Abstract
We have shown previously that epimorphin (EPM), a protein expressed on the surface of myoepithelial and fibroblast cells of the mammary gland, acts as a multifunctional morphogen of mammary epithelial cells. Here, we present the molecular mechanism by which EPM mediates luminal morphogenesis. Treatment of cells with EPM to induce lumen formation greatly increases the overall expression of transcription factor CCAAT/enhancer binding protein (C/EBP)β and alters the relative expression of its two principal isoforms, LIP and LAP. These alterations were shown to be essential for the morphogenetic activities, since constitutive expression of LIP was sufficient to produce lumen formation, whereas constitutive expression of LAP blocked EPM-mediated luminal morphogenesis. Furthermore, in a transgenic mouse model in which EPM expression was expressed in an apolar fashion on the surface of mammary epithelial cells, we found increased expression of C/EBPβ, increased relative expression of LIP to LAP, and enlarged ductal lumina. Together, our studies demonstrate a role for EPM in luminal morphogenesis through control of C/EBPβ expression.
Keywords: transcription factor balance, mammary morphogenesis, epithelial–stromal interactions, CCAAT/enhancer binding protein, transgenic mice
Introduction
The majority of mammary gland development occurs postnatally. During puberty, a system of branching ducts penetrates the fatty stroma; in pregnancy, the epithelium continues to proliferate, developing additional complexity and lobuloalveolar structures. Many of these developmental processes are dependent on reciprocal communication between the stroma and the epithelium (Hennighausen and Robinson 1998; Wiesen et al. 1999), although the signaling molecules that mediate the reciprocal interactions are only beginning to be identified.
Epimorphin (EPM) was originally characterized as a stromal cell surface molecule involved in embryonic epithelial morphogenesis (Hirai et al. 1992; Hirai 1993); a later study also showed that the same gene encoded a member of the syntaxin family (Bennett et al. 1993; Pelham 1993). In its capacity as an extracellular morphogen, EPM has been shown to control developmental processes in endothelial (Oka and Hirai 1996), liver parenchymal (Hirose et al. 1996; Watanabe et al. 1998), embryonic lung (Koshida and Hirai 1997), and pancreatic carcinoma cells (Lenhert et al. 2001). In the mammary gland, EPM is present at the surface of both stromal fibroblasts and myoepithelial cells (Hirai et al. 1998) and thus is poised to play a role in mammary morphogenesis. Previously, we used a three-dimensional collagen assay to characterize the function of EPM in normal mammary epithelial cell morphogenesis (Hirai et al. 1998). When presented in a polar basal fashion, EPM produced branching morphogenesis, whereas apolar presentation triggered formation of structures with large central lumina. However, the mechanism of its morphogenic activities remained to be identified.
The transcription factor CCAAT/enhancer binding protein (C/EBP)β has been implicated in many developmental processes (for review see Lekstrom-Hines and Xanthopolous 1998); however, no upstream effector of C/EBPβ has, as yet, been identified (Robinson et al. 1998). C/EBPβ is found in several isoforms that possess altered transactivation potentials: LAP, the full-length 34-kD isoform and LIP, a truncated isoform of 20 kD (Descombes and Schibler 1991). The relative expression of these isoforms changes throughout development of the mammary gland (Raught et al. 1995). Investigations of mice deficient for expression of C/EBPβ have revealed that this transcription factor is essential for normal mammary gland morphogenesis (Robinson et al. 1998; Seagroves et al. 1998). Among other defects, C/EBPβ−/− mice were found to have bloated ducts with enlarged central lumina, reminiscent of the luminal structures formed in our culture assays when mammary epithelial cells were presented with EPM in an apolar fashion. We hypothesized that this transcription factor might participate in the luminal morphogenesis triggered by apolar presentation of EPM to cultured mammary epithelial cells and that EPM may play a similar role in vivo. This hypothesis led to several specific predictions. First, presentation of EPM to mammary epithelial cells in vitro should regulate C/EBPβ, and the morphogenic effects of EPM should be reproduced by appropriate artificial regulation of C/EBPβ in cultured mammary epithelial cells. Second, EPM should be present at the apical surface of the mammary epithelial cells in vivo during the development of alveolar lumina, and apolar presentation of EPM to mammary epithelial cells in vivo should regulate C/EBPβ and produce enlarged lumina. Here, we present experimental results that confirm these predictions.
Materials and Methods
Cells and Tissue Culture
SCp2 cells, a normal mammary epithelial cell line (Desprez et al. 1993; Roskelley et al. 1994; Hirai et al. 1998), and primary mammary epithelial cells were maintained in growth medium (DME/F12 [GIBCO BRL] supplemented with 5% FBS [Hyclone], 5 μg/ml insulin [Sigma-Aldrich], and 50 μg/ml gentamycin). SCp2 tetracycline supresses epimorphin cells, generated as described in Hirai et al. 1998, were maintained in growth medium supplemented with 5 μg/ml of tetracycline. For generation of SCp2 cells expressing LIP and LAP under control of a tetracycline-repressible promoter, cDNAs were isolated from cytomegalovirus (CMV)–LIP and CMV–LAP constructs and were cloned into the EcoRI site of the eukaryotic expression vector pTetT-Splice (Life Technologies). SCp2 cells (5 × 105) were transfected with 5 μg of this vector, 5 μg of pTet.tTAK vector (Life Technologies), and 0.5 μg of pSV40neo (Schmidhauser et al. 1992) using lipofectamine (Life Technologies) according to the manufacturer's instructions. After selection for neomycin-resistant clones in the continuous presence of tetracycline, the expression of LIP and LAP was analyzed by Western blotting in the presence and absence of 5 μg/ml tetracycline. SCp2/LIP1, SCp2/LIP2, SCp2/LIP3, and SCp2/LAP cell lines were isolated using this procedure.
Functional Assays
Assays for formation of luminal structures were performed essentially as described previously (Hirai et al. 1998). In brief, cells were grown in serum-free medium either containing 100 μg/ml soluble recombinant EPM (rEPM) (for SCp2 cells) or lacking tetracycline (for PTSE cells). In some wells, rEPM (H123) was coated before seeding the cells (Hirai et al. 1998). The level of protein expression of C/EBPβ gene products was measured by immunoblotting the total cell lysates at day 3 or as described. Morphogenesis assays were performed using clustered cells that were embedded in collagen gels as described previously (Hirai et al. 1998) with slight modifications. In brief, cell clusters were prepared on agarose gels and suspended in a mixture of 8.5 vol of collagen type I (I-PC; Koken Corp.) and 1 vol of 10× serum-free medium, adjusted to pH 7.4 with 0.5 vol of alkaline solution. After addition of 1 vol of PBS, cell clusters were suspended in the collagen solution (50–100 clusters in 100 μl) and poured onto basal collagen gels in individual wells of 48-well plates. Growth medium containing 50 ng/ml EGF and the appropriate amount of tetracycline was added to each well. As indicated, soluble rEPM was added to a final concentration of 50 μg/ml.
Generation of rEPM
All rEPM proteins could be solubilized in 1.5 mM HCl, whereas solubility of the proteins was increased by COOH-terminal truncation. By removal of all of domain 3 (amino acids 189–264), rEPM (termed H12, amino acids 1–188) is freely soluble in PBS or growth medium. By three-dimensional collagen assay (Hirai et al. 1998), the truncated proteins retained all original morphogenic activities. All rEPM polypeptides were produced in Escherichia coli BL21 and purified in the presence of 8 M urea as described previously (Oka and Hirai 1996) with slight modifications. Deletion mutants of EPM tagged with a 6× His sequence were generated by PCR, inserted into the prokaryotic expression vector PET3a (Novagen), and introduced into bacterial cells. All the recombinant proteins purified from the bacteria with Ni-NTA–agarose beads (QIAGEN) were dialyzed either directly against 1.5 mM HCl or gradually against 1, 0.8, 0.5, 0.25, and 0.125 M urea in ice-cold PBS, followed by urea-free PBS. The soluble fraction in each sample was sterile filtered and assayed for protein concentration.
Antibodies
To prepare antibodies specific for individual domains of EPM, antiserum raised against untagged EPM (Hirai 1994) was affinity purified with nitrocellulose membranes precoated with purified rEPM fragments (1, amino acids 1–104; 2, amino acids 105–188; 3, amino acids 189–264) as described previously (Hirai et al. 1998). To prepare antibodies recognizing just the COOH-terminal sequence of EPM, affinity purified anti-H123 antibodies were absorbed with a column (mixture of Affigel 10 and 15; Bio-Rad Laboratories) immobilized with histidine-tagged rEPM deletion mutant H1–230 (amino acids 1–230). The antibodies bound to the column were eluted with 0.25 M glycine-HCl (pH 2.7), immediately neutralized with 1 M phosphate buffer (pH 8.0), dialyzed against PBS, and used as anti–1–230. These antibodies were used for immunoblotting at a concentration of 10 μg/ml. For preparation of the anti-LAPonly antibody, a construct containing the nucleotide sequence of C/EBPβ from 190–582 was fused with an ATG and a 6× CAT sequence (for 6× His) at the 5′ end was cloned into bacterial expression vector pet 3C′ and expressed in E. coli strain BL21. The recombinant LAPonly peptide was purified on Ni-NTA gel in the presence of 4 M urea, dialyzed against PBS (final purity was >95%), and used for injecting rats. Antibodies to C/EBPα and C/EBPβ gene products were from Santa Cruz Biotechnology, Inc., and antibody to β-actin was from Sigma-Aldrich. These reagents were used for immunoblotting at the dilution of 1:200. The antibody to T7 peptide (Novagen) was used at a 1:1,000 dilution.
Analysis of EPM Cleavage
Lactating mammary gland tissue (1 g) was sonicated in 5 ml of 20 mM Tris-HCl (pH 8.0), 0.5 mM CaCl2, and 25 mM NaCl on ice. After centrifugation at 200 g for 1 min, the supernatant was collected and centrifuged at 3,000 g for 30 min at 4°C. The pellet was then washed several times with PBS and resuspended in 500 μl serum-free medium. A 100-μl portion of this membrane-enriched fraction was mixed with 10 μg of recombinant full-length EPM (isoform I), tagged with 6× histidine at the NH2 terminus (Oka and Hirai 1996), and incubated at 37°C. After 48 h, 800 μl of 8 M urea (pH 8.0) was added to the reaction to dissolve all the insoluble materials. Ni-NTA–agarose beads (QIAGEN) were then added to the supernatant to collect the His-tagged products. After washing the beads several times with 8 M urea (pH 8.0), the collected products were analyzed by immunoblotting with anti-EPM antibodies. Untagged rEPM was used as a control. For transfection experiments, the expression vector SRα-296 (Takebe et al. 1988), containing the full-length cDNA for EPM isoform I tagged with T7 peptide at the NH2 terminus (SRαTEPM) (Hirai 1994), was electrophoretically transfected into SCp2, SCp2′, and primary mammary epithelial cells using a Bio-Rad Laboratories GenePulser. Cells were incubated in growth medium for 24 h, then in serum-free DME/F12 supplemented with 5 μg/ml insulin (Sigma-Aldrich), 3 μg/ml prolactin (National Institutes of Health, Bethesda, MD), and 1 μg/ml hydrocortisone (Sigma-Aldrich) for an additional 2 d. The cDNA products in the cells were analyzed by immunoblotting using an anti-T7 tag monoclonal antibody (Novagen). Supernatants from cultured cells were concentrated by immunoprecipitation using anti-EPM antibodies and protein A–Sepharose (Bio-Rad Laboratories) and analyzed by immunoblot.
Estimation of LIP/LAP Ratios in Cells
Cells were directly dissolved in SDS sample buffer, electrophoresed in SDS-PAGE gels, and blotted onto polyvinylidene difluoride filter membrane; C/EBPβ gene products were visualized with anti-C/EBPβ antibodies. Nuclear extracts were prepared as described by Deryckere and Gannon 1994. The estimation of intensity of LIP and LAP signals was carried out with the ChemiImager 4000 low light imaging system (Alpha Innotech). The relative amount of LIP/LAP was compared with control, which was loaded on the same blot.
Transgenic Mice
For generation of whey acidic protein (WAP)–EPM mice, EPM cDNA was tagged with a mouse IL-2 signal peptide sequence (5′-ATG TAC AGC ATG CAG CTC GCA TCC TGT GTC ACA TTG ACA CTT GTG CTC CTT GTC AAC AGC GCT CCC-3′) generated by PCR as described previously (Hirai et al. 1998). The construct was subcloned into the HindIII site of the CA10 vector, which contains a WAP gene promoter and a sequence for the 3′ untranslated region of WAP protein (Sympson et al. 1994). After linearization with NotI, the target gene was purified with glass milk (Bio-Rad Laboratories) and microinjected into fertilized eggs. The injected embryos were then transferred to pseudopregnant Friend virus B mice. To determine the presence and integrity of the transgene, DNA was isolated from the tail of offspring of transgenic mice and analyzed by PCR using 5′-AGCACCAGGAGAAGTCAC-3′ (from the WAP promoter sequence) as 5′ primer and 5′-CGACAGCAGTGTCTGCATCG-3′ (from the EPM sequence) as 3′ primer. For the positive lines, the expression of the transgene was further analyzed by reverse transcription (RT)-PCR. In brief, total RNA from pregnant mice was isolated, treated with DNase, reverse transcribed, and analyzed using 5′-ATGCAGCTCGCATCCTCTGTC-3′ (from the IL-2 signal peptide sequence) as 5′ primer and 5′-CGACAGCAGTGTCTGCATCG-3′ (from the EPM sequence) as 3′ primer.
Results
Apolar Presentation of EPM Regulates LIP
When PTSE mammary epithelial cells, which express EPM under control of the tetracycline transactivator, were cultured in the absence of tetracycline for 4 d, they expressed EPM in an apolar fashion and formed structures composed of a single cell layer and a central lumen (Fig. 1 A, a). Addition of tetracycline at this point repressed the EPM transgene and caused the luminal structures to collapse (Fig. 1 A, c). However, addition of soluble and rEPM (H12) along with tetracycline caused the luminal structures to continue to expand and to develop even larger central lumina (Fig. 1 A, b). Using this assay, we found that EPM elevated the overall levels of C/EBPβ and increased the relative ratio of the 20-kD isoform (LIP) to the 34-kD isoform (LAP) (Fig. 1 B). A similar effect was observed in primary mammary epithelial cells (Fig. 1 C). EPM also increased the expression of C/EBPα, although the truncated form of this protein was not observed (Fig. 1 D), suggesting that the alteration in the LIP/LAP ratio was not due to increased expression of nonspecific proteases.
Figure 1.


Effects of apolar presentation of EPM. (A) EPM activates luminal morphogenesis. PTSE cell clusters were cultured in collagen for 4 d in the absence of tetracycline (EPM ON) (a), then cultured for an additional 4 d in the presence of tetracycline (EPM OFF) and with (b) or without (c) H12 form of EPM in the medium. The luminal diameter of >20 clusters in each category was measured; graph bar indicates SD. (B) EPM increases C/EBPβ expression and the LIP/LAP ratio. PTSE cell clusters were cultured on plastic in the presence or absence of tetracycline, and SCp2 cells were cultured on recombinant full-length EPM (rEPM) or collagen (Hirai et al. 1998) or in the presence of rEPM/H12. Analyses of unclustered SCp2 cells incubated on tissue culture plastic or plastic coated with collagen are shown as controls. CRM, cross-reactive material (Seagroves et al. 1998). Estimated LIP/LAP ratios relative to the control samples are indicated. (C) LIP and LAP are dramatically upregulated by both EPM transfection (sig-EPM) and addition of rEPM to primary mammary epithelial cells. The identity of the band between CRM and LAP is unknown. (D) SCp2 cells cultured in the presence of rEPM also upregulate C/EBPα. For B, C, and D, the results are typical of three independent experiments. Bar, 100 μm.
EPM-induced LIP Is Not Produced by Proteolysis
To verify that increased LIP did not result from specific in vitro proteolysis, we generated an antiserum against the NH2-terminal 14 kD of C/EBPβ, the region contained within LAP that is absent in LIP (Fig. 2 A). This antiserum was used to probe a blot containing extracts of control SCp2 cells, SCp2 cells treated with rEPM, SCp2 cells transiently transfected with LAP (SCp2/LAP cells), or SCp2/LAP cells treated with rEPM (Fig. 2 B). We found the expected increase in 34-kD LAP in both the rEPM-treated samples and in the LAP transfectants but did not find any 14-kD band that would have resulted from proteolytic cleavage of LAP. A parallel blot probed with a commercial antibody directed against a region of C/EBPβ common to LAP and LIP (Fig. 2 C) showed a comparable increase in LIP expression in both EPM-treated samples. Despite the considerably higher expression of LAP in the SCp2/LAP cells, there was only a very faint band corresponding to LIP. A third blot containing extracts from SCp2 cells transiently transfected with either LAP or LIP constructs was probed with the commercial antibody, and no significant degradation of LAP was observed (Fig. 2 C). Together, these experiments show that our extraction protocol produces little or no proteolysis of C/EBPβ.
Figure 2.
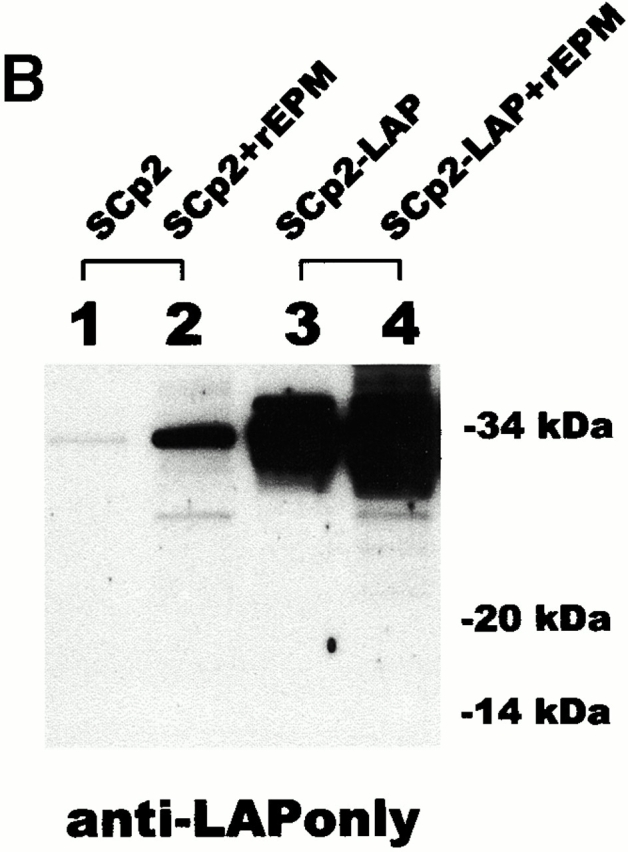
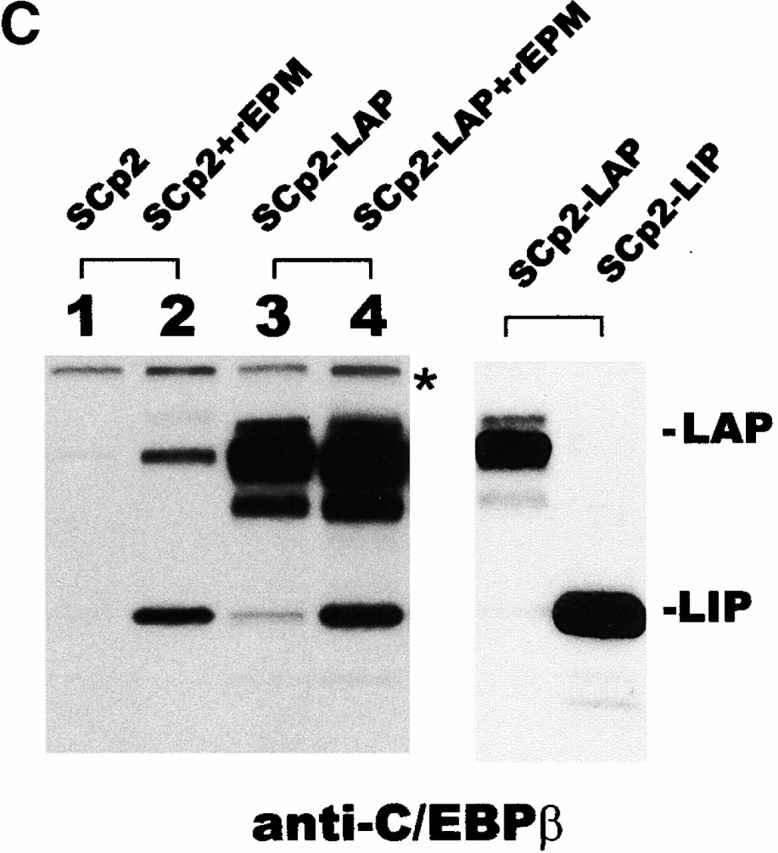
Minimal proteolysis of LAP occurs during sample preparation. (A) Diagram depicting the targeted location of the anti-LAPonly antibody and of the commercial anti-C/EBPβ antibody. (B) Western blot probed with the anti-LAPonly antibody. (C) Western blots probed with commercial anti-C/EBPβ antibody. Left: Parallel blot to B; asterisk, cross-reactive material. Right: Blot of SCp2 cells transiently transfected with LAP or LIP expression plasmids. Results shown are typical of two independent experiments.
LIP Mimics and LAP Blocks the Effects of EPM
We found that regulation of C/EBPβ isoform expression was sufficient to mediate luminal morphogenesis of cultured mammary epithelial cells using constructs with tetracycline-regulated expression of LIP or LAP (Fig. 3 A). In collagen assays, untransfected or mock-transfected cells formed aggregates. Moderate expression of LIP achieved through attenuation of expression in SCp2/LIP1 and SCp2/LIP2 cells and through incomplete repression in SCp2/LIP3 cells promoted luminal morphogenesis. However, high expression of LIP caused apoptosis, assayed by accumulation of fragmented DNA (data not shown). In several experiments, we found that increases in the LIP/LAP ratio between ∼2–10 triggered luminal morphogenesis (Fig. 3 B, c [0.5 μg/ml tetracycline] and C, b), and increases in ratios that were >10 led to apoptosis (Fig. 3 B, b [0.02 μg/ml tetracycline] and C, c). By contrast, overexpression of LAP led to formation of compact colonies with no lumina that were resistant to EPM-mediated luminal morphogenesis (Fig. 3 D, c), possibly through the ability of LAP to neutralize LIP (Descombes and Schibler 1991; Buck et al. 1994). Taken together, these results demonstrate that EPM upregulates the relative expression of LIP to LAP, constitutive expression of LIP is sufficient to produce luminal morphogenesis, and constitutive expression of LAP can block EPM from producing this morphogenic activity.
Figure 3.
Characterization of clones that conditionally express LIP and LAP. (A) Analysis of C/EBPβ gene products in LIP and LAP transfectants cultured in the presence (5 μg/ml) or absence of tetracycline (tet). Results shown are typical of three independent experiments. (B and C) Behavior of LIP-transfected cells cultured in various concentrations of tetracycline. Clusters of SCp2 controls (B, a) and of SCp2/LIP1 and SCp2/LIP2 in 0.5 μg/ml tet (B, b) formed compact colonies in collagen (shown in C, a for SCp2/LIP1; 0.5 μg/ml tetracycline). SCp2/LIP3 expresses moderate levels of LIP and forms lumina in the presence of tetracycline (B c and C b). Reduction of tetracycline in medium of SCp2/LIP1 and SCp2/LIP2 cells results in apoptotic cell death (B, b; shown in C, c for SCp2/LIP1; 0.02 μg/ml tetracycline). (D) Induction of LAP transgene inhibits luminal morphogenesis. (a) LAP expression in clustered parental SCp2 cells. (b and c) Clustered parental Scp2 cells and SCp2/LAP cells cultured in collagen gels in the absence of tetracycline (tet) and in the presence and absence of rEPM. For B, C, and D, ≥20 colonies from each condition were examined. Bar, 100 μm.
Soluble EPM Is Present in Milk
In mammary glands from nulliparous animals, we had previously detected EPM only on the stromal fibroblasts and myoepithelial cells (Hirai et al. 1998). However, as apolar presentation of EPM was important for luminal morphogenesis in culture, we investigated the possibility that endogenous EPM might also be present at the apical surface of luminal epithelial cells during normal development. When unfixed lactating glands from wild-type mice were stained with a gentle washing protocol, EPM could be detected also in the lumina (Fig. 4 A). This localization suggested a soluble form of EPM, and Western blot analysis of normal mouse milk with anti-EPM antibodies identified an ∼30-kD band that was smaller than full-length 34-kD EPM (Fig. 4 B, b). The presence of this protein was not due to simple cell lysis, since β-actin, a diagnostic for burst cells (Mather and Keenan 1998), was not found in the milk (Fig. 4 B, a). Western analyses using antibodies specific for domains within EPM suggested that the ∼30-kD protein is a COOH-terminal truncation (Fig. 4 C).
Figure 4.
Identification of ∼30-kD soluble EPM in vivo. (A) Frozen sections of prefixed (a) and unfixed (b) lactating mammary glands labeled with anti-EPM antibodies. The tissue in b was fixed on the slide immediately after sectioning, and the staining was carried out with mild washing so as not to remove soluble proteins in the lumina (asterisk). (B) Immunoblot analysis of the lactating mammary gland tissue (T) and milk (M) with anti–β-actin and anti-EPM antibodies. 5 (×5) or 1 μg protein samples of mammary gland extract (T) or milk (M) collected from lactating wild-type mice were probed with anti–β-actin (a) and EPM (b) antibodies. (C) 30-kD soluble EPM reacts with all anti-EPM antibodies except those directed against the COOH terminus. (a) Schematic diagram of affinity purified antibodies targeted to different domains of EPM. (b) Immunoblot analyses of milk and recombinant full-length 34-kD EPM (r-EPM) using the affinity purified antibodies. Anti-1, -2, -3, -1–230, and -c are specific to EPM amino acids 1–104, 105–188, 189–264, 1–230, and 231–264, respectively. Bar, 30 μm.
We reasoned that the soluble form of EPM might result from proteolysis of membrane-bound EPM. Consistent with this hypothesis, when full-length rEPM containing an NH2-terminal 6× His tag was incubated with a membrane extract derived from whole lactating mammary tissue, the protein was cleaved to form a single ∼30-kD product (Fig. 5 B, b). Although it was not possible to isolate sufficient quantities of the ∼30-kD species to verify its activity, other COOH-terminal truncations are fully functional in morphogenesis assays (Hirai et al. 1998).
Figure 5.
Production of ∼30-kD soluble EPM in vitro. (A) The products of EPM cDNA tagged with T7 peptide at the NH2 terminus in transfected primary mammary cells (a), SCp2 cells (b), and SCp2′ cells (c) were analyzed by immunoblot with monoclonal anti-T7 antibody. Cells (C) and supernatants (S) were analyzed separately. For detection in the supernatant, an immunocomplex with anti-EPM antibodies was collected with protein A–Sepharose beads. Untagged rEPM was used as a control. (B) rEPM-tagged with 6× His at the NH2 terminus was incubated with membranes derived from lactating mammary glands (a) or from either SCp2 or SCp2′ cells (b). Products were collected with a Ni-agarose column and analyzed by immunoblotting with anti-EPM antibodies. For a, untagged rEPM was used as a control. In b, the MMP inhibitor GM6001 or the inactive structural homologue C1004 was added to a final concentration of 10 μM. Results are typical of three independent experiments.
When an expression construct containing full-length EPM with an NH2-terminal T7 tag was transfected into primary mammary epithelial cells derived from pregnant animals, the cells produced both ∼30- and 34-kD products (Fig. 5 A, a). Although the general population of SCp2 cells when transfected with the expression construct did not normally produce the soluble ∼30-kD EPM species (Fig. 5 A, b), a subclone of transfectants named SCp2′ possessed the ability to do so (Fig. 5 A, c). Cleavage of tagged EPM by SCp2′ cell extracts was inhibited by the metalloproteinase (MMP) inhibitor GM6001 (Fig. 5 B, b), suggesting that an MMP could mediate the conversion to the soluble ∼30-kD species. The identity of this protease is under investigation.
WAP–EPM Transgenic Mice Showed Upregulation of LIP
To investigate EPM-mediated mammary luminal morphogenesis in vivo, we generated transgenic mice that expressed membrane-tethered EPM on the surface of luminal epithelial cells. This was accomplished by fusing the interleukin 2 signal peptide to the EPM cDNA and then sandwiching this construct between the promoter and 3′ untranslated region of the WAP gene (Pittius et al. 1988; Sympson et al. 1994) to generate the WAP–EPM construct (Fig. 6 A). We had previously used a similar construct to localize the EPM to the surface of cultured mammary epithelial cells (Hirai et al. 1998).
Figure 6.

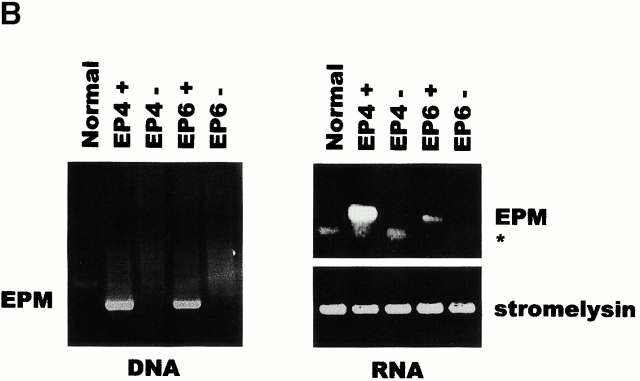
WAP–EPM transgenic mice have enlarged ductal lumina and altered C/EBPβ expression. (A) Schematic diagram of the transgene construct. N, NotI; H, HindIII. (B) PCR analysis of the integration of the transgene into genomic DNA and RT-PCR analysis of EPM transgene expression. As a control for RT-PCR, endogenous stromelysin 1 (SL-1) was analyzed in the same samples. Lower bands (asterisk) are unreacted primers that remained in control samples after RT-PCR. (C) Phenotypic appearance of the mammary gland (circled) from midpregnant (7 d) normal (a) and transgenic (b) mice. Whole-mount stained mammary gland of normal (c) and transgenic (d) mice. (D) Analysis of C/EBPβ gene products in mammary tissue of normal and transgenic mice using commercial anti-C/EBPβ antibody. CRM, cross-reactive material. Bars: (B) 5 μm; (C) 300 μm.
Two out of six founder mice, EP4 and EP6, showed incorporation of the transgene (Fig. 6 B). Nulliparous heterozygotes from these lines displayed no detectable increase in EPM expression and had no distinctively aberrant phenotype (data not shown). Pregnant and lactating heterozygotes did express EPM and also had dramatically enlarged ducts (Fig. 6 C, b). In addition, transgenic animals had large and disorganized secretory alveoli. A similar phenotype with varying severity was observed in all of the WAP–EPM mice but not in any of the transgene-negative littermates examined (n = 25), and the phenotype of all the transgenic mice became stronger as animals aged (data not shown). Lactating transgenic mice displaying the strongest phenotype had problems producing milk and would cannibalize their young.
When analyzed by Western blot, mammary glands from WAP–EPM mice showed a dramatic increase in C/EBPβ relative to wild-type mice, both in total protein and in the relative ratio of LIP to LAP (Fig. 6 D). Thus, the WAP–EPM mice paralleled the culture studies, since apolar expression of EPM on mammary epithelial cells upregulated the LIP/LAP ratio and led to enlarged ductal lumina.
Discussion
In this study, we have identified a functional relationship between EPM-triggered luminal morphogenesis and increased relative expression of the LIP isoform of C/EBPβ. This was both necessary and sufficient for the morphogenic activity in cultured cells as expression of LIP induced luminal morphogenesis, whereas expression of LAP blocked luminal morphogenesis induced by EPM. Since apical presentation of EPM seemed to be important for this morphogenic process, we examined EPM expression in vivo and found a truncated soluble form present in mouse milk. Analysis of the WAP–EPM transgenic mice supported the role of EPM as an effector of LIP-mediated luminal morphogenesis. These animals express EPM in an apolar fashion on luminal epithelial cells and also have increased LIP/LAP ratios and greatly enlarged ductal lumina in pregnant and lactating animals.
It is unclear if EPM-induced luminal morphogenesis is caused by a positive activity of LIP or by the downregulation of LAP by LIP. The latter possibility is supported by the similarities between mammary ductal morphology of WAP–EPM and C/EBPβ−/− mice. However, it should be noted that the phenotype of the WAP–EPM mice is significantly different from the phenotype of the C/EBPβ2/− mice. The latter have severe ovarian dysfunction and consequent female sterility (Sterneck et al. 1997), defective differentiation of myeloid cells (Screpanti et al. 1995; Tanaka et al. 1995), adipocytes (Tanaka et al. 1997), hepatocytes (Lee et al. 1997), and keratinocytes (Zhu et al. 1999), and aberrant expression of lactogenic hormone receptors (Seagroves et al. 2000). Even when comparisons are limited to the mammary gland, there are still substantial differences, since lobuloalveolar development was inhibited in the C/EBPβ−/− mice (Seagroves et al. 1998). These differences may be partially attributed to the spatiotemporal modulation of EPM expression by the WAP promoter and the consequent limitation of the affected cell population. Furthermore, differences in mouse strain backgrounds may also be relevant.
Soluble EPM in Murine Ductal Lumina
Examination of normal mouse milk revealed a soluble molecule of ∼30 kD that was cross-reactive with all antibodies to EPM except those generated against the COOH-terminal sequence. The hypothesis that the truncated EPM could result from proteolytic cleavage at the cell surface was examined by reconstitution experiments in which membrane fractions from mammary glands of lactating mice were incubated with tagged recombinant EPM. We found that this treatment resulted in selective generation of the ∼30-kD form (Fig. 6 B). Several other signaling molecules, including the kit ligand and TGF-β, are released by membrane-bound MMPs (Blobel 1997; Werb 1997), and we found that a specific inhibitor of MMPs inhibited the conversion of full-length EPM to the ∼30-kD form in SCp2′ cells.
It is unclear how soluble extracellular EPM, if produced by stromal cells, is transported to the ductal lumina. Possibly, after removal of the membrane-anchoring sequence by proteolysis EPM could be delivered by transcytosis across the epithelial membrane, or it could pass through temporary junctions between luminal epithelial cells. Such mechanisms are involved in the delivery of other stromal proteins to the luminal space (Linzell and Peaker 1973; Grosvenor et al. 1992; Stelwagen et al. 1997; Ollivier-Bousquet 1998). Alternatively, since some mammary epithelial cell lines are able to express EPM in culture (Hirai et al. 1998) it is possible that a subpopulation of luminal epithelial cells produce soluble EPM during development. In this case, secretion of the large volume of milk proteins and lipid droplets by the lactating gland (Burgoyne and Duncan 1998; Mather and Keenan 1998) may act as a vehicle for efficient distribution of EPM. Currently, there are no data to distinguish between these possibilities.
Regulation of the LIP/LAP Ratio
LAP and LIP are mutually antagonistic isoforms of C/EBPβ (Descombes and Schibler 1991; Buck et al. 1994). In our culture system, moderate expression of LIP led to lumen formation, whereas expression of LAP blocked this process. That high expression of LIP produced apoptosis is an intriguing observation in light of the role of apoptosis in the formation of lumina in terminal endbuds and alveolar structure in culture (Humphreys et al. 1996). The possibility that LIP may contribute to this process warrants careful analysis in three-dimensional cultures and in vivo. However, as morphogenic processes are likely to be orchestrated within microdomains of the mammary gland, specific antibodies and quantitative real time imaging may be required.
LIP has been implicated in other physiological processes as diverse as inflammation (An et al. 1996), liver regeneration (Timchenko et al. 1998; Welm et al. 2000), development (Diehl et al. 1994; Darlington 1999), and aging (Hseih et al. 1998; Timchenko et al. 1998). LIP was originally proposed to arise through alternative translation by leaky ribosome scanning (Descombes and Schibler 1991; Ossipow et al. 1993). Subsequent investigations implicated a small, out-of-frame ORF in the 5′ sequence of C/EBPβ as a regulator of LIP/LAP ratios and determinant of cell differentiation (Calkhoven et al. 2000 and references therein). Some studies have suggested that a specific RNA-binding protein may control the alternative translation initiation (Timchenko et al. 1999; Welm et al. 2000). Other investigations have shown that LIP can result from a mechanism that is regulated by C/EBPα (Welm et al. 1999), and in this regard it is relevant that apolar presentation of EPM leads to upregulation of C/EBPα as well (Fig. 1 D). In some cases, observation of the LIP isoform may be due to in vitro proteolysis of LAP (Baer et al. 1998; Lincoln et al. 1998; Baer and Johnson 2000), but we excluded this possibility in our system (Fig. 2).
EPM Is a Member of the Syntaxin Family
The gene that encodes EPM also encodes syntaxin-2, a protein that has been shown to function during targeting/fusion of intracellular vesicles with the plasma membrane (Bennett et al. 1993; Pelham 1993). Although syntaxins have been shown to act at the cytoplasmic face of membranes (Brose 1993; Pelham 1993; Rothman 1994; Wheeler et al. 1996; Jagadish et al. 1997), the subcellular localization of syntaxins can be modulated through various mechanisms (Hui et al. 1997; Jagadish et al. 1997; Rodger et al. 1998; Quiñones et al. 1999), and syntaxins have been found at the cell surface at least transiently (Hirai et al. 1993, Hirai et al. 1998; Smirnova et al. 1993a,Smirnova et al. 1993b; Butt et al. 1996; Nagamatsu et al. 1997; Guo et al. 1998). Evidently, these complex and versatile proteins can perform multiple functions, and it will be a considerable challenge to discover how these diverse roles are related. However, functional relationships of known protein motifs within EPM/syntaxin-2 may provide some clues. The SNARE motif is a highly conserved domain within the syntaxin family that is located between amino acids 188 and 253 of EPM (Weimbs et al. 1997). Although domain analyses of syntaxins demonstrate the SNARE motif is essential for formation of competent membrane fusion complexes (Sutton et al. 1998; Jahn and Südhof 1999), we have found that this motif is not required for the morphogenic activity of EPM in mammary epithelial cells, since a genetically engineered EPM (H12) lacking the SNARE motif is fully competent in our assays (Fig. 1; Hirai et al. 1998). These findings support a model in which syntaxin/EPM molecules harbor distinct functional domains that allow the molecules to function in more than one cellular context.
The mechanism by which EPM is translocated to the outside of the plasma membrane is not known, although cell surface localization of other membrane-associated molecules lacking signal peptides has been reported (Wen et al. 1992; Skach et al. 1993; Ostapchuk et al. 1994; Guo et al. 1998). In such cases, the membrane topology depends on the three-dimensional conformation of the NH2-terminal sequence (Denzer et al. 1995; Spiess 1995; Wahlberg and Spiess 1997). Similarly, intramolecular interactions within the EPM sequence (Hirai 1994) may allow a subpopulation to orient itself on the outer surface of the plasma membrane.
In conclusion, we have shown that EPM can mediate luminal morphogenesis of mammary epithelial cells in vitro by control of C/EBPβ, and we have implicated this mechanism in mammary morphogenesis. The conclusive link will require further analyses of WAP–EPM, WAP–LIP, and WAP–LAP transgenic mice, generated in the same strain background, and a knockout of C/EBPβ in our assay system. Through such experiments, it would be possible to determine if C/EBPβ is the only mediator of EPM-triggered luminal morphogenesis in vivo. Characterization of the molecular mechanism by which EPM controls C/EBPβ isoform levels will require analysis of the epithelial receptor for extracellular EPM; it should be easier to identify this receptor by using the soluble form of EPM, identified in this study as a probe. These investigations are currently underway.
Acknowledgments
We thank Jinger Xie and Dewight Williams for help in breeding the transgenic mice, William Johansen and Amy Ukena for their administrative support, and Zena Werb, Eva Turley, Joan Brugge, John Muschler, Karen Schmeichel, and Jimmie Fata for critical reading of the manuscript. The eukaryotic expression vectors CMV–LIP and CMV–LAP were generous gifts from Ueli Schibler.
This work was supported by the U.S. Department of Energy, Office of Biological and Environmental Research (contract DE-AC03-76SF00098), and the National Institutes of Health (grant CA57621), by a Distinguished Hollaender Postdoctoral fellowship to D. Radisky, and by support from the Science and Technology Agency of Japan to Y. Hirai.
Footnotes
M.E. Stevens' present address is Bayer Corp., Berkeley, CA 94701.
Abbreviations used in this paper: C/EBP, CCAAT/enhancer binding protein β; CMV, cytomegalovirus; EPM, epimorphin; MMP, metalloproteinase; rEPM, recombinant EPM; RT, reverse transcription; WAP, whey acidic protein.
References
- An M.R., Hseih C.-C., Reisner P.D., Rabek J.P., Scott S.G., Kuninger D.T., Papaconstantinou J. Evidence for posttranscriptional regulation of C/EBPα and C/EBPβ isoform expression during the lipopolysaccharide-mediated acute-phase response. Mol. Cell. Biol. 1996;16:2295–2306. doi: 10.1128/mcb.16.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer M., Johnson P.F. Generation of truncated C/EBPβ isoforms by in vitro proteolysis. J. Biol. Chem. 2000;275:26582–26590. doi: 10.1074/jbc.M004268200. [DOI] [PubMed] [Google Scholar]
- Baer B.M., Williams S.C., Dillner A., Schwartz R.C., Johnson P.F. Autocrine signals control CCAAT/enhancer binding protein β expression, localization, and activity in macrophages. Blood. 1998;92:4325–4369. [PubMed] [Google Scholar]
- Bennett M.K., Garcia-Arraras J.E., Elferink L.A., Peterson K., Fleming A.M., Hazuka C.D., Sheller R.H. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Blobel C.P. Metalloprotease-disintegrinslinks to cell adhesion and cleavage of TNF alpha and Notch. Cell. 1997;90:589–592. doi: 10.1016/s0092-8674(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Brose N. Membrane fusion takes an excitatory turnsyntaxin, vesicle docking protein, or glutamate receptor? Cell. 1993;75:1043–1044. doi: 10.1016/0092-8674(93)90313-f. [DOI] [PubMed] [Google Scholar]
- Buck M., Turler H., Chojkier M. LAP (NF-IL-6), a tissue specific transcriptional activator, is an inhibitor of hepatoma cell proliferation. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:851–860. doi: 10.1002/j.1460-2075.1994.tb06328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R.D., Duncan J.S. Secretion of milk proteins. J. Mammary Gland Biol. Neoplasia. 1998;3:275–286. doi: 10.1023/a:1018763427108. [DOI] [PubMed] [Google Scholar]
- Butt K.I., Manabe M., Yaguchi H., Tsuboi R., Ogawa H. Immunolocalization of epimorphin in skin. J. Dermatol. Sci. 1996;13:193–201. doi: 10.1016/s0923-1811(96)00534-8. [DOI] [PubMed] [Google Scholar]
- Calkhoven C.F., Müller C., Leutz A. Translational control of C/EBPα and C/EBPβ isoform expression. Genes Dev. 2000;14:1920–1932. [PMC free article] [PubMed] [Google Scholar]
- Darlington G.J. Molecular mechanisms of liver development and differentiation. Curr. Opin. Cell Biol. 1999;11:678–682. doi: 10.1016/s0955-0674(99)00035-6. [DOI] [PubMed] [Google Scholar]
- Denzer A.J., Nabholz C.E., Spiess M. Transmembrane orientation of signal-anchor proteins is affected by the folding state but not the size of the N-terminal domain. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:6311–6317. doi: 10.1002/j.1460-2075.1995.tb00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryckere F., Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques. 1994;16:405. [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Desprez P.-Y., Roskelley C., Campisi J., Bissell M.J. Isolation of functional cell lines from a mouse mammary epithelial cell strainthe importance of basement membrane and cell-cell interaction. Mol. Cell Differ. 1993;1:99–110. [Google Scholar]
- Diehl A.M., Michaelson P., Yang S.Q. Selective induction of CCAAT/enhancer binding protein isoforms occurs during rat liver development. Gastroenterology. 1994;106:1625–1637. doi: 10.1016/0016-5085(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Grosvenor C.E., Picciano M.F., Baumrucker C.R. Hormones and growth factors in milk. Endocrine Rev. 1992;14:710–728. doi: 10.1210/edrv-14-6-710. [DOI] [PubMed] [Google Scholar]
- Guo Z., Tumer C., Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- Hennighausen L., Robinson G.W. Think globally, act locallythe making of a mouse mammary gland. Genes Dev. 1998;12:449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- Hirai Y. Molecular cloning of human epimorphinidentification of isoforms and their unique properties. Biochem. Biophys. Res. Commun. 1993;191:1332–1337. doi: 10.1006/bbrc.1993.1363. [DOI] [PubMed] [Google Scholar]
- Hirai Y. Sodium-dodecyl-sulfate-resistant complex formation of epimorphin monomers and interaction of the 150kDa complex with the cell surface. Eur. J. Biochem. 1994;225:1133–1139. doi: 10.1111/j.1432-1033.1994.1133b.x. [DOI] [PubMed] [Google Scholar]
- Hirai Y., Takebe K., Takashina M., Kobayashi S., Takeichi M. Epimorphina mesenchymal protein essential for epithelial morphogenesis. Cell. 1992;69:471–481. doi: 10.1016/0092-8674(92)90448-l. [DOI] [PubMed] [Google Scholar]
- Hirai Y., Nakagawa S., Takeichi M. Re-examination of the properties of epimorphin and its possible roles. Cell. 1993;73:426–427. doi: 10.1016/0092-8674(93)90129-e. [DOI] [PubMed] [Google Scholar]
- Hirai Y., Lochter A., Galosy S., Koshida S., Niwa S., Bissell M.J. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J. Cell Biol. 1998;140:159–169. doi: 10.1083/jcb.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M., Watanabe S., Oide H., Kitamura T., Miyazaki A., Sato N. A new function of ito cells in liver morphogenesisEvidence using a novel morphogenic protein, epimorphin, in vitro. Biochem. Biophys. Res. Commun. 1996;225:155–160. doi: 10.1006/bbrc.1996.1146. [DOI] [PubMed] [Google Scholar]
- Hseih C.-C., Xiong W., Xie Q., Rabek J.P., Scott S.G., An M.R., Reisner P.D., Kuniger D.T., Papaconstantinou J. Effects of age on the posttranscriptional regulation of CCAAT/enhancer binding protein alpha and CCAAT/enhancer binding protein beta isoform synthesis in control and LPS-treated livers. Mol. Biol. Cell. 1998;9:1479–1494. doi: 10.1091/mbc.9.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui N., Nakamura N., Sonnichsen B., Shima D.T., Nilsson T., Warren G. An isoform of Golgi-tSNARE, syntaxin 5, with an endoplasmic reticulum retrieval signal. Mol. Cell. Biol. 1997;8:1777–1787. doi: 10.1091/mbc.8.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys R.C., Krajewska M., Krnacik S., Jæger R., Weiher H., Krajewski S., Reed J.C., Rosen J.M. Apoptosis in the terminal endbud of the mammary glanda mechanism of ductal morphogenesis. Development. 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- Jagadish M.N., Tellam J.T., Macaulay S.L., Gough K.H., James D.E., Ward C.W. Novel isoform of syntaxin 1 is expressed in mammalian cells. Biochem. J. 1997;321:151–156. doi: 10.1042/bj3210151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Südhof T.C. Membrane fusion and exocytosis. Annu. Rev. Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Koshida S., Hirai Y. Identification of cellular recognition sequence of epimorphin and critical role of cell/epimorphin interaction in lung branching morphogenesis. Biochem. Biophys. Res. Commun. 1997;234:522–525. doi: 10.1006/bbrc.1997.6673. [DOI] [PubMed] [Google Scholar]
- Lee Y.-H., Gonzales S.C., Baer M., Sterneck E., Gonzales F.J., Johnson P.F. The ability of C/EBPβ but not C/EBPα to synergize with an Sp1 protein is specified by the leucine zipper and activation domain. Mol. Cell. Biol. 1997;17:2038–2047. doi: 10.1128/mcb.17.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekstrom-Hines J., Xanthopolous K.G. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- Lenhert L., Lerch M.M., Hirai Y., Kruse M.L., Schmiegel W., Kalthoff H. Autocrine stimulation of human pancreatic duct-like development by soluble isoforms of epimorphin in vitro. J. Cell Biol. 2001;152:911–921. doi: 10.1083/jcb.152.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln A.J., Monczak Y., Williams S.C., Johnson P.F. Inhibition of CCAAT/enhancer-binding protein α and β translation by upstream open reading frames. J. Biol. Chem. 1998;273:9552–9560. doi: 10.1074/jbc.273.16.9552. [DOI] [PubMed] [Google Scholar]
- Linzell J.L., Peaker M. Changes in mammary gland permeability at the onset of lactation in the goatan effect on tight junction? J. Physiol. 1973;230:13p–14p. [PMC free article] [PubMed] [Google Scholar]
- Mather I.H., Keenan T.W. Origin and secretion of milk lipids. J. Mammary Gland Biol. Neoplasia. 1998;3:259–274. doi: 10.1023/a:1018711410270. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S., Sawa H., Nakamichi Y., Kondo Y., Mastsushima S., Watanabe T. Non-functional role of syntaxin2 in insulin exocytosis by pancreatic β cells. Cell Biochem. Funct. 1997;15:237–242. doi: 10.1002/(SICI)1099-0844(199712)15:4<237::AID-CBF746>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Oka Y., Hirai Y. Inductive influences of epimorphin on endothelial cells in vitro . Exp. Cell Res. 1996;222:189–198. doi: 10.1006/excr.1996.0024. [DOI] [PubMed] [Google Scholar]
- Ollivier-Bousquet M. Transferrin and prolactin transcytosis in lactating mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 1998;3:303–313. doi: 10.1023/a:1018767528017. [DOI] [PubMed] [Google Scholar]
- Ossipow V., Descombes P., Schibler U. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc. Natl. Acad. Sci. USA. 1993;90:8219–8223. doi: 10.1073/pnas.90.17.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Hearing P., Ganem D. A dramatic shift in the transmembrane topology of a viral envelope glycoprotein accompanies hepatitis B viral morphogenesis. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1048–1057. doi: 10.1002/j.1460-2075.1994.tb06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H.R. Is epimorphin involved in vesicular transport? Cell. 1993;73:425–426. doi: 10.1016/0092-8674(93)90128-d. [DOI] [PubMed] [Google Scholar]
- Pittius C.W., Sankaran L., Tropper Y.J., Hennighausen L. Comparison of the regulation of the whey acidic protein gene with that of a hybrid gene containing the whey acidic protein gene promoter in transgenic mice. Mol. Endocrinol. 1988;2:1027–1032. doi: 10.1210/mend-2-11-1027. [DOI] [PubMed] [Google Scholar]
- Quiñones B., Riento K., Olkkonen V.M., Hardy S., Bennett M. Syntaxin 2 splice variants exhibit differential expression patterns, biochemical properties, and subcellular localizations. J. Cell Sci. 1999;112:4291–4304. doi: 10.1242/jcs.112.23.4291. [DOI] [PubMed] [Google Scholar]
- Raught B., Liao W.S.L., Rosen J.M. Developmentally and hormonally regulated CCAAT/enhancer binding protein isoforms influence β-casein gene expression. Mol. Endocrinol. 1995;9:1223–1232. doi: 10.1210/mend.9.9.7491114. [DOI] [PubMed] [Google Scholar]
- Robinson G.W., Johnson P.F., Hennighausen L., Sterneck E. The C/EBPβ transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1998;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J., Davis S., Laroche S., Mallet J., Hicks A. Induction of long term potentiation in vivo regulates alternate splicing to alter syntaxin 3 isoform expression in rat dentate gyrus. J. Neurochem. 1998;7:666–675. doi: 10.1046/j.1471-4159.1998.71020666.x. [DOI] [PubMed] [Google Scholar]
- Roskelley C.D., Desprez P.Y., Bissell M.J. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl. Acad. Sci. USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Schmidhauser C., Casperson G.F., Myers C.A., Sanzo K.T., Bolten S., Bissell M.J. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of β-casein gene expression. Mol. Biol. Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti I., Romani L., Musiani P., Modesti A., Fattori E., Lazzaro D., Sellitto C., Scarpa S., Bellavia D., Lattanzio G. Lymphoproliferative disorder and imbalanced T-helper response in C/EBPβ-deficient mice. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves T.N., Krnacik S., Raught B., Gay J., Burgess-Beusse B., Darlington G.J., Rosen J.M. C/EBPβ, but not EBPα, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves T.N., Lydon J.P., Hovey R.C., Vonderhaar B.K., Rosen J.M. C/EBPβ (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol. Endocrinol. 2000;14:359–368. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- Skach W.R., Calayag M.C., Lingappa V.R. Evidence for an alternate model of human P-glycoprotein structure and biogenesis. J. Biol. Chem. 1993;268:6903–6908. [PubMed] [Google Scholar]
- Smirnova T., Stinnakre J., Mallet J. Characterization of a presynaptic glutamate receptor Science 262 1993. 430 433a [DOI] [PubMed] [Google Scholar]
- Smirnova T., Laroche S., Errington M.L., Hicks A.A., Bliss T.V.P., Mallet J. Transsynaptic expression of presynaptic glutamate receptor during hippocampal long-term potentiation Science. 262 1993. 433 435b [DOI] [PubMed] [Google Scholar]
- Spiess M. Heads or tailwhat determines the orientation of proteins in the membrane? FEBS Lett. 1995;369:76–79. doi: 10.1016/0014-5793(95)00551-j. [DOI] [PubMed] [Google Scholar]
- Stelwagen K., Farr V.C., McFadden H.A., Prosser C.G., Davis S.R. Time course of milk accumulation-induced opening of mammary tight junction, and blood clearance of milk components. Am. J. Physiol. 1997;273:R379–R386. doi: 10.1152/ajpregu.1997.273.1.R379. [DOI] [PubMed] [Google Scholar]
- Sterneck E., Tessarollo L., Johnson P.F. An essential role for C/EBPβ in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer D., Jahn R., Brunger A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Sympson C.J., Talhouk R.S., Alexander C.M., Chin J.R., Clift S.M., Bissell M.J., Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Akai K., Yoshida M., Arai N. SRα promoteran efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and R-U5 segment of human T-cell leukemia virus type I long terminal repeat. Mol. Cell. Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Akira S., Yoshida K., Umemoto M., Yoneda Y., Shirafuji N., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yoshida N., Kishimoto T., Akira S. Defective adipocyte differentiation in mice lacking the C/EBPβ and/or the C/EBPδ gene. EMBO (Eur. Mol. Biol. Organ.) J. 1997;18:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko N.A., Wilde M., Kosai K., Heydari A.R., Bilyeu T.A., Finegold M.H., Mohamedali K., Richardson A., Darlington G.J. Regenerating livers of old rats contain high levels of C/EBPα that correlate with altered expression of cell cycle associated proteins. Nucleic Acids Res. 1998;26:3293–3299. doi: 10.1093/nar/26.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko N.A., Welm A.L., Lu X., Timchenko L.T. CUG repeat binding protein (CUGBP1) interacts with the 5′ region of C/EBPβ mRNA and regulates translation of C/EBPβ isoforms. Nucleic Acids Res. 1999;27:4517–4525. doi: 10.1093/nar/27.22.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J.M., Spiess M. Multiple determinants direct the orientation of signal-anchor proteinsthe topogenic role of the hydrophobic signal domain. J. Cell Biol. 1997;137:555–562. doi: 10.1083/jcb.137.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Hirose M., Wang X.-E., Ikejima K., Oide H.T., Kitamura T., Takei Y., Miyazaki A., Sato N. A novel hepatic stellate (Ito) cell-derived protein, epimorphin plays a key role in the late stages of liver regeneration. Biochem. Biophys. Res. Commun. 1998;250:486–490. doi: 10.1006/bbrc.1998.9339. [DOI] [PubMed] [Google Scholar]
- Weimbs T., Low S.H., Chapin S.J., Mostov K.E., Bucher P., Hofmann K. A conserved domain is present in different families of vesicular fusion proteinsa new superfamily. Proc. Natl. Acad. Sci. USA. 1997;94:3046–3051. doi: 10.1073/pnas.94.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welm A.L., Timchenko N.A., Darlington G.J. C/EBPα regulates generation of C/EBPβ isoforms through activation of specific proteolytic cleavage. Mol. Cell. Biol. 1999;19:1695–1704. doi: 10.1128/mcb.19.3.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welm A.L., Mackey S.L., Timchenko L.T., Darlington G.J., Timchenko N.A. Translational induction of liver-enriched transcriptional inhibitory protein during acute phase response leads to repression of CCAAT/enhancer binding protein α mRNA. J. Biol. Chem. 2000;275:27406–27413. doi: 10.1074/jbc.M002343200. [DOI] [PubMed] [Google Scholar]
- Wen D., Peles E., Cupples R., Suggs S.V., Bacus S.S., Luo Y., Trail G., Hu S., Silbiger S.M., Levy R.B. New differentiation factora transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysisregulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Wheeler M.B., Sheu L., Ghai M., Bouquillon A., Grondin G., Weller U., Beaudoin A.R., Bennett M.K., Trimble W.S., Gaisano H.Y. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology. 1996;137:1340–1348. doi: 10.1210/endo.137.4.8625909. [DOI] [PubMed] [Google Scholar]
- Wiesen F.J., Young P., Werb Z., Cunha G.R. Signaling through the epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- Zhu S., Oh H.-S., Shim M., Sterneck E., Johnson P.F., Smart R.C. C/EBPβ modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol. Cell. Biol. 1999;19:7181–7190. doi: 10.1128/mcb.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]