smad4 along with GFP expression plasmids on day 3 and then immunostained by MF20 on day 12. (a) Hoechst dye; (b) GFP; (c) MF20. Arrows, a GFP-positive cell; arrowheads, a GFP-negative cell. Bar, 20 μm. (D) Expression of cardiac-specific genes in P19CL6noggin cells overexpressing Smad1 and/or Smad4. RNA was extracted from parental P19CL6 cells and P19CL6noggin cells transfected with Smad1 and/or Smad4 on day 14. RT-PCR was performed for the analysis of Csx/Nkx-2.5 mRNA. 10 μg of RNA from each sample was subjected to Northern blot analysis for other genes. Ethidium bromide staining of rRNA is presented at the bottom to show that the same amount of intact RNA was loaded in each lane. (E and F) Ligand stimulation enhanced the differentiation efficiency of P19CL6noggin cells transfected with Smad1/4. (E) Immunostaining with MF20. P19CL6noggin cells were cultured with (c and d) or without BMP (a and b) in the culture media at the concentration of 100 ng/ml from day 0. The cells were transfected with Smad1/4 on day 3 by the lipofection method (b and d). The cells were then immunostained with MF20 on day 14. By the treatment with BMP, P19CL6noggin cells transfected with Smad1/4 more efficiently differentiated into MF20-positive cardiomyocytes. Bar, 250 μm. (F) Quantification of the areas stained by MF20 in P19CL6noggin cells. The areas of at least three fields were measured for each cell line under the same conditions. The results are expressed as the mean (%) ± SD.
Translocation of Smads into the nucleus and subsequent transcriptional activation have been reported to require heterooligomer formation of ligand-specific Smads and the common mediator Smad4 after ligand stimulation and phosphorylation of ligand-specific Smads by the receptors (Lagna et al. 1996; Macias-Silva et al. 1996). To clarify whether overexpression of exogenous Smad1 and Smad4 causes nuclear accumulation of the Smad proteins, we examined the localization of exogenous FLAG-tagged Smad1 in P19CL6noggin cells using anti-FLAG antibody. P19CL6noggin cells were transfected with both expression plasmids carrying FLAG-tagged Smad1 and those containing Smad4, and immunostained by anti-FLAG antibody 12 h later. In the absence of BMP stimulation, the staining was highly positive in the cytosol, and less but surely positive in the nucleus (Fig. 1 B, a–d). On the other hand, FLAG signals were detected predominantly in the nucleus after BMP stimulation (Fig. 1 B, e and f). These observations suggest that a part of overexpressed Smads can move into the nucleus even in the absence of BMP stimulation and that the sufficient ligand stimulation accelerates the translocation of exogenous Smads into the nucleus.
To characterize the correlation between overexpression of Smads and the P19CL6 differentiation into cardiomyocytes at the cellular level, we examined whether the differentiation is surely induced in the cells overexpressing exogenous Smads. P19CL6noggin cells were transfected simultaneously with expression vectors carrying Smad1/4 and those containing GFP on day 3 and then were stained by MF20 on day 12. The immunostaining by MF20 were detected in GFP-positive cells (Fig. 1 C, arrows) but not in GFP-negative cells (Fig. 1 C, arrowheads), suggesting that the myocyte differentiation was induced in the cells overexpressing exogenous Smads but not in the cells which were not transfected with Smads.
In parental P19CL6 cells, expression of cardiac transcription factors such as Csx/Nkx-2.5, GATA-4, and MEF2C was detected from day 6 and expression of contractile protein genes such as MHC and MLC2v was observed on day 12 (Fig. 1 D, lane 5; Fig. 2 B, lanes 1–3). Expression of these genes was induced also in P19CL6noggin cells transfected simultaneously with Smad1 and Smad4, but not in the control P19CL6noggin cells or the cells transfected with Smad1 or Smad4 alone (Fig. 1 D, lanes 1–4). These results indicate that cooverexpression of Smad1 and Smad4 restored the ability of P19CL6noggin cells to differentiate into cardiomyocytes with concomitant expression of some set of cardiac-specific genes, suggesting that Smads may mediate the BMP-induced cardiomyocyte differentiation.
Figure 2.

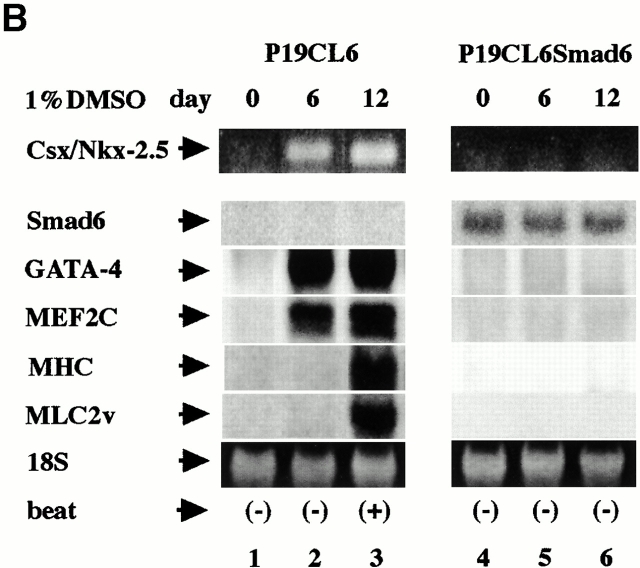
Inhibition of cardiomyocyte differentiation by overexpression of Smad6. (A) In the absence of DMSO, P19CL6Smad6 cells grew well and did not differentiate into any specific cells like parental P19CL6 cells (a and b). When 1% DMSO was added to the media, P19CL6Smad6 cells did not differentiate into MF20-positive beating cardiomyocytes in contrast to parental P19CL6 cells (c). The cells were stained with MF20 (a and c) or Hoechst dye (b). Bar, 50 μm. (B) Expression of cardiac-specific genes was detected in P19CL6 cells but not in P19CL6Smad6 cells. RNA was prepared from parental P19CL6 cells and P19CL6Smad6 cells on day 0 (before DMSO treatment) (lanes 1 and 4), day 6 (lanes 2 and 5), and day 12 (lanes 3 and 6). RT-PCR was performed to analyze Csx/Nkx-2.5 mRNA. 10 μg of RNA from each sample was subjected to Northern blot analysis for other genes. Ethidium bromide staining of rRNA is presented at the bottom to show that the same amount of intact RNA was loaded in each lane.
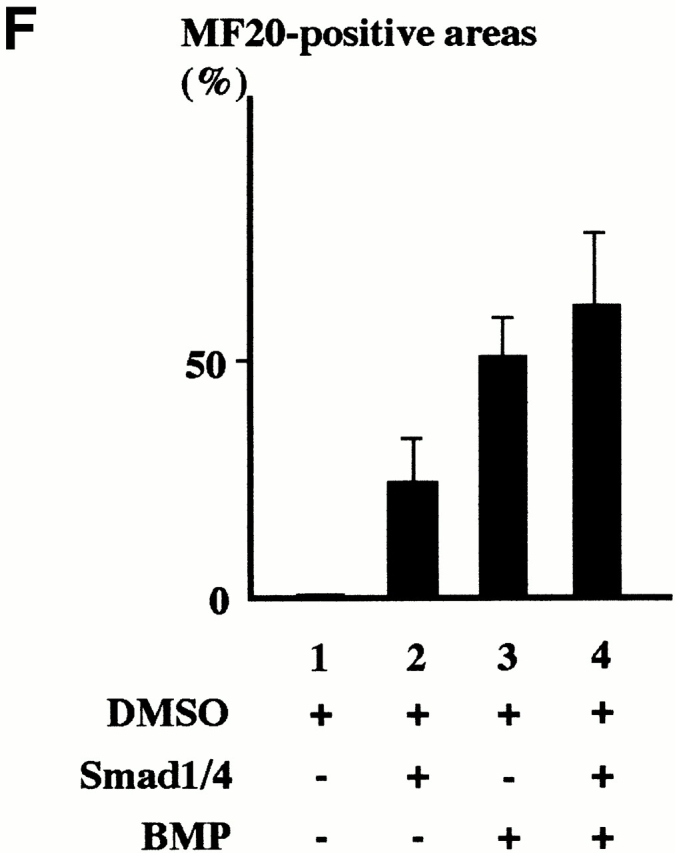
We further examined whether ligand stimulation enhances the differentiation efficiency of P19CL6noggin cells transfected with Smad1 and Smad4. The cells were cultured with or without addition of the BMP protein to the culture media at the concentration of 100 ng/ml from day 0. The cells were transfected with Smad1 and Smad4 on day 3 by the lipofection method. To estimate the differentiation efficiency, the percentage of areas positive for MF20 were measured on day 14. When the BMP protein was added to the media, some P19CL6noggin cells differentiated into cardiomyocytes as we observed previously (Monzen et al. 1999), implying that a sufficient amount of BMP protein restored the ability of P19CL6noggin cells to differentiate into cardiomyocytes. P19CL6noggin cells transfected with Smad1 and Smad4 more efficiently differentiated into cardiomyocytes stained by MF20 in the presence of the sufficient ligand stimulation compared with the transfected cells in the absence of BMP (Fig. 1 E). The percentage of positive areas was increased from ∼25 to 60% by the addition of BMP in the Smad1/4-transfected P19CL6noggin cells and from ∼49 to 60% by overexpression of Smads in the presence of BMP stimulation, although the latter increase was not significant (Fig. 1 F). These results suggest that excessive ligand stimulation overcomes the antagonistic effect of noggin and enhances the differentiation efficiency through the Smad pathway and further confirm the notion that Smads are involved in BMP-mediated cardiomyocyte differentiation.
Overexpression of Smad6, an Inhibitory Smad, Blocked Cardiomyocyte Differentiation of P19CL6 Cells
Smad6 has been identified and characterized as an inhibitory Smad, which forms stable association with BMP type I receptors and interferes with the phosphorylation of Smad1 (Imamura et al. 1997). Subsequent studies have revealed that Smad6 also competes with Smad4 for binding to Smad1 to block BMP signaling (Hata et al. 1998). To elucidate the requirement of the Smad pathway for cardiomyocyte differentiation, we isolated P19CL6 clones which permanently overexpress murine Smad6 under the control of human cytomegalovirus enhancer and designated them P19CL6Smad6. Northern blot analysis revealed that abundant expression of exogenous Smad6 mRNA was observed throughout differentiation in P19CL6Smad6 cells (Fig. 2 B, lanes 4–6). In contrast to parental P19CL6 cells, P19CL6Smad6 cells did not differentiate into MF20-positive beating cardiomyocytes by the treatment with DMSO (Fig. 2 A). The same results were obtained with at least four independent P19CL6Smad6 cell lines.
Furthermore, RT-PCR and Northern blot analyses revealed that, unlike parental P19CL6 cells, neither expression of cardiac transcription factors such as Csx/Nkx-2.5, GATA-4, and MEF2C nor that of contractile protein genes such as MHC and MLC2v was detected in P19CL6Smad6 cells during the course of the observation (Fig. 2 B). These results suggest that the Smad pathway is essential for expression of cardiac-specific genes and differentiation of P19CL6 cells into cardiomyocytes.
The Expression Levels and the Activity of ATF-2 Were Increased during the Course of Differentiation of P19CL6 Cells
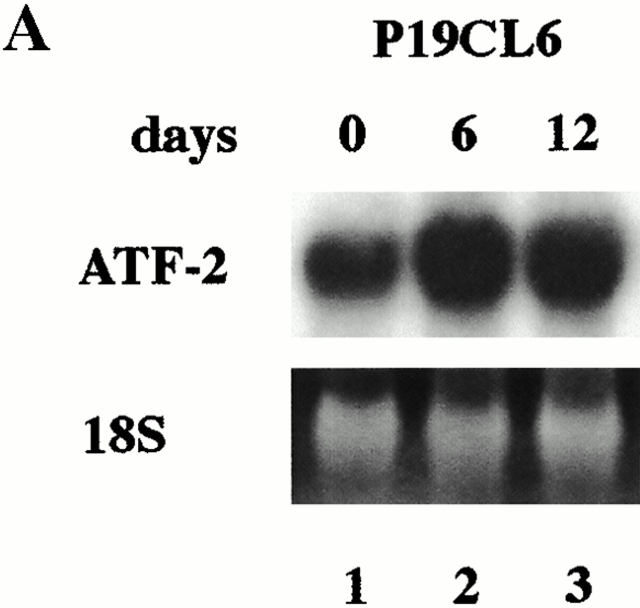
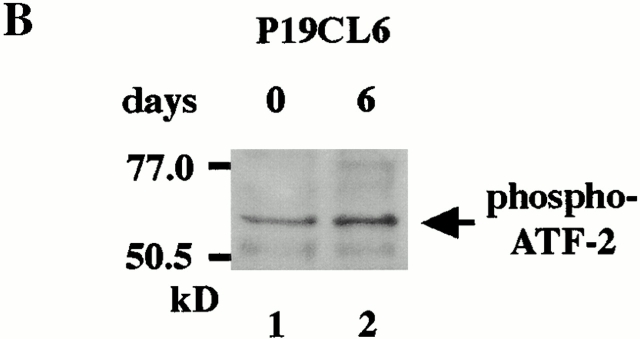
Although we have clarified that both the Smad pathway and the TAK1 pathway play an important role in BMP-induced cardiomyocyte differentiation in this study and the previous study (Monzen et al. 1999), the relation between both pathways has been largely unknown. Recently, however, the transcription factor ATF-2 has been reported to be a common nuclear target of the Smad and the TAK1 pathways and play a central role in TGF-β signaling (Sano et al. 1999). Therefore, we examined the role of ATF-2 in BMP-induced cardiomyocyte differentiation. First, we performed Northern blot analysis to examine the expression level of ATF-2 mRNA during cardiomyocyte differentiation of P19CL6. Expression of ATF-2 mRNA was detected even before the treatment with DMSO (on day 0), but became more abundant after DMSO treatment (Fig. 3 A). We further examined whether the ATF-2 protein was activated during differentiation of P19CL6. Western blot analysis using anti–phospho-ATF-2 antibody, which recognizes only activated ATF-2, revealed that ATF-2 was slightly activated even before the differentiation and its activity was enhanced on day 6 (Fig. 3 B). These results suggest that not only expression of ATF-2 mRNA but also the activity of the ATF-2 protein are upregulated during differentiation induced by DMSO treatment.
Figure 3.
The expression levels and the activity of ATF-2 were increased during differentiation of P19CL6 cells. (A) RNA was prepared from parental P19CL6 cells on day 0 (lane 1), day 6 (lane 2), and day 12 (lane 3). 10 μg of RNA from each sample was subjected to Northern blot analysis for ATF-2 mRNA. Ethidium bromide staining of rRNA is presented at the bottom to show that the same amount of intact RNA was loaded in each lane. (B) Whole cell extracts from P19CL6 cells on day 0 (lane 1) and day 6 (lane 2) of differentiation were prepared and analyzed by SDS-PAGE followed by Western blotting. The phosphorylation of ATF-2 was examined using anti-phospho-ATF-2 antibody which detects phosphorylation of Thr-71 specifically.
ATF-2 Was Involved in Transactivation of the βMHC Promoter with Synergistic Enhancement by Smads and TAK1
To examine the role of ATF-2 in the regulation of cardiac-specific gene promoters, cotransfection experiments were performed using reporter plasmids containing luciferase gene driven by the βMHC promoter in the presence or the absence of BMP stimulation. Expression vectors encoding Smad1/4, the constitutively active form of TAK1 (caTAK1), and ATF-2 were used as effectors. P19CL6 cells cultured with 1% DMSO were transfected with effector and reporter plasmids on day 5 by the calcium phosphate method. The cell lysates were extracted on day 7 (48 h after transfection) and the luciferase activities were analyzed. The BMP treatment was performed at a final concentration of 100 ng/ml for 24 h before the lysate preparation. The βMHC promoter was slightly activated by BMP treatment (1.8-fold; Fig. 4 A, column 1). Smad1/4 and caTAK1 mildly stimulated this promoter activity, and cooverexpression of Smad1/4 and caTAK1 synergistically enhanced the activity by 3.9-fold in the presence of BMP treatment (Fig. 4 A, columns 2–4). ATF-2 stimulated this promoter activity by 2.1-fold in the absence of BMP treatment and by 2.5-fold in the presence of BMP treatment (Fig. 4 A, column 5). The degree of activation of this promoter by ATF-2 was slightly enhanced by cooverexpression of Smad1/4 or caTAK1 (Fig. 4 A, columns 6 and 7). Furthermore, this promoter activity was strongly enhanced by cooverexpression of all three effectors together, resulting in a 3.1- and 6.4-fold stimulation in the absence and presence of BMP treatment, respectively (Fig. 4 A, column 8). These results suggest that cooverexpression of ATF-2, Smad1/4, and caTAK1 synergistically activates the βMHC promoter during the course of differentiation in P19CL6 cells.
Figure 4.
ATF-2 stimulated the promoter activity of βMHC gene by the synergistic manner with Smad1/4 and TAK1. P19CL6 cells were cultured in the presence of 1% DMSO and were transfected on day 5 by the calcium phosphate method with reporter plasmids containing luciferase gene driven by βMHC promoter and expression plasmids carrying ATF-2, Smad1, Smad4, and caTAK1 (A) and ATF-2, Smad6, and dnTAK1 (B) as effector plasmids. The cell lysates were extracted on day 7 and the luciferase activities were analyzed. The degree of activation is indicated as means ± SD. Dotted columns, BMP treatment (−). Closed columns, BMP treatment (+).
To confirm that ATF-2, Smads, and TAK1 cooperatively activate the βMHC promoter, we next examined whether Smad6 (an inhibitory Smad) or the dominant negative form of TAK1 (dnTAK1) inhibits transactivation of this promoter by ATF-2. Overexpression of Smad6 or dnTAK1 partially inhibited the ATF-2–induced promoter activation both in the presence or in the absence of BMP treatment (Fig. 4 B). These results suggest that the blockade either of the Smad or the TAK1 pathway can inhibit the stimulatory effect of ATF-2 on the βMHC promoter and that ATF-2 induces the transactivation of this promoter through BMP signaling.
Overexpression of the Dominant Negative Form of ATF-2 Inhibited Cardiomyocyte Differentiation of P19CL6
To further clarify the role of ATF-2 in cardiomyocyte differentiation, we transfected expression plasmids containing two ATF-2 mutants, ATF-2Ala and ATF-2Δ107, into parental P19CL6 cells. ATF-2Ala is the mutant in which the three SAPK phosphorylation sites (Thr-69, Thr-71, and Ser-90) are replaced by alanine, and ATF-2Δ107 is the truncated mutant which lacks the NH2-terminal 107 amino acids including all three SAPK phosphorylation sites (Sano et al. 1999). These two mutants cannot be phosphorylated by TGF-β signaling via TAK1 and p38 and are expected to act as dominant negative forms. The cells cultured with 1% DMSO were transfected on day 3 by the lipofection method, and then the differentiation ability was assessed at day 14. P19CL6 cells transfected with either of the two ATF-2 mutants less efficiently differentiated into MF20-positive beating cardiomyocytes than the control P19CL6 cells (Fig. 5 A). The percentage of positive areas stained by MF20 was estimated to be ∼50% lower in the cells transfected with the mutants than in the control P19CL6 cells (Fig. 5 B).
Figure 5.

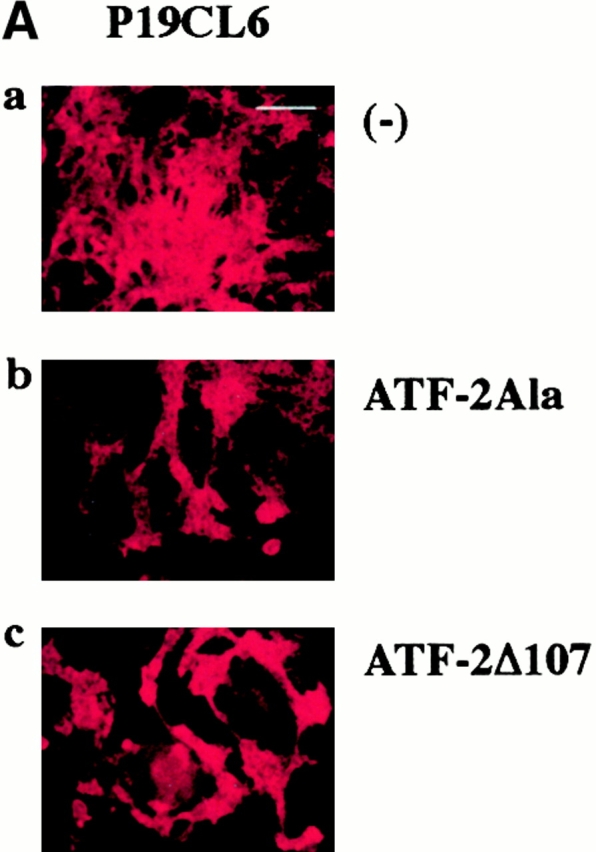
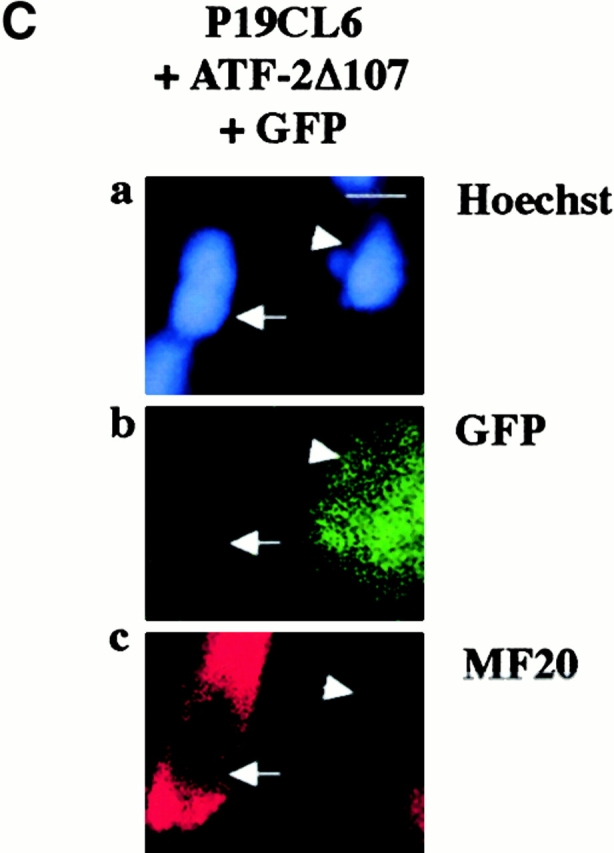
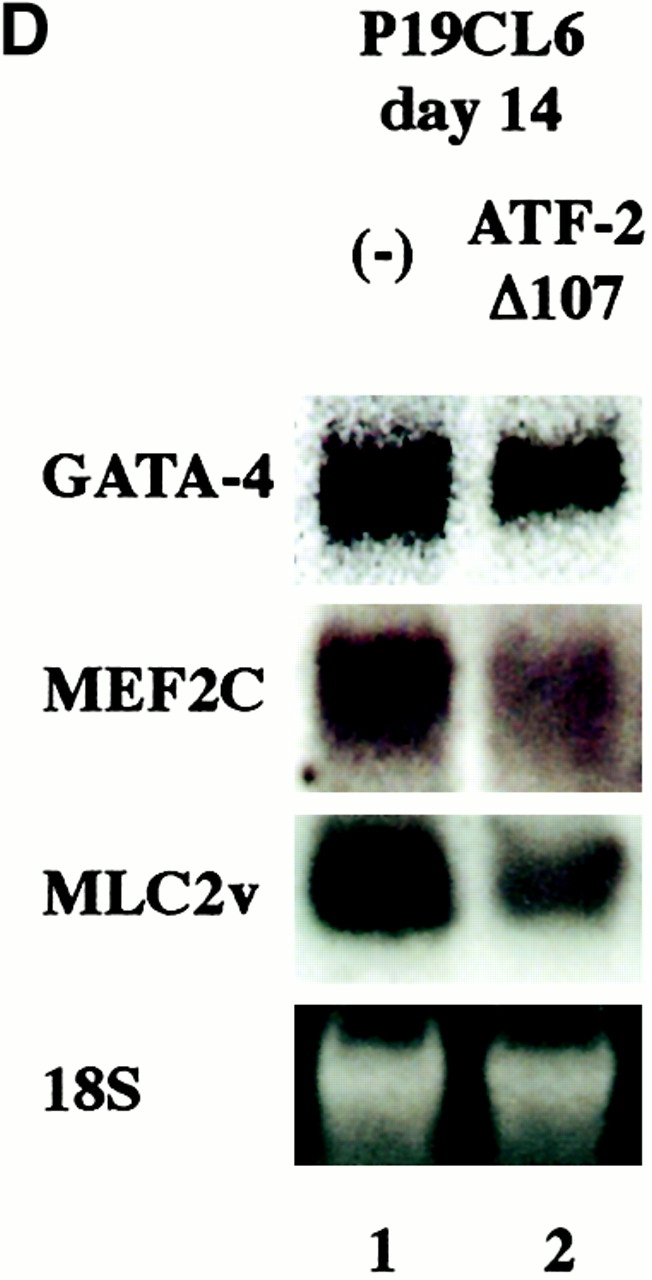
The dominant negative forms of ATF-2 inhibited expression of cardiac-specific genes and terminal cardiomyocyte differentiation. (A) P19CL6 cells were cultured with 1% DMSO and transfected with either of two ATF-2 mutants, ATF-2Ala (b) and ATF-2Δ107 (c), on day 3 by the lipofection method. The cells were then immunostained with MF20 on day 14. Bar, 250 μm. (B) Quantification of the areas stained by MF20 in P19CL6 cells. The percentage of areas positive for MF20 in the P19CL6 cells transfected with the ATF-2 mutants (lanes 2 and 3) was ∼50% lower than the control P19CL6 cells (lane 1). The areas of at least three fields were measured for each cell line under the same conditions. The results are expressed as means (%) ± SD. (C) The correlation between overexpression of the ATF-2 mutant and the P19CL6 differentiation. P19CL6 cells were transfected with expression plasmids encoding ATF-2Δ107 along with GFP expression plasmids on day 3 and then immunostained by MF20 on day 12. (a) Hoechst dye; (b) GFP; (c) MF20. Arrows, a GFP-negative cell; arrowheads, a GFP-positive cell. Bar, 20 μm. (D) Expression of cardiac-specific genes in the control P19CL6 cells (lane 1) and P19CL6 cells transfected with ATF-2Δ107 (lane 2). RNA was prepared on day 14 and 10 μg of RNA from each sample was subjected to Northern blot analysis. Ethidium bromide staining of rRNA is presented at the bottom to show that the same amount of intact RNA was loaded in each lane. (E) P19CL6 cells were cultured with 1% DMSO and transfected on day 5 by the calcium phosphate method with effector plasmids carrying ATF-2Δ107 and reporter plasmids containing promoters of several cardiac genes such as βMHC, ANP, BNP, and skeletal α-actin. The cell lysates were extracted on day 7 and the luciferase activities were analyzed. The degree of activation is indicated as means ± SD.
To clarify the correlation between expression of the ATF-2 mutants and the phenotype of the P19CL6 differentiation at the cellular level, we examined whether cardiomyocyte differentiation was inhibited in the cells overexpressing the ATF-2 mutants. P19CL6noggin cells were transfected simultaneously with expression vectors encoding ATF-2Δ107 and those containing GFP on day 3, and then were stained by MF20 on day 12. The MF20-positive signals were detected in GFP-negative cells (Fig. 5 C, arrows) but not in GFP-positive cells (Fig. 5 C, arrowheads), suggesting that the myocyte differentiation was inhibited in the cells overexpressing the dominant negative form of ATF-2 but not in the cells which were not transfected with the ATF-2 mutant.
Northern blot analysis revealed that the expression level of cardiac genes such as GATA-4, MEF2C, and MLC2v was reduced in the cells transfected with ATF-2Δ107 (Fig. 5 D). To confirm the inhibitory effects of these dominant negative mutants on cardiomyocyte differentiation, we further examined the promoter activities of cardiac genes. P19CL6 cells were cultured with 1% DMSO and transfected on day 5 by the calcium phosphate method with effector plasmids carrying ATF-2Δ107 and reporter plasmids containing promoters of several cardiac genes such as βMHC, ANP, BNP, and skeletal α-actin. The cell lysates were extracted on day 7 and the luciferase activities were analyzed. The ATF-2 mutant significantly inhibited the βMHC, ANP, and BNP promoter activities whereas the promoter activity of skeletal α-actin was not affected (Fig. 5 E). All these results suggest that overexpression of the dominant negative form of ATF-2 inhibits expression of at least some set of cardiac-specific genes and terminal cardiomyocyte differentiation in this P19CL6 system.
Overexpression of ATF-2 Enhanced the BMP-induced Cardiomyocyte Differentiation of P19CL6noggin
To examine whether ATF-2 promotes cardiomyocyte differentiation of P19CL6, we transfected expression plasmids encoding wild-type ATF-2 along with those carrying Smads and TAK1 mutants into P19CL6noggin cells. The cells were transfected with the expression vectors on day 3 by the lipofection method and then were immunostained by MF20 to evaluate the differentiation efficiency. Overexpression of dnTAK1 partially inhibited Smad1/4-induced cardiomyocyte differentiation of P19CL6noggin whereas Smad6 blocked caTAK1-induced differentiation (Fig. 6, columns 2–5), suggesting that Smads and TAK1 act in parallel, at least partially, against their downstream targets. Cooverexpression of Smad1/4 and caTAK1 enhanced the differentiation efficiency (Fig. 6, column 6). Overexpression of wild-type ATF-2 alone restored the ability of P19CL6noggin to differentiate into cardiomyocytes (Fig. 6, column 7), and the differentiation efficiency was enhanced by simultaneous overexpression along with Smad1/4 or caTAK1 (Fig. 6, columns 8 and 9). Furthermore, differentiation of P19CL6noggin into cardiomyocytes was most strongly induced by overexpression of all inducers together (Fig. 6, column 10). These results suggest that ATF-2, Smad1/4, and caTAK1 cooperatively induce differentiation of P19CL6noggin into cardiomyocytes.
Figure 6.

Overexpression of ATF-2, Smad1/4, and caTAK1 cooperatively rescued the ability of P19CL6noggin to differentiate into cardiomyocytes. P19CL6 cells were cultured in the presence of 1% DMSO and were transfected on day 3 by the lipofection method with expression plasmids carrying ATF-2, Smads, and the TAK1 mutants. The cells were then immunostained with MF20 on day 14. The percentage of areas positive for MF20 in the P19CL6 cells transfected with respective plasmids was measured at least three fields under the same conditions. The results are expressed as means (%) ± SD.
Discussion
In this study, we obtained the following results: (a) The Smad pathway is necessary for BMP-induced cardiomyocyte differentiation in P19CL6; (b) ATF-2, a common nuclear target of the Smad and the TAK1 pathways, is involved in transcriptional activation of some cardiac-specific genes and terminal cardiomyocyte differentiation in P19CL6; and (c) Smads, TAK1, and ATF-2 cooperatively induce differentiation of P19CL6 cells into cardiomyocytes.
Smads Are Required for BMP-induced Cardiomyocyte Differentiation
We previously reported that BMPs induce cardiomyocyte differentiation through TAK1 (Monzen et al. 1999). In the study, we established a permanent P19CL6 cell line, P19CL6noggin, which constitutively overexpresses the BMP antagonist noggin. The secreted protein noggin was first identified as a dorsalizing factor localized in the Spemann organizer in Xenopus embryos (Smith and Harland 1992). Subsequent studies have demonstrated that noggin binds specifically to BMP-2 and BMP-4 with high affinity and also to BMP-7 with lower affinity, thereby abolishing the activity of BMPs by blocking the binding of BMPs to cognate cell surface receptors (Zimmerman et al. 1996). Although almost all parental P19CL6 cells differentiated into beating cardiomyocytes when treated with 1% DMSO, P19CL6noggin cells did not differentiate into beating cardiomyocytes nor express cardiac transcription factors or contractile protein genes. The failure of differentiation was rescued by overexpression of BMP-2 using adenovirus-mediated gene delivery or addition of BMP protein to the culture media, indicating that BMPs are indispensable for cardiomyocyte differentiation in this system. Furthermore, overexpression of TAK1 restored the ability of P19CL6noggin cells to differentiate into cardiomyocytes and concomitantly express some cardiac genes, whereas overexpression of the dominant negative form of TAK1 in parental P19CL6 cells inhibited cardiomyocyte differentiation. These results suggest that TAK1 plays a pivotal role in the cardiogenic BMP signaling pathway (Monzen et al., 1999).
In this study, coexpression of Smad1 and Smad4 also restored the ability of P19CL6noggin cells to differentiate into cardiomyocytes with concomitant expression of cardiac transcription factors such as Csx/Nkx-2.5, GATA-4, and MEF2C, and contractile protein genes such as MHC and MLC2v. These results suggest that Smads also mediate BMP-induced transactivation of cardiac-specific genes and induction of terminal differentiation of P19CL6 cells into cardiomyocytes. Expression of endogenous Smad1 and Smad4 was not detected in the differentiating P19CL6 cells by Northern blot analysis (data not shown), implying that expression levels of these Smads were relatively low in this cell line. The correlation between overexpression of Smads and the P19CL6 differentiation at the cellular level was confirmed by the cotransfection experiments using Smad1/4 and GFP expression plasmids followed by immunostaining with MF20, which demonstrated that only GFP-positive cells (i.e., Smads-overexpressing cells) were stained positively by MF20. The reasons why overexpression of Smad1 alone could not restore the differentiation ability remains unknown. One possibility is that only overexpression of one component of heterooligomers are not sufficient for effective heterooligomer formation and subsequent nuclear translocation in the absence of ligand stimulation. In fact, immunofluorescence experiments revealed that the majority of exogenous Smad1 was localized in the cytoplasm in P19CL6noggin cells, suggesting that only a small part of the Smad proteins can translocate into the nucleus in the absence of BMP function. Excessive amounts of both ligand-specific and common-partner Smads may be necessary for effective hetero-oligomer formation.
Overexpression of Smad6, an inhibitory Smad which has been reported to block the TGF-β superfamily signal transduction, inhibited expression of some set of cardiac-specific genes and terminal differentiation of P19CL6 cells into cardiomyoctes. These results suggest that the Smad pathway is indispensable for normal cardiomyocyte differentiation. To date, several studies have shown the importance of the Smad pathway in normal cardiac development in vivo. Gene targeting experiments in mice have revealed that Smad5 knockout mice exhibit morphological defects in the developing amnion, gut, and heart which are similar to those of BMP-2 knockout mice (Chang et al. 1999). Smad6-deficient mice also have multiple cardiovascular abnormalities such as hyperplasia of the cardiac valves and outflow tract septation defects, indicating a function for Smad6 in the regulation of endocardial cushion transformation (Galvin et al. 2000). The latter study suggests that excess activation of the Smad pathway also causes abnormal heart development. It has been reported recently that expression of Smad6 itself is regulated by BMP-activated Smad1/5 (Ishida et al. 2000). These observations and our results imply that temporal and spacial precise regulation of the Smad activities may be important for normal cardiac development from initial cardiomyocyte differentiation to terminal cardiac morphogenesis.
ATF-2 Induces Transcriptional Activation of Cardiac Genes through the Smad and the TAK1 Pathways during Cardiomyocyte Differentiation
In the previous paper (Monzen et al. 1999) and this paper, we have provided several lines of evidence suggesting that both the Smad and the TAK1 pathways play a crucial role in cardiomyocyte differentiation. We next focused on the molecular mechanisms of how both pathways cooperatively regulate expression of cardiac genes and induce terminal cardiomyocyte differentiation. In Xenopus embryos, overexpression of kinase-negative TAK1 has been reported to inhibit the Smad1-induced ventralization (Shibuya et al. 1998), suggesting that the regulation by BMPs requires cooperative actions of Smads and TAK1. It is also noteworthy that MEKK-1, a component of the SAPK pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells (Brown et al. 1999). TAK1, which also stimulates the SAPK/JNK pathway similar to MEKK-1, may activate the Smad pathway similarly. However, little had been known about the molecules that connect the Smad and the TAK1 pathways, until the transcription factor ATF-2 was proposed to be a common nuclear target of both pathways. ATF-2 has been demonstrated to bind directly to heterooligomers of Smads and be phosphorylated by TGF-β signaling via TAK1 and p38 (Hanafusa et al. 1999; Sano et al. 1999). Both pathways have been shown to synergistically stimulate ATF-2 transactivating capacity (Sano et al. 1999). From this viewpoint, we examined the involvement of ATF-2 in BMP-induced cardiomyocyte differentiation.
ATF-2 has been shown to be ubiquitously expressed in various human embryonic tissues and cell lines, with the highest expression level being observed in brain (Maekawa et al. 1989; Takeda et al. 1991). Northern blot analysis revealed that ATF-2 mRNA was abundantly expressed during the course of differentiation in P19CL6 cells and that the expression level was higher on day 6 and day 12 than on day 0 (i.e., before the initiation of DMSO treatment). Furthermore, Western blot analysis demonstrated that the phosphorylation of the ATF-2 protein was detected during differentiation of P19CL6. These results suggest that ATF-2 is one of the DMSO-inducible factors (see below) and that it may play an important role in the induction of differentiation of P19CL6.
The results of cotransfection experiments followed by reporter gene assays demonstrated that overexpression of ATF-2 alone stimulated the promoter activity of βMHC gene and that cooverexpression of ATF-2, Smad1/4, and caTAK1 synergistically activated this promoter. Furthermore, overexpression of Smad6, an inhibitory Smad, and the dominant negative form of TAK1 partially inhibited transactivation of βMHC gene by ATF-2, suggesting that ATF-2-induced transactivation of βMHC gene depends on both the Smad and the TAK1 pathways. These results are consistent with the previous reports showing synergistic enhancement of ATF-2 activity by Smads and TAK1 using the CRE-containing promoters (Sano et al. 1999), although the βMHC promoter we used does not contain the CRE site (Strehler et al. 1985). It is possible that ATF-2 may indirectly stimulate this promoter via transactivation of some other genes such as cardiac-specific transcription factors. Further studies are necessary to elucidate the molecular cascade from ATF-2 to related genes needed for the full differentiation of cardiac precursor cells into cardiomyocytes.
ATF-2 Plays a Pivotal Role in Cardiomyocyte Differentiation of P19CL6
To test the importance of ATF-2 for the actual differentiation of P19CL6, we transfected ATF-2 mutants into P19CL6 and examined the differentiation efficiency, expression of cardiac-specific genes, and activation of promoters. Our results demonstrated that overexpression of the dominant negative form of ATF-2 (dnATF-2) partially inhibited (a) promoter activities of some cardiac genes such as βMHC, ANP, and BNP, (b) mRNA expression of GATA-4, MEF2C, and MLC2v, and (c) terminal cardiomyocyte differentiation of P19CL6 cells, indicating that ATF-2 plays a crucial role in differentiation into cardiomyocytes. The reduction of promoter activities and the differentiation efficiency by overexpression of dnATF-2 was estimated to be ∼50%. This result was thought to be compatible with the transfection efficiency by the lipofection method, which was estimated to range from ∼40 to 60% by counting GFP-positive cells 1 d after the transfection of GFP expression plasmids (data not shown). All these results strongly suggest that ATF-2 plays a pivotal role in transactivation of some cardiac-specific genes and terminal cardiomyocyte differentiation.
We further established the permanent P19CL6 cell line which constitutively overexpresses one of the dnATF-2 mutants, ATF-2Δ107 (P19CL6ATF-2Δ107). Unexpectedly, a part of P19CL6ATF-2Δ107 cells differentiated into beating cardiomyocytes by the treatment with DMSO, although the differentiation efficiency was reduced compared with parental P19CL6 (data not shown). This is probably because endogenous ATF-2 was so abundantly expressed in P19CL6 cells that stable expression of dnATF-2 could not completely block its activity. In fact, Northern blot analysis revealed that the expression level of dnATF-2 was almost the same as that of endogenous ATF-2 on day 6 in P19CL6ATF-2Δ107 cells (data not shown). On the other hand, transient transfection provides much higher expression of exogenous genes, so that it is probable that dnATF-2 could completely inhibit the effects of endogenous ATF-2 in the P19CL6 cells when transiently transfected. The transient transfection experiments clearly suggest that ATF-2 plays a critical role in cardiomyocyte differentiation.
In the in vivo situation, ATF-2 knockout mice generated by gene targeting exhibit lowered postnatal viability and growth, in addition to a defect in endochondrial ossification and a reduced number of cerebellar Purkinje cells (Reimold et al. 1996). Another group subsequently reported that mouse ATF-2 null mutants display features of a severe type of meconium aspiration syndrome (Maekawa et al. 1999). Neither embryonic lethality nor abnormal cardiac phenotype has been reported in these knockout mice, which is inconsistent with our in vitro results. This is possibly because genetic redundancies among the ATF/CREB family members, all of which bind to the CRE site, may rescue the phenotypes of these knockout mice. The understanding of the precise molecular requirement of the ATF/CREB family members for differentiation of cardiac precursor cells in vivo awaits further investigation.
Cotransfection experiments into P19CL6noggin cells using wild-type ATF-2 along with Smad1/4 and caTAK1 revealed that overexpression of wild-type ATF-2 alone restored the ability of P19CL6noggin to differentiate into cardiomyocytes, although the efficiency was relatively low probably because of the lack of BMP stimulation, and that the differentiation efficiency was most strongly enhanced when Smad1/4 and caTAK1 were also overexpressed. These results indicate that cooverexpression of ATF-2, Smad1/4, and caTAK1 cooperatively promotes the ability of P19CL6noggin to differentiate into cardiomyocytes and suggest that ATF-2 is involved in differentiation of P19CL6 into cardiomyocytes downstream of the Smad and the TAK1 pathways.
Both the binding of Smads with ATF-2 and phosphorylation of ATF-2 by TAK1 and p38 are thought to contribute to the enhancement of the DNA binding affinity of ATF-2 and subsequent transactivational responses. The heterooligomer of Smad3/4 has been reported to bind directly to ATF-2 through the MH1 region of Smad3/4 and the b-ZIP region of ATF-2 and enhance the transactivating capacity of ATF-2 (Sano et al. 1999). In this sense, Smads resemble adenovirus E1A, which stimulates CRE-dependent transcription via binding to the b-ZIP region of ATF-2 (Liu and Green 1994). Expression of E1A has been shown to induce differentiation of several other cell lines such as F9 cells (Montano and Lane 1987; Velcich and Ziff 1989). We have preliminary data demonstrating that overexpression of E1A strongly restored the ability of P19CL6noggin cells to differentiate into cardiomyocytes, suggesting that E1A, instead of Smads, can induce cardiomyocyte differentiation through the activation of ATF-2 (Monzen and Komuro, unpublished data). Meanwhile, it has been reported that CREB-binding protein (CBP), which was originally identified as a coactivator of CREB, directly binds to the b-ZIP region of ATF-2 and potentiates transactivation of ATF-2 (Sano et al. 1998). However, E1A also has been demonstrated to suppress the CREB-mediated transcription by competing with p300/CBP-associated factor (P/CAF) for binding to p300/CBP (Yang et al. 1996). Moreover, it has been reported recently that ATF-2 has intrinsic histone acetyltransferase (HAT) activity as well as p300/CBP and P/CAF and that phosphorylation of ATF-2 controls its intrinsic HAT activity and its action on CRE-dependent transcription (Kawasaki et al. 2000). Further studies are necessary to dissect the molecular mechanisms of how these transcriptional cofactors work during differentiation into cardiomyocytes.
General Consideration
A speculative diagram of the regulatory cascade controlling differentiation of P19CL6 cells into cardiomyocytes is shown in Fig. 7 as a summary of our study. BMPs induce expression of some cardiac-specific genes and terminal cardiomyocyte differentiation through the Smad and the TAK1 pathways. Both pathways cooperatively activate the transcription factor ATF-2, resulting in the transactivation of some set of genes related to terminal cardiomyocyte differentiation. As we showed in the previous report (Monzen et al. 1999), some other DMSO-inducible factors independent of BMP signaling are necessary for the terminal differentiation (indicated as “X” and “Y” on Fig. 7), because only activation of BMP signaling is not sufficient to induce differentiation in the absence of DMSO. Thus, BMP signal transduction pathways and its downstream transcription factors are the central molecules of this regulatory network controlling cardiac differentiation as well as unknown DMSO-inducible factors. The identification of cardiac genes regulated by ATF-2 and signals induced by DMSO in this system will provide new insights into the understanding of the precise molecular mechanisms by which cardiomyocyte differentiation is regulated.
Figure 7.
Schematic representation of the regulatory cascade controlling differentiation of P19CL6 cells into cardiomyocytes.
Acknowledgments
We thank Dr. H. Shibuya, Dr. D.B. Wilson, and Dr. E.N. Olson for providing plasmids, and Ms. C. Masuo and Ms. K. Abe for their excellent technical assistance.
This study was supported by a Grant-in-Aid for Scientific Research and Developmental Science Research from the Ministry of Education, Science and Culture of Japan and the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan (to I. Komuro).
Footnotes
Abbreviations used in this paper: ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; BMP, bone morphogenetic protein; CBP, CREB-binding protein; CRE, cAMP response element; GFP, green fluorescent protein; RT, reverse transcription; SAPK, stress-activated protein kinase.
References
- Arceri R.J., King A.A.J., Simon M.C., Orkin S.H., Wilson D.B. Mouse GATA-4a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol. Cell. Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano L., Wrana J.L. Mads and Smads in TGF beta signalling. Curr. Opin. Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D.A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.J., DiChiara M.R., Anderson K.R., Gimbrone M.A., Jr., Topper J.N. MEKK-1, a component of the stress (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells. J. Biol. Chem. 1999;274:8797–8805. doi: 10.1074/jbc.274.13.8797. [DOI] [PubMed] [Google Scholar]
- Busch S.J., Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990;6:36–40. doi: 10.1016/0168-9525(90)90071-d. [DOI] [PubMed] [Google Scholar]
- Chang H., Huylebroeck D., Verschueren K., Guo Q., Matzuk M.M., Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–467. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- Galvin K.M., Donovan M.J., Lynch D.A., Meyer R.I., Paul R.J., Lorenz J.N., Fairchild-Huntress V., Dixon K.L., Dunmore J.H., Gimbrone M.A., Jr. A role for Smad6 in development and homeostasis of the cardiovascular system. Nat. Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Gupta S., Campbell D., Dérijard B., Davis R.J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Habara-Ohkubo A. Differentiation of beating cardiac muscle cells from a derivative of P19 embryonal carcinoma cells. Cell Struct. Funct. 1996;21:101–110. doi: 10.1247/csf.21.101. [DOI] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Dennis J.E., Cohen-Gould L., Bader D.M., Fischman D.A. Expression of sarcomeric myosin in the presumptive myocardium of chicken embryos occurs within six hours of myocyte commitment. Dev. Dyn. 1992;193:257–265. doi: 10.1002/aja.1001930306. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Ninomiya-Tsuji J., Masuyama N., Nishita M., Fujisawa J., Shibuya H., Matsumoto K., Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-β-induced gene expression. J. Biol. Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Hata A., Lagna G., Massagué J., Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C., Miyazono K., ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Imamura T., Takase M., Nishihara A., Oeda E., Hanai J., Kawabata M., Miyazono K. Smad6 inhibits signaling by the TGF-β superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Ishida W., Hamamoto T., Kusanagi K., Yagi K., Kawabata M., Takehara K., Sampath T.K., Kato M., Miyazono K. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J. Biol. Chem. 2000;275:6075–6079. doi: 10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Schiltz L., Chiu R., Itakura K., Taira K., Nakatani Y., Yokoyama K.K. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature. 2000;405:195–200. doi: 10.1038/35012097. [DOI] [PubMed] [Google Scholar]
- Lagna G., Hata A., Hemmati-Brivanlou A., Massague J. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- Lin Q., Schwarz J., Bucana C., Olson E.N. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Green R. Promoter targeting by adenovirus E1a through interaction with different cellular DNA-binding domains. Nature. 1994;368:520–525. doi: 10.1038/368520a0. [DOI] [PubMed] [Google Scholar]
- Lyons I., Parsons L.M., Hartley L., Li R., Andrews J.E., Robb L., Harvey R.P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- Macgregor P.F., Abate C., Curran T. Direct cloning of leucine zipper proteinsJun binds cooperatively to the CRE with CRE-BP1. Oncogene. 1990;5:451–458. [PubMed] [Google Scholar]
- Macias-Silva M., Abdollah S., Hoodless P.A., Pirone R., Attisano L., Wrana J.L. MADR2 is a substrate of the TGFbeta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Sakura H., Kanei-Ishii C., Sudo T., Yoshimura T., Fujisawa J., Yoshida M., Ishii S. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:2023–2028. doi: 10.1002/j.1460-2075.1989.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T., Bernier F., Sato M., Nomura S., Singh M., Inoue Y., Tokunaga T., Imai H., Yokoyama M., Reimold A. Mouse ATF-2 null mutants display features of a severe type of meconium aspiration syndrome. J. Biol. Chem. 1999;274:17813–17819. doi: 10.1074/jbc.274.25.17813. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Maekawa T., Ishii S. Identification of the functional domains of the transcriptional regulator CRE-BP1. J. Biol. Chem. 1991;266:18188–18193. [PubMed] [Google Scholar]
- Montano X., Lane D. The adenovirus E1a gene induces differentiation of F9 teratocarcinoma cells. Mol. Cell. Biol. 1987;7:1782–1790. doi: 10.1128/mcb.7.5.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzen K., Shiojima I., Hiroi Y., Kudoh S., Oka T., Takimoto E., Hayashi D., Hosoda T., Habara-Ohkubo A., Nakaoka T. Bone morphogenetic protein induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol. Cell. Biol. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascone N., Mercola M. An inductive role for the endoderm in Xenopus cardiogenesis. Development. 1995;121:515–523. doi: 10.1242/dev.121.2.515. [DOI] [PubMed] [Google Scholar]
- Olson E.N., Srivastava D. Molecular pathway controlling heart development. Science. 1996;272:671–676. doi: 10.1126/science.272.5262.671. [DOI] [PubMed] [Google Scholar]
- Reimold A.M., Grusby M.J., Kosaras B., Fries J.W., Mori R., Maniwa S., Clauss I.M., Collins T., Sidman R.L., Glimcher M.J., Glimcher L.H. Chondrodysplasia and neurological abnormalities in ATF-2-deficient mice. Nature. 1996;379:262–265. doi: 10.1038/379262a0. [DOI] [PubMed] [Google Scholar]
- Rosenquist G.C., Dehaan R.L. Migration of precardiac cells in the chick embryoa radioautographic study. Carnegie Inst. Wash. Contrib. Embryol. 1966;38:111–121. [Google Scholar]
- Sano Y., Tokitou F., Dai P., Maekawa T., Yamamoto T., Ishii S. CBP alleviates the intramolecular inhibition of ATF-2 function. J. Biol. Chem. 1998;273:29098–29105. doi: 10.1074/jbc.273.44.29098. [DOI] [PubMed] [Google Scholar]
- Sano Y., Harada J., Tashiro S., Gotoh-Mandeville R., Maekawa T., Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J. Biol. Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- Schultheiss T.M., Xydas S., Lassar A.B. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- Schultheiss T.M., Burch J.B.E., Lassar A.B. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Iwata H., Masuyama N., Gotoh Y., Yamaguchi K., Irie K., Matsumoto K., Nishida E., Ueno N. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1019–1028. doi: 10.1093/emboj/17.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojima I., Komuro I., Oka T., Hiroi Y., Mizuno T., Takimoto E., Monzen K., Aikawa R., Akazawa H., Yamazaki T. Context-dependent transcriptional cooperation mediated by cardiac transcription factors Csx/Nkx-2.5 and GATA-4. J. Biol. Chem. 1999;274:8231–8239. doi: 10.1074/jbc.274.12.8231. [DOI] [PubMed] [Google Scholar]
- Smith W.C., Harland R.M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Strehler E.E., Mahdavi V., Periasamy M., Nadal-Ginard B. Intron positions are conserved in the 5′ end region of myosin heavy-chain genes. J. Biol. Chem. 1985;260:468–471. [PubMed] [Google Scholar]
- Takano H., Komuro I., Oka T., Shiojima I., Hiroi Y., Mizuno T., Yazaki Y. The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell. Biol. 1998;18:1580–1589. doi: 10.1128/mcb.18.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda J., Maekawa T., Sudo T., Seino Y., Imura H., Saito N., Tanaka C., Ishii S. Expression of the CRE-BP1 transcriptional regulator binding to the cyclic AMP response element in central nervous system, regenerating liver, and human tumors. Oncogene. 1991;6:1009–1014. [PubMed] [Google Scholar]
- Velcich A., Ziff E.B. The adenovirus-5 12S E1a protein, but not the 13S induces expression of endoA differentiation marker in F9 cells. Oncogene. 1989;4:707–713. [PubMed] [Google Scholar]
- Winnier G., Blessing M., Labosky P.A., Hogan B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Xu X., Yin Z., Hudson J.B., Ferguson E.L., Frasch M. Smad proteins act in combination with synergistic and antagonistic regulators to target Dpp responses to the Drosophila mesoderm. Genes Dev. 1998;12:2354–2370. doi: 10.1101/gad.12.15.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Shirakabe K., Shibuya H., Irie K., Oishi I., Ueno N., Taniguchi T., Nishida E., Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yang X., Ogryzko V.V., Nishikawa J., Howard B.H., Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zhu W., Zou Y., Aikawa R., Harada K., Kudoh S., Uozumi H., Hayashi D., Gu Y., Yamazaki T., Nagai R., Yazaki Y., Komuro I. MAPK superfamily plays an important role in daunomycin-induced apoptosis of cardiac myocytes. Circulation. 1999;100:2100–2107. doi: 10.1161/01.cir.100.20.2100. [DOI] [PubMed] [Google Scholar]
- Zimmerman L.B., Jesus-Escobar J.D., Harland R.M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein-4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]