Abstract
Abnormal spindle (Asp) is a 220-kD microtubule-associated protein from Drosophila that has been suggested to be involved in microtubule nucleation from the centrosome. Here, we show that Asp is enriched at the poles of meiotic and mitotic spindles and localizes to the minus ends of central spindle microtubules. Localization to these structures is independent of a functional centrosome. Moreover, colchicine treatment disrupts Asp localization to the centrosome, indicating that Asp is not an integral centrosomal protein. In both meiotic and mitotic divisions of asp mutants, microtubule nucleation occurs from the centrosome, and γ-tubulin localizes correctly. However, spindle pole focusing and organization are severely affected. By examining cells that carry mutations both in asp and in asterless, a gene required for centrosome function, we have determined the role of Asp in the absence of centrosomes. Phenotypic analysis of these double mutants shows that Asp is required for the aggregation of microtubules into focused spindle poles, reinforcing the conclusion that its function at the spindle poles is independent of any putative role in microtubule nucleation. Our data also suggest that Asp has a role in the formation of the central spindle. The inability of asp mutants to correctly organize the central spindle leads to disruption of the contractile ring machinery and failure in cytokinesis.
Keywords: Asp, meiosis, central spindle, cytokinesis, Drosophila melanogaster
Introduction
In most animal cells, spindle formation is mediated by the centrosome, a specialized multiprotein organelle that nucleates astral arrays of microtubules (Zimmerman et al. 1999). Nucleation of microtubules is achieved using templates that contain γ-tubulin, a member of the tubulin family localized to centrosomes. These templates, called γ-tubulin–containing ring complexes (γ-TuRCs), bind with other proteins to an insoluble centrosomal matrix and mediate formation of new microtubules (for reviews see Zimmerman et al. 1999; Gunawardane et al. 2000). However, in most cells the majority of microtubules nucleated by the γ-TuRCs are released from the centrosome and held in the vicinity of the spindle pole through interactions with a minus end–directed motor complex containing dynein, dynactin, and nuclear mitotic apparatus protein (NuMA) (Merdes et al. 1996, Merdes et al. 2000; Gaglio et al. 1997; Heald et al. 1997). At the same time, plus end–directed motors act to align microtubules such that their plus ends are in the vicinity of chromatin (Sawin et al. 1992; Gaglio et al. 1996). The balance set up between the minus end–directed complexes and the plus end–directed motors form a polarized and focused spindle that is capable of chromosome segregation (Gaglio et al. 1996; Compton 1998; Sharp et al. 1999). In systems without centrosomes, the chromatin itself is responsible for microtubule nucleation, but otherwise the mechanism of spindle assembly appears to be similar to that of centrosome-containing cells (Gaglio et al. 1997; Heald et al. 1997; Merdes and Cleveland 1997).
Much less is known about the formation during late anaphase of the central spindle, the bundle of microtubules between the two daughter ana-telophase nuclei. Whether the source of microtubules for this structure is the spindle on which the chromatids have already been separated or whether new microtubules are nucleated at the site of central spindle formation is unclear. However, in Drosophila male meiotic cells of asterless (asl) mutants, which lack functional centrosomes and hence asters, a normal central spindle can self-assemble in the absence of properly oriented microtubules emanating from the spindle poles (Bonaccorsi et al. 1998). In addition, recent data have suggested that a cooperative interaction exists between the central spindle and the contractile ring during Drosophila male meioisis; when one of these structures is disrupted, the proper assembly of the other is also affected (Giansanti et al. 1998; Gatti et al. 2000).
Asp is a microtubule-binding protein of 220 kD that localizes to the polar regions of the mitotic spindle in early embryos and larval neuroblasts of Drosophila (Saunders et al. 1997; Avides and Glover 1999). It possesses cdc2 kinase and MAP kinase phosphorylation sites and putative calmodulin and actin-binding sites (Saunders et al. 1997). Studies carried out on Drosophila embryonic and larval brain cells have shown that this protein is required for correct spindle structure (Gonzalez et al. 1990; Saunders et al. 1997; Avides and Glover 1999). Moreover, recent work on isolated embryonic centrosomes has led to the suggestion that Asp is required for microtubule nucleation in vitro (Avides and Glover 1999). The phenotypic effects of asp mutations have also been examined in male meiosis. It has been reported that asp mutant males exhibit abnormally shaped meiotic spindles and high levels of nondisjunction (Ripoll et al. 1985; Casal et al. 1990; Gonzalez et al. 1990).
Here, we have reevaluated the role of the Asp protein by analyzing its localization during neuroblast mitosis, female meiosis, and male meiosis and by examining the cytological phenotype of asp mutants in greater detail. To gain further insight into the Asp function, we have also studied Asp localization in asl mutants and characterized the cytological phenotype of asl asp double mutants. Taken together, our observations indicate that Asp functions by cross-linking microtubule minus ends during cell division, helping to organize both the spindle poles and the central spindle.
Materials and Methods
Drosophila Stocks
The stocks used in this study were y/y + Y, red asp /TM6 (Ripoll et al. 1985), e aspE3/TM6B (Gonzalez et al. 1990; Carmena et al. 1991), asl /TM6B and asl 2 /TM6B (Bonaccorsi et al. 1998), and the Oregon R wild-type strain that served as control. In addition, we used an asl 2 asp double mutant, which was constructed by recombination and then balanced over TM6B. Homozygous asp larvae were identified by the red malphigian tubules produced by the red mutation. asl 1/asl1, asl 2/asl2, and asl 2asp1/asl2 asp larvae were discriminated from their TM6B-bearing sibs, since they do not exhibit the body shape phenotype elicited by the Tubby-dominant mutation carried by the TM6B balancer. red asp/e aspE3 male larvae were generated by crossing red asp/TM6B males to e aspE3/TM6B females and sorted from TM6B-bearing sibs on the basis of their non-Tubby phenotype. Balancers and markers are described in Lindsley and Zimm 1992. The flies were reared on standard Drosophila medium at 25 ± 1°C, and dissections were performed at room temperature.
Brain and Testis Immunostaining
For brain preparation, we followed the method described by Bonaccorsi et al. 2000b. Testis preparations were made with testes dissected from third instar larvae or young pupae. Testes were fixed according to Bonaccorsi et al. 1998 for γ-tubulin plus α-tubulin imunostaining, to Cenci et al. 1994 for either centrosomin or KLP3A plus α-tubulin immunostaining, and to Gunsalus et al. 1995 for α-tubulin immunostaining followed by actin staining with phalloidin. For Asp plus α-tubulin immunostaining, testes were fixed with formaldehyde and acetic acid as described by Giansanti et al. 1999. All of these testis fixation techniques are described in detail in Bonaccorsi et al. 2000a.
Before incubation with antibodies, slides were rinsed several times in PBS. Both brain and testis preparations were first incubated overnight at 4°C with any of the following rabbit primary antibodies diluted in PBT (PBS containing 0.1% Triton X-100) containing 1% BSA: anti-Asp (1:50; Saunders et al. 1997); anti–γ-tubulin (1:200; Callaini et al. 1997); anticentrosomin (1:500; Li and Kaufman 1996); anti-KLP3A (1:500; Williams et al. 1995). Primary antibodies were detected by a 2-h incubation at room temperature with TRITC-conjugated anti–rabbit IgG (Cappel Laboratories) diluted 1:100 in PBT. Slides were then incubated for 1 h at room temperature with a monoclonal anti–α-tubulin antibody (Amersham Pharmacia Biotech) diluted 1:50 in PBS, which was detected by FLUOS-conjugated sheep anti–mouse IgG (Boehringer) diluted 1:10 in PBS. For actin plus tubulin staining, testes were first immunostained for α-tubulin as described and then incubated with rhodamine-phalloidin (Molecular Probes) for 2 h at 37°C according to Gunsalus et al. 1995. Immunostained preparations were air dried and mounted with Vectashield mounting medium H-1200 with DAPI (Vector Laboratories).
Embryo Immunostaining
0–3-h Drosophila embryos were fixed with methanol and processed for indirect immunofluorescence according to Kellogg et al. 1989. This 0–3-h collection yielded embryos that were sufficiently young to contain meiosis II spindles and older syncytial stage embryos. For colchicine and taxol experiments, 1–3-h embryos were treated with colchicine (5 mM) or taxol (25 μM) for 20 min before fixation as described previously (Raff et al. 1993). Fixed embryos were incubated overnight at 4°C with primary antibodies (anti-Asp 1:50; anti–γ-tubulin 1:500; anti-centrosomin 1:500). In experiments using drug-treated embryos, it was not possible to compare the localization of γ-tubulin (or centrosomin) and Asp in the same embryo, since all of these antibodies were raised in rabbits. Instead, batches of embryos were fixed, split, and stained with the DM1a mouse monoclonal antiboby to α-tubulin (1:500; Sigma-Aldrich) and with either anti-Asp, anti–γ-tubulin, or anticentrosomin. RNase A was included with the primary antibodies, and propidium iodide was used to stain the DNA (Gonzalez and Glover 1993). Cy5 anti–rabbit (Jackson ImmunoResearch Laboratories) and Alexa 488 anti–mouse (Molecular Probes) secondary antibodies were used at 1:500.
Microscopy
All brain and testis preparations were examined with an Axioplan (ZEISS) microscope equipped with an HBO 50-W mercury lamp for epifluorescence and with a cooled charge-coupled device (CCD; Photometrics Inc.). DAPI, FLUOS, and TRITC fluorescence were detected as described (Gunsalus et al. 1995; Giansanti et al. 1999; Bonaccorsi et al. 2000b). Grayscale digital images were collected separately using IP Lab Spectrum software. Embryo imaging was performed using a Bio-Rad Laboratories MRC 1024 scanning confocal head as described previously (Gergely et al. 2000). Images were then converted to Photoshop® 2.5 or 4 format (Adobe® System, Inc.), pseudocolored, and merged.
Results
Asp Is Enriched at the Minus Ends of Spindle Microtubules
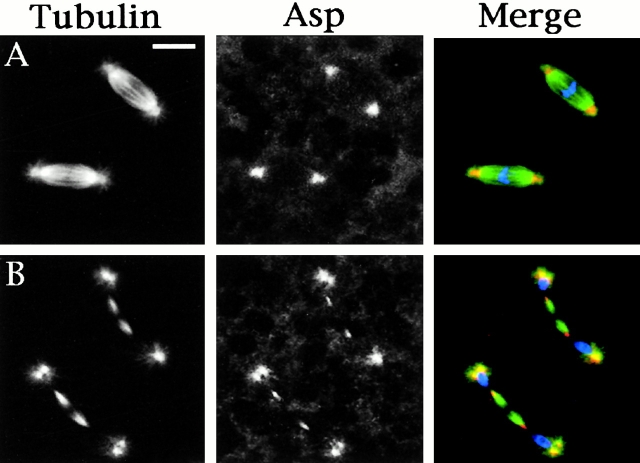
Meiotic spindles of Drosophila males are much larger than those of mitotic cells and particularly suitable for immunolocalization studies (Bonaccorsi et al. 2000a). Thus, we initially analyzed Asp localization during Drosophila male meiosis. Wild-type testes were immunostained both with an antibody produced against the NH2-terminal portion of Asp (Saunders et al. 1997) and with an antibody to α-tubulin to label the microtubules (Fig. 1). During meiotic interphase I, the Asp antibodies stain the nucleus (not shown), but upon entry into the first meiotic division the signal relocates to the cytoplasm and weakly illuminates the spindle poles. As meiosis proceeds, the spindle pole staining increases until the spindle has formed completely (Fig. 1 A). After the chromosomes have been segregated, the polar staining of Asp begins to fade (Fig. 1 B). During late anaphase and telophase I, Asp dramatically relocalizes to the minus ends of the central spindle microtubules (Fig. 1C and Fig. D). This central spindle staining remains until the central spindle is disassembled after cytokinesis. In addition to microtubules, the antibodies also localize to the reforming daughter nuclei during anaphase and telophase I (Fig. 1C and Fig. D). An identical immunostaining pattern was observed in secondary spermatocytes undergoing the second meiotic division (not shown).
Figure 1.
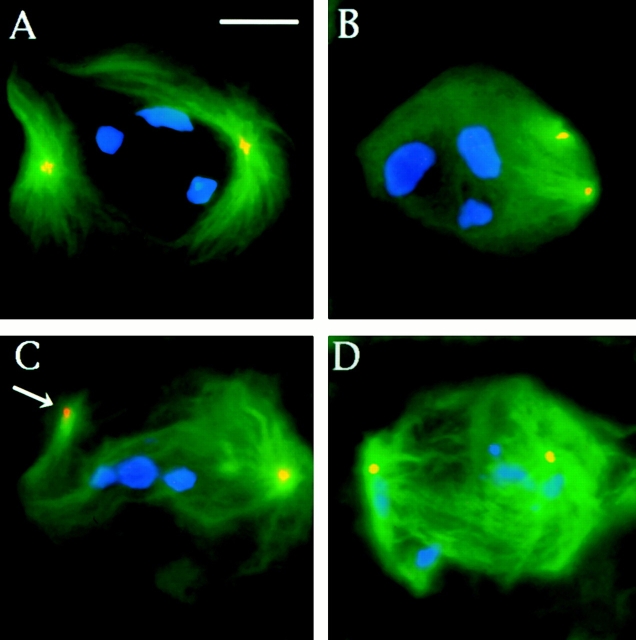
Asp localization during the first meiotic division of wild-type, asp, and asl males. Cells were fixed according to Giansanti et al. 1999 (described in Materials and Methods) and stained for α-tubulin, Asp, and DNA (by DAPI). (A–D) Wild-type meiotic cells. (A) Metaphase; (B) early anaphase; (C) late anaphase; (D) telophase. Note that in metaphase (A) figures, Asp is localized at the spindle poles. In telophase cells (D), Asp predominantly accumulates at the extremities of the central spindle but not in the central spindle midzone. (E) An asl anaphase-like figure showing Asp staining at the spindle poles. (F) An asl telophase with a well-organized central spindle showing a regular Asp immunostaining at its extremities. Bar, 10 μm.
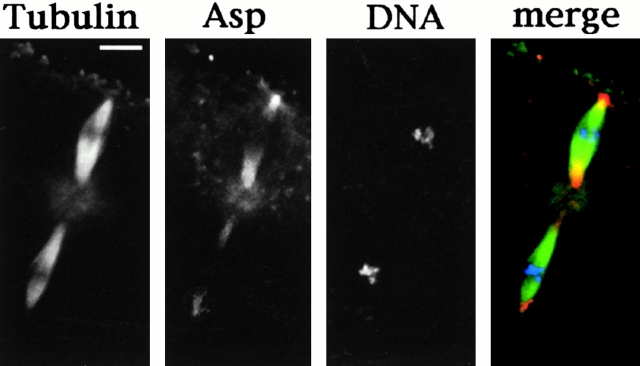
To investigate whether Asp localizes to the minus ends of central spindle microtubules in other cell types, we stained early Drosophila embryos and larval neuroblasts with the anti-Asp antibody. In agreement with previous studies, we found that during spindle formation Asp is present at centrosomes and at the area of the spindle adjacent to the centrosome (Fig. 2 A and Fig. 3A and Fig. B; Saunders et al. 1997; Avides and Glover 1999). However, in addition Asp exhibits a striking enrichment at the minus ends of central spindle microtubules similar to that seen during male meiosis (Fig. 2 B and Fig. 3 C). Interestingly, in both embryonic and neuroblast telophases the two daughter nuclei are not immunostained by the anti-Asp antibody, indicating that the nuclear staining observed in meiotic telophases is a peculiar feature of this type of cell.
Figure 2.
Asp localization in cells of wild-type Drosophila embryos. In the merged images, DNA is colored in blue, tubulin in green, and Asp in orange. (A) Metaphases showing Asp localization at the spindle poles; (B) telophases showing Asp accumulation at the minus ends of central spindle microtubules. Bar, 10 μm.
Figure 3.
Asp localization in neuroblasts of wild-type and asl larval brains. Cells were stained for α-tubulin, Asp, and DNA (by DAPI). In the merged images, DNA is colored in blue, tubulin in green, and Asp in orange. (A–C) Wild-type neuroblasts; (D–F) asl neuroblasts; (A and D) metaphases; (B and E) anaphases; (C and F) telophases. Note that both wild-type and asl neuroblasts divide asymmetrically, giving rise to two daughter cells of different sizes (C and F). For details on unequal neuroblast division see Giansanti et al. 2001. In metaphase and anaphase figures, Asp is localized at the spindle poles, wheras in telophases (C and F) it accumulates at both the poles and the extremities of the central spindle. Note that wild-type and asl neuroblasts exhibit similar patterns of Asp accumulation. Bar, 5 μm.
Asp Accumulates at the Minus Ends of Microtubules in Cells Lacking Functional Centrosomes
To determine whether centrosomes are required for Asp localization to the spindle poles and to the outer regions of the central spindle, we examined cells in which functional centrosomes are absent. In Drosophila, two such cell types have been described: oocytes undergoing female meiosis (Theurkauf and Hawley 1992) and cells that carry a mutation in the asl gene (Bonaccorsi et al. 1998, Bonaccorsi et al. 2000b).
Drosophila female meiosis is mediated by anastral spindles assembled from microtubules nucleated near the chromosomes (Theurkauf and Hawley 1992). During meiosis II, two spindles are tandemly arranged, perpendicular to the oocyte cortex (Riparbelli and Callaini 1996; Endow and Komma 1997). Between these two spindles there is an aster-like structure that contains centrosomal components such as γ-tubulin and CP190 (Riparbelli and Callaini 1996, Riparbelli and Callaini 1998). In contrast, the two outer poles, although well focused, do not contain any reported centrosomal or microtubule-associated proteins (Theurkauf and Hawley 1992; Matthies et al. 1996; Riparbelli and Callaini 1996, Riparbelli and Callaini 1998). When oocytes possessing these meiotic II spindles were stained with the Asp antibody, the central aster was recognized only weakly. However, the regions corresponding to the minus ends of the focused spindle poles showed strong Asp staining (Fig. 4).
Figure 4.
Asp localization in cells undergoing the second meiotic division in wild-type Drosophila oocytes. In the merged image, DNA is colored in blue, tubulin in green, and Asp in orange. Note that Asp accumulates at the poles of both meiotic spindles. Bar, 10 μm.
asl spermatocytes and larval neuroblasts are both defective in centrosome structure and fail to nucleate astral microtubules. In both cell types, the loss of centrosomal integrity leads to the formation of anastral spindles that are built around the chromatin. In asl spermatocytes, meiotic spindles are very poorly focused; chromosomes fail to align on a metaphase plate and often missegregate. Nonetheless, they assemble perfectly normal central spindles that are able to stimulate cytokinesis (Bonaccorsi et al. 1998; Fig. 1 F). The anastral spindles of asl neuroblasts are, in contrast, very well focused and are able to mediate cell division correctly (Bonaccorsi et al. 2000b; Giansanti et al. 2001; Fig. 3, D–F). The localization of Asp in asl spermatocytes and asl neuroblasts is shown in Fig. 1E and Fig. F and Fig. 3D–F, respectively. In metaphase I asl spermatocytes, a clear Asp signal is present at the poorly focused spindle poles (Fig. 1 E). During meiotic telophase I, the anti-Asp antibody immunostains the minus ends of the central spindle microtubules (Fig. 1 F). Similarly, in asl mutant neuroblasts undergoing either metaphase, anaphase, or telophase (Fig. 3, D–F) Asp localization is comparable to that seen in wild-type controls (Fig. 3, A–C). Thus, on the basis of our observations both of female meiosis and of asl mutant cells we conclude that the Asp protein does not require centrosomes to accumulate at or near the minus ends of spindle microtubules.
Asp Is Not Present at the Centrosome in the Absence of Microtubules
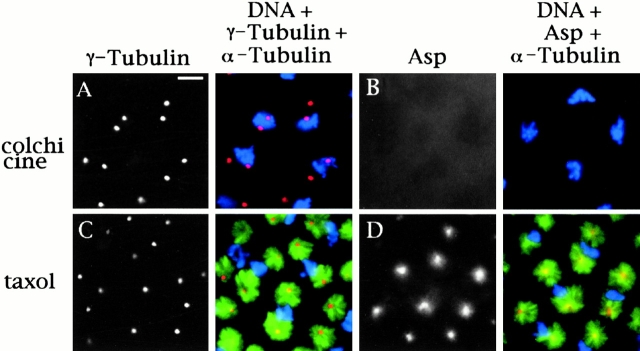
To understand the basis of Asp localization to the centrosome in greater detail, we treated embryos either with colchicine or taxol (to depolymerize or stabilize microtubules, respectively), and then compared the localization of Asp to that of γ-tubulin. As shown in Fig. 5, when microtubules are depolymerized or stabilized γ-tubulin staining remains tightly associated with the centrosomes. Similar results (not shown) were obtained using antibodies to the integral centrosomal protein centrosomin (Li and Kaufman 1996). However, Asp was not visible at the centrosomes in embryos treated with colchicine (Fig. 5). Furthermore, in embryos treated with taxol Asp staining was not restricted to the centrosome but included a broader region at the minus ends of the stabilized microtubules (Fig. 5). Asp is therefore not an integral centrosomal component but is dependent upon microtubules for its localization to the centrosome.
Figure 5.
Localization of Asp in embryos treated with colchicine or taxol. Embryos were incubated in 5 mM colchicine or 25 μM taxol for 20 min before fixation (described in Materials and Methods). Embryos from the same batch were stained with antibodies to either γ- and α-tubulin (A and C) or Asp and α-tubulin (B and D) and with propidium iodide to show the mitotic chromatin (A–D). In the merged images, chromatin is colored in blue, tubulin in green, and Asp or γ-tubulin in red/orange. (A and B) 5 mM colchicine. (A) α-Tubulin staining is diffuse, suggesting all microtubules have been depolymerised. γ-Tubulin, as an integral centrosomal protein, is tightly focused at the centrosome. (B) α-Tubulin is not detectable, and Asp does not show any centrosomal localization. (C and D) 25 μM taxol. (C) Microtubules are stabilized and form astral arrays surrounding each centrosome. γ-Tubulin is found only at the centrosomes. (D) Asp staining is found throughout the area corresponding to the minus ends of the microtubules, and it is not focused at the centrosome. Bar, 10 μm.
The Role of Asp in Neuroblast Spindle Assembly
To define the functional role of Asp in neuroblast spindle formation, we examined cell division in larval brains of asp 1/asp1 homozygotes and asp 1/aspE3 heterozygotes. The asp and asp E3 alleles are both semilethal mutations; they are two of the strongest extant asp mutant alleles and exhibit comparable mitotic phenotypes (Gonzalez et al. 1989b; Carmena et al. 1991; White-Cooper et al. 1996). When we looked at brains of asp 1/asp1 and asp 1/aspE3 larvae, we also observed very similar phenotypes (data not shown). Thus, we decided to focus on asp 1/asp1 neuroblasts for detailed cytological analysis.
In asp brains, most dividing neuroblasts arrest in metaphase. To analyze the structure of the spindle poles, we double stained both wild-type and asp mutant brains for either α- and γ-tubulin or α-tubulin and centrosomin. We found that the γ-tubulin and centrosomin signals observed in asp metaphases are of similar intensity to those of controls (Fig. 6, A–D). However, although there is always a single signal at the spindle poles of control metaphases (Fig. 6A and Fig. C), in ∼40% of asp metaphases we see two centrosomal signals at each pole (Fig. 6 B). These results indicate that asp mutants normally accumulate γ-tubulin and centrosomin at their centrosomes. In addition, they show that in a substantial fraction of the asp metaphases there is a precocious centrosome splitting.
Figure 6.
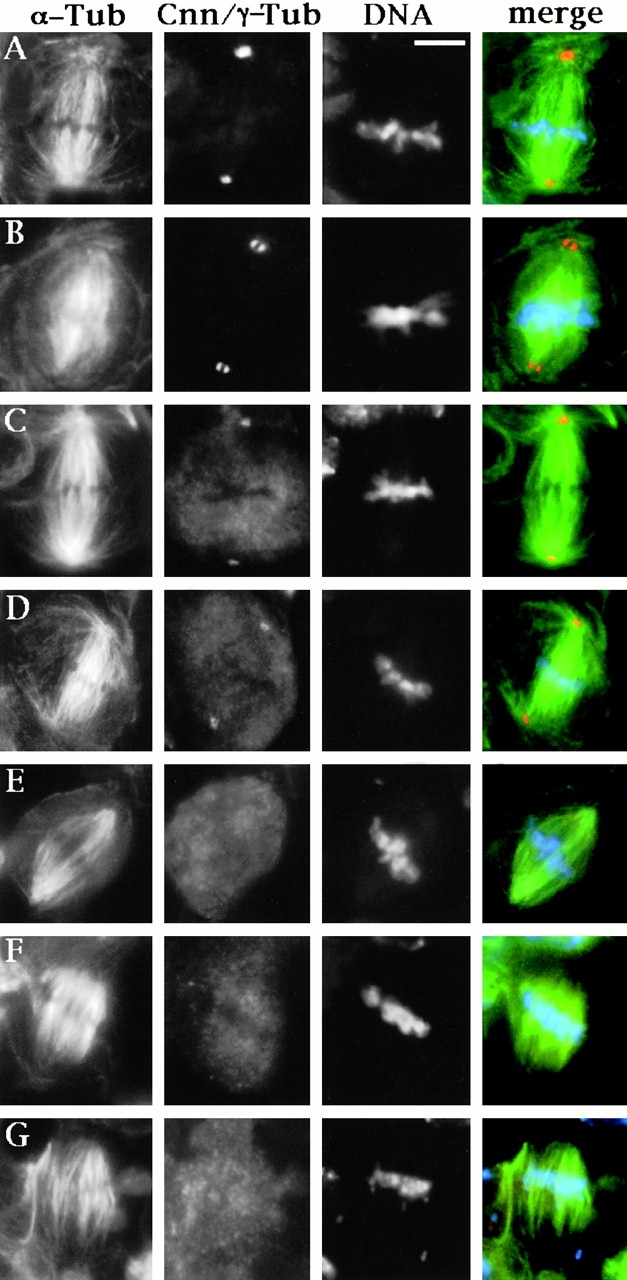
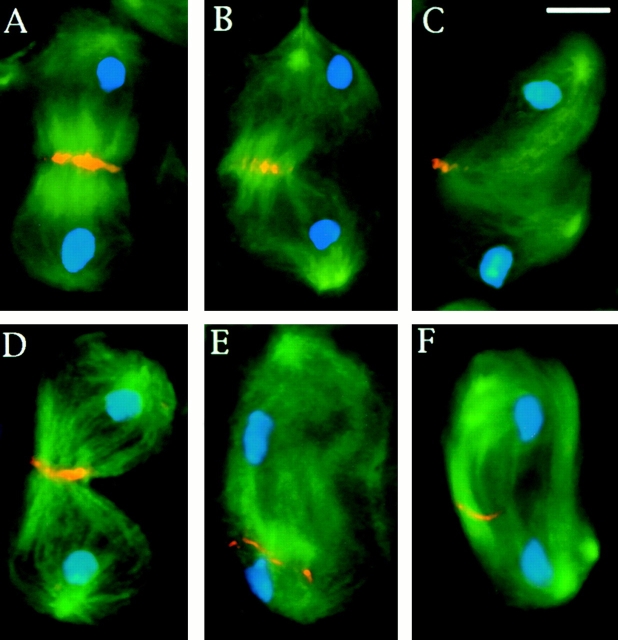
Spindle pole organization in asp neuroblast metaphases. Cells were stained for α-tubulin, DNA, and either centrosomin (Cnn; A and B) or γ-tubulin (C–G). In the merged images, chromatin is colored in blue, α-tubulin in green, and Cnn or γ-tubulin in orange. (A and C) Wild-type neuroblast metaphases; (B and D) asp; (E) asl 2; (F and G) asl 2 asp neuroblast metaphases. Note in B the splitting of the Cnn signal and in E–G the lack of γ-tubulin accumulation in the centrosomes. The anastral asl 2 mutant metaphases (E) exhibit well-focused spindle poles. By contrast, in asl 2 asp metaphases spindle microtubules fail to converge into the poles and are splayed outward (F and G). Bar, 5 μm.
The precocious centrosome separation observed in asp metaphases is not surprising, since asp-dividing neuroblasts remain arrested in metaphase for a long time. This is likely to ungear the centrosome cycle from the spindle dynamics, leading to centrosome splitting in metaphase. Our results are in apparent contrast with those of Avides and Glover 1999 who reported that asp mutant neuroblasts have disorganized γ-tubulin clumps at the spindle poles. However, to document their data they showed an asp neuroblast metaphase with two γ-tubulin spots at each pole. It is thus likely that they interpreted the precocious centrosome separation that occurs in asp metaphases as an aberrant γ-tubulin distribution at the spindle poles caused by its dissociation from the centrosome.
Although asp and wild-type metaphases contain similar amounts of centrosomal material, mutant metaphases have fewer and generally shorter astral microtubules than their wild-type counterparts (Fig. 6, A–D). Moreover, in asp metaphases many spindle microtubules are poorly focused and do not appear to terminate at the centrosome (compare Fig. 6A and Fig. C with B and D). One interpretation of these observations is that Asp is required for correct microtubule nucleation from the centrosome. Alternatively, Asp could function directly at the microtubule minus ends, mediating microtubule focusing and centrosome attachment to the spindle poles.
By examining the cytological phenotype of asl 2 asp double mutants, we sought to discriminate between these possibilities. In asl 2 mutants, despite the absence of functional centrosomes and astral microtubules, metaphase spindles of larval neuroblasts are very well focused at their poles (Fig. 6 E; see also Bonaccorsi et al. 2000b). In contrast, in neuroblast metaphases of asl asp double mutants the microtubule minus ends are splayed apart and fail to converge into focused spindle poles (Fig. 6F and Fig. G). This suggests that the poorly focused spindles observed in asp mutants are not a consequence of centrosome abnormalities. Instead, the above results indicate that Asp is a microtubule minus end–associated protein that mediates spindle pole formation independently of centrosomes.
The Role of Asp during Male Meiosis
Drosophila male meiosis offers a significant advantage over mitotic larval brain cells for the phenotypic analysis of mutants defective in spindle formation. In larval brain cells, the presence of disorganized spindles activates the spindle integrity checkpoint, precluding the observation of cell division subsequent to arrested metaphases (Avides and Glover 1999). However, in spermatocytes the spindle checkpoint is not stringent and causes only a small delay in progression through meiosis (Basu et al. 1999; Rebollo and Gonzalez 2000). Thus, by analyzing meiotic divisions in asp males, in addition to the role of asp in spindle pole formation, we could also address its role in central spindle assembly.
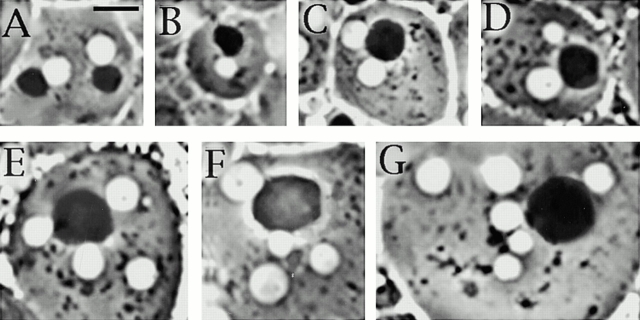
As an initial step to characterize male meiosis in asp mutants, we analyzed “onion stage” spermatids in living preparations of asp 1/asp1 and asp 1/aspE3 mutant testes by phase–contrast microscopy. Because these males displayed comparable meiotic abnormalities, we focused on asp 1/asp1 testes for detailed cytological analysis. In wild type, each spermatid consists of one phase–light nucleus and one phase–dense mitochondrial derivative (called the Nebenkern) of similar size (for review see Fuller 1993). The regular size of both nuclei and Nebenkern depends on the correct execution of both meiotic divisions. Errors in chromosome segregation result in the formation of nuclei of abnormal size (Gonzalez et al. 1989a). Failures in cytokinesis produce aberrant spermatids composed of an abnormally large Nebenkern associated with two or four nuclei of regular size (Fuller 1993).
In asp mutant males (Fig. 7 and Table ), 41% of the spermatids consist of a normal-sized nucleus associated with a normal Nebenkern and are likely to be the product of regular meiotic divisions. 15% of the spermatids contain a normal Nebenkern associated with an abnormally sized nucleus and are probably the consequence of a failure in chromosome segregation but not in cytokinesis. Conversely, 23% of spermatids result from failures in cytokinesis but not in chromosome segregation, since they exhibit two or four normal-sized nuclei associated with a single Nebenkern of twice or four times the size of a normal Nebenkern, respectively. Finally, we observed spermatids consisting of a single large Nebenkern associated with two (12%), four (3%), or more than four (6%) nuclei of different sizes. These peculiar spermatids are likely to be the consequence of failures in both chromosome segregation and cytokinesis. Taken together, these results strongly suggest that asp mutants are defective in both processes.
Figure 7.
Abnormal spermatids observed in living asp mutant testes. (A) Two regular spermatids showing nuclei (white circles) and Nebenkern (dark circles) of similar sizes. (B) An abnormal spermatid with the nucleus smaller than the Nebenkern. (C and D) Spermatids with two nuclei of similar sizes (C) or of different sizes (D) associated with a single Nebenkern, which is twice the size of a normal Nebenkern. (E and F) Abnormal spermatids containing four equally sized (E) or differently sized (F) nuclei associated with only one Nebenkern that is four times larger than a regular Nebenkern. (G) A spermatid containing only one abnormally large Nebenkern associated with multiple nuclei. Bar, 10 μm.
Table 1.
Abnormal Spermatid Morphology in asp1 Mutants
| Genotype | Number of spermatids | Type of spermatids (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:1 | 2:1 | 4:1 | Other irregular | Total irregular | |||||
| A | B | A | B | A | B | ||||
| asp1/asp1 | 925 | 41.2 | 14.9 | 18.8 | 12.2 | 3.9 | 3.0 | 6.0 | 58.8 |
| Oregon R | 500 | 99.6 | 0.2 | 0.2 | 0 | 0 | 0 | 0 | 0.4 |
To determine the primary defects that cause the formation of aberrant spermatids, we examined meiotic cell division in asp males by staining fixed preparations for chromatin, α-tubulin, and either γ-tubulin or centrosomin. Several phenotypes were observed. First, during prometaphase the asters are smaller than their wild-type counterparts and often are not attached to the nuclear envelope as in wild type. In addition, the two asters are usually close to each other, suggesting a delayed migration to the opposite sides of the nucleus (Fig. 8, compare A and B). Second, as described previously asp spermatocytes form abnormally shaped and poorly focused bipolar spindles (Fig. 8C and Fig. D; Casal et al. 1990; Gonzalez et al. 1990). Third, in many cells the centrosomes do not appear to be attached to the ends of these spindles but float free in the cytosol (Fig. 8 C). Fourth, γ-tubulin is present at the centrosomes in asp spermatocytes throughout meiosis, and we could detect no difference in immunostaining intensity between asp and wild-type cells (Fig. 8). Staining with antibodies to centrosomin gave similar results (not shown). Thus, asp spermatocytes behave similarly to asp neuroblasts. Although they have small asters and poorly focused spindle poles, their centrosomes recruit normal amounts of γ-tubulin and centrosomin.
Figure 8.
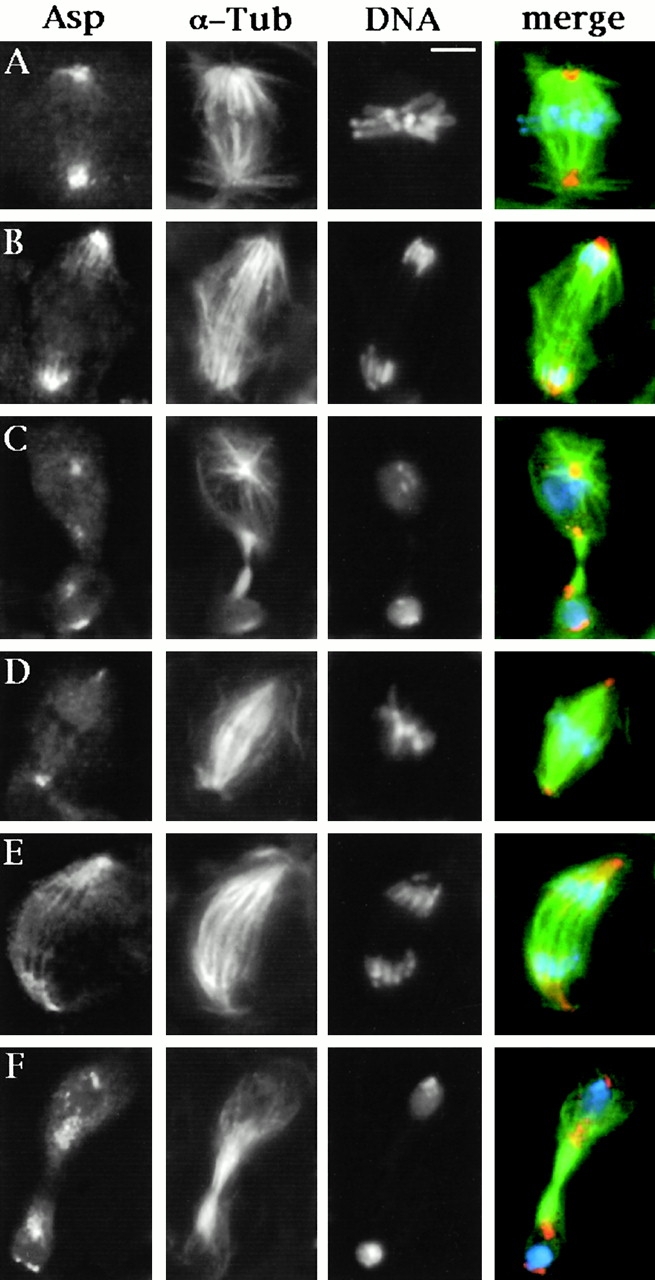
Aster positioning and spindle pole structure in asp primary spermatocytes. Cells were fixed according to Bonaccorsi et al. 1998 (described in Materials and Methods) and stained for DNA (blue), α-tubulin (green), and γ-tubulin (orange). (A) A wild-type prophase/prometaphase at the M1 stage showing two robust asters closely apposed to the nuclear envelope and located at the opposite sides of the nucleus. (B) An asp prophase/prometaphase with small asters detached from the nucleus that have failed to migrate to the cell poles. (C) An asp prometaphase-like figure with one centrosome (arrow) detached from a spindle pole. (D) An anaphase-like figure with broad spindle poles and abnormally segregating chromosomes. Note that the centrosomes of the asp mutant cells accumulate normal amounts of γ-tubulin. Bar, 10 μm.
To determine whether the Asp protein plays a role in central spindle assembly, we examined asp telophases for the presence and normality of the central spindle (Table and Fig. 9). Although central spindle morphology looked rather normal in about one half of the telophases, in the other half it was severely affected. In a fraction of the abnormal telophases, the central spindle fails to form completely, whereas in the remaining cells some interzonal microtubules can be discerned, which are organized in small and irregular bundles that do not completely traverse the cell (Fig. 9E and Fig. F). To better define central spindle structure in asp mutant telophases, we immunostained asp testes for KLP3A, a plus end–directed microtubule motor that localizes to the equatorial region of the central spindle and is required for meiotic cytokinesis (Williams et al. 1995). We observed a KLP3A distribution that reflects the organization of interzonal microtubules; this protein was not detected in areas with sparse and unbundled microtubules but accumulated in the center of the irregular microtubule bundles (Table and Fig. 9B and Fig. C). We also examined the actin-enriched contractile ring of asp telophases by staining cells with rhodamine-phalloidin. In wild type, this procedure reveals a clear acto-myosin ring that surrounds the central spindle midzone (Gunsalus et al. 1995; Giansanti et al. 1998; Fig. 9 D). In asp telophases with a normal central spindle, actin localized correctly at the cell equator. However, in cells where the central spindle failed to form properly actin localization was severely disrupted (Fig. 9E and Fig. F), and where the central spindle was completely absent we did not observe any actin staining.
Table 2.
Central Spindle Defects in asp1 Telophases Immunostained for Tubulin and KLP3A
| Genotype | Number of telophases | Types of central spindles (%) | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | Total irregular | ||
| asp1/asp1 | 74 | 52.7 | 28.3 | 14.9 | 4.1 | 47.3 |
| Oregon R | 68 | 98.5 | 1.5 | 0 | 0 | 1.5 |
Figure 9.
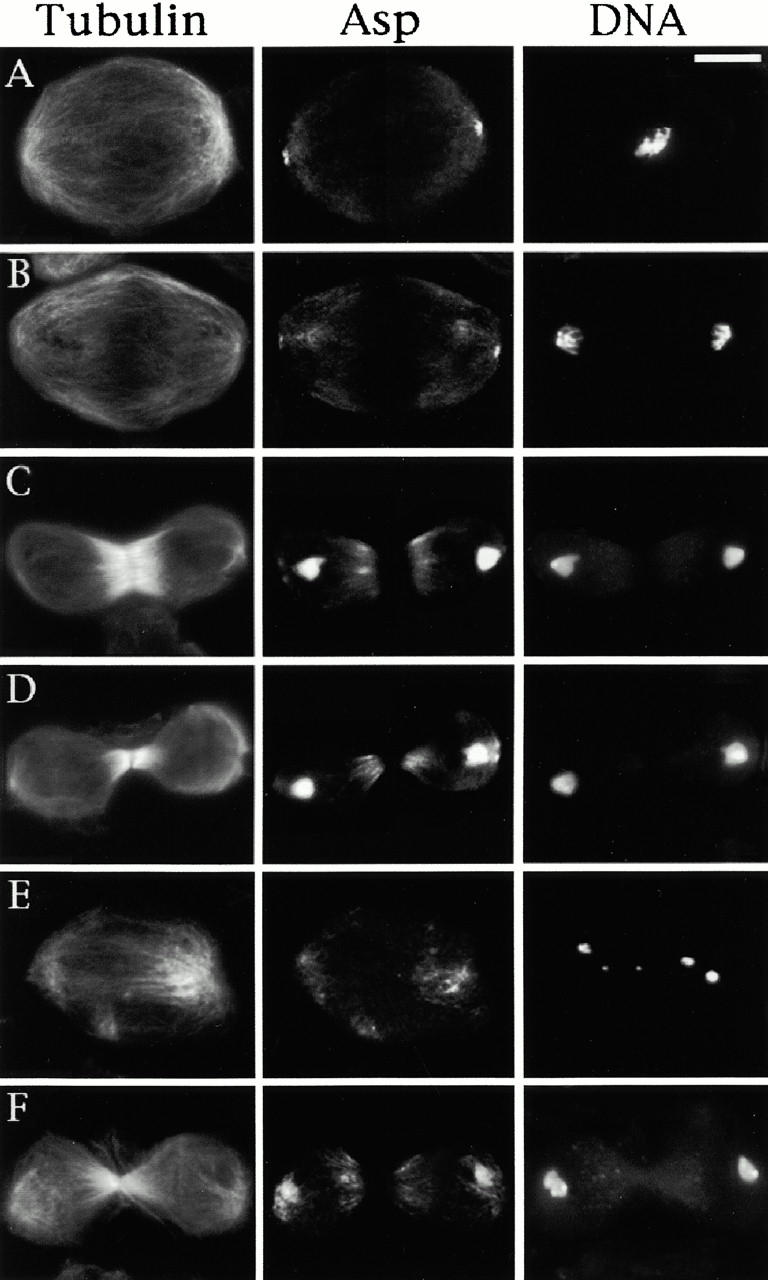
Central spindle morphology and acto-myosin ring formation in meiotic telophases I of asp males. (A–C) Cells fixed according to Cenci et al. 1994 (described in Materials and Methods) and stained for DNA (blue), α-tubulin (green), and KLP3A (orange). (A) A wild-type telophase showing a prominent central spindle displaying a normal KLP3A accumulation at its midzone. (B and C) asp telophases with abnormal central spindles. In B, the central spindle microtubules are irregularly arranged, and KLP3A accumulation in the midzone is partially disrupted. In C, the central spindle is virtually absent, and only clumps of KLP3A can be observed. (D–F) Cells fixed according to Gunsalus et al. 1995 (described in Materials and Methods) stained for DNA (blue), α-tubulin (green), and F actin (orange). (D) A wild-type telophase displaying a normal actin-enriched contractile ring. (E and F) asp telophases with poorly organized central spindles and irregularly positioned and incomplete actin rings. Bar, 10 μm.
The defects in central spindle formation observed in asp mutants could be either a secondary consequence of a global disorganization of the meiotic spindle or a direct consequence of an impairment of the Asp function. To discriminate between these possibilities, we examined larval testes of asl 2 asp double mutants. In asl 2/asl 2 testes, telophases display a morphologically normal central spindle and cytokinesis occurs normally (Bonaccorsi et al. 1998; data not shown). In the asl 2 asp double mutant, the frequency of cells undergoing meiosis is similar to that observed in asl 2/asl 2 homozygotes. However, in contrast to asl single mutants most if not all asl 2 asp double mutant telophase-like figures have highly disorganized central spindles, which exhibit only small and irregular KLP3A and F actin patches (data not shown). These results support the hypothesis that Asp contributes towards central spindle organization during anaphase and telophase. The defects in central spindle formation could then disrupt the assembly of the contractile apparatus, causing defective cytokinesis.
Discussion
The Role of Asp in Spindle Pole Organization
We have shown that Asp accumulates at the spindle poles of both mitotic and meiotic Drosophila cells. This result is consistent with those obtained previously on embryonic and larval brain cells (Saunders et al. 1997; Avides and Glover 1999). In addition, we have demonstrated for the first time that cells that are devoid of functional centrosomes still accumulate Asp at the spindle poles. Furthermore, we have shown that in the absence of microtubules Asp does not accumulate at the centrosome. These findings suggest Asp may function at the minus ends of mitotic and meiotic spindles rather than as part of the core centrosome.
We have shown that both centrosome-containing asp mutant neuroblasts and spermatocytes display common phenotypical features. They form smaller astral microtubule arrays than their wild- type counterparts, have poorly focused spindle poles, and accumulate normal amounts of γ-tubulin and centrosomin at their centrosomes. In addition, in a substantial fraction of mutant cells centrosomes are disconnected from the rest of the spindle. Previous studies have shown that free centrosomes can also be observed in embryos produced by homozygous asp mothers (Gonzalez et al. 1990). An interpretation consistent with all the phenotypes observed in asp mutant cells is that the Asp protein mediates interactions between microtubule minus ends and the centrosome. In asp mutants, the microtubules would be normally nucleated by the centrosome but would not be held in its vicinity after their release from this organelle. This would account for the small size of asters and for the detachment of the centrosomes from the spindle poles.
This interpretation is supported by the analysis of neuroblast division in asl asp double mutants, which provides a trenchant demonstration of Asp function in spindle pole formation in the absence of centrosomes. In asl mutant neuroblasts, despite the absence of functional centrosomes and astral microtubule arrays, microtubule minus ends converge into well-focused spindle poles. However, when the asl and asp functions are simultaneously impaired, the microtubule minus ends are splayed outward and fail to converge into poles. This indicates that Asp plays a role in microtubule focusing at the spindle poles that is independent of any putative function in microtubule nucleation.
If our results indicate that Asp plays an essential role in microtubule bundling at the spindle poles, how can we reconcile them with the in vitro experiments using purified centrosomes that point towards a microtubule nucleating function for Asp (Avides and Glover 1999)? In those in vitro experiments, embryo extracts were added to purified salt-stripped centrosomes, and microtubule nucleation from the centrosome was measured. When wild-type extract was added, asters were formed. However, when either asp extracts or extracts immunodepleted of Asp were used asters did not form. Instead, many linear arrays of microtubules were seen that did not appear to have a focus. This led the authors to suggest that Asp functions by holding together γ-TuRCs at the centrosome, thus facilitating microtubule nucleation. Clearly, this hypothesis cannot fully account for the results described here. An alternative model is that purified centrosomes do nucleate microtubules in an asp mutant background but that the microtubules are released very soon afterwards where they could continue to grow, producing the linear arrays of microtubules.
The functions we have proposed for Asp during spindle formation are very similar to those thought to be played by the dynein–dynactin–NuMA complex in a variety of vertebrate cell systems (Merdes and Cleveland 1997; Compton 1998). Disruption of the activity of either dynein or NuMA during the in vitro assembly of Xenopus acentrosomal spindles results in splayed spindle poles comparable to those seen in asl asp double mutants (Heald et al. 1996, Heald et al. 1997; Merdes et al. 2000). When the function of either dynein, dynactin, or NuMA is disrupted in centrosome-containing systems, the centrosomes retain their nucleating ability and form small asters, but spindle pole organization is disrupted; spindles display unfocused poles, which are often disconnected from the centrosomes (Gaglio et al. 1995; Echeverri et al. 1996; Merdes et al. 1996; Gaglio et al. 1997; Heald et al. 1997; Quintyne et al. 1999). These findings suggest that vertebrates and Drosophila exploit similar mechanisms for microtubule tethering at the spindle poles and that NuMA and Asp play similar roles in these processes.
The Role of Asp in Central Spindle Assembly and Cytokinesis
Besides its function at the spindle poles, Asp appears to perform an additional function during central spindle assembly. We have shown that Asp is not uniformly distributed along the central spindle but exhibits a striking enrichment at the microtubule minus ends of this structure. Many proteins have been found to bind to the equatorial region of the central spindle, and some of them have been shown to be necessary for central spindle stability (for review see Gatti et al. 2000). In contrast, as far as we are aware only two proteins, Asp and γ-tubulin, are known to accumulate at the minus ends of central spindle microtubules. The localization of γ-tubulin to the central spindle extremities has been seen only in mammalian cells. Accordingly, γ-tubulin depletion by either antibody injection or antisense RNA disrupts the central spindle and causes failure in cytokinesis (Julian et al. 1993; Shu et al. 1995). However, we and others have never been able to detect the presence of γ-tubulin at the central spindle extremities in various Drosophila cell types (Raff et al. 1993; Callaini et al. 1997; Bonaccorsi et al. 1998; Bonaccorsi et al. 2000b). This suggests that in Drosophila the role played by γ-tubulin in mammalian central spindle assembly is fulfilled by other proteins, one of which could be Asp.
When Asp function is disrupted, a large fraction of spermatocyte telophases display severe defects in central spindle morphology. These defects are unlikely to be an indirect consequence of abnormal microtubule organization in earlier stages of meiotic cell division in asp mutants. asl spermatocytes, which exhibit highly disorganized metaphase and anaphase spindles, nonetheless form central spindles that are undistinguishable from their wild-type counterparts (Fig. 1 F; Bonaccorsi et al. 1998). However, in asl asp double mutants, although metaphase and anaphase spindles are comparable to those of asl single mutants, central spindles either fail to form or are severely defective. Thus, we conclude that the Asp protein plays a specific role in central spindle organization and stabilization.
The abnormalities seen in the central spindle of asp spermatocytes suggest that the Asp protein may help to cross-link the minus ends of central spindle microtubules, preventing them from sliding and splaying apart. Central spindle assembly during male meiosis also requires KLP3A, a kinesin-like protein that accumulates in the central spindle midzone (Williams et al. 1995). It is therefore likely that central spindle formation depends on both plus end– and minus end–associated proteins, which work in concert to ensure proper orientation, alignment, and stabilization of central spindle microtubules.
There is growing evidence that the assembly of the contractile ring depends on the integrity of the central spindle (for review see Gatti et al. 2000). Our observations on asp mutant telophases provide additional support for this view. In asp telophases with a normal central spindle, we also observe an apparently regular F actin–enriched ring. In cells where the central spindle is completely disrupted, there is no F actin accumulation at the cell equator. Finally, in asp telophases that possess irregular bundles of microtubules between the two daughter nuclei, we usually observe patches of F actin that are always associated with bundled microtubules but which never form a continuous contractile ring. We thus conclude that the failures in cytokinesis observed in asp spermatids are brought about by a primary defect in central spindle organization that leads to a partial or complete failure in the acto-myosin ring assembly.
To summarize, we propose that Asp binds at or near the microtubule minus ends of both the spindle poles and the central spindle. At the spindle poles, Asp may cross-link the microtubules, ensuring both their focusing into polarized arrays and their attachment to centrosomes after release from these nucleating centers. During anaphase and telophase, Asp could also serve to cross-link the minus ends of central spindle microtubules, thus stabilizing this structure and allowing formation of the acto-myosin contractile apparatus required for cytokinesis.
Acknowledgments
We thank D.M. Glover, W.G. Whitfield, T.C. Kaufman, and B.C. Williams for anti-Asp, anti–γ-tubulin, anticentrosomin, and anti-KLP3A antibodies, respectively, S. Pimpinelli and P. Ripoll for the asp mutant strains, and M.L. Goldberg for comments on the manuscript. We also thank J.W. Raff in whose laboratory the embryo experiments were initially carried out.
J.G. Wakefield was supported by a Training and Mobility of Researchers grant of the European Union.
Footnotes
J.G. Wakefield's present address is Department of Biochemistry, School of Medical Sciences, University of Bristol, Bristol BS8 1TD, UK.
Abbreviations used in this paper: asl, asterless; Asp, abnormal spindle; γ-TuRC, γ-tubulin–containing ring complex; NuMA, nuclear mitotic apparatus protein.
References
- Avides M.C., Glover D.M. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 1999;283:1733–1735. doi: 10.1126/science.283.5408.1733. [DOI] [PubMed] [Google Scholar]
- Basu J., Bousbaa H., Logarinho E., Li Z., Williams B.C., Lopes C., Sunkel C., Goldberg M.L. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila . J. Cell Biol. 1999;146:13–28. doi: 10.1083/jcb.146.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi S., Giansanti M.G., Gatti M. Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster . J. Cell Biol. 1998;142:751–761. doi: 10.1083/jcb.142.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi S., Giansanti M.G., Cenci G., Gatti M. Cytological analysis of spermatocyte growth and male meiosis in Drosophila melanogaster. Sullivan W., Ashburner M., Hawley R.S. Drosophila Protocols 2000. 87 109 Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: a [Google Scholar]
- Bonaccorsi S., Giansanti M.G., Gatti M. Spindle assembly in Drosophila neuroblasts and ganglion mother cells Nat. Cell Biol. 2 2000. 54 56b [DOI] [PubMed] [Google Scholar]
- Callaini G., Whitfield W.G., Riparbelli M.G. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila . Exp. Cell Res. 1997;234:183–190. doi: 10.1006/excr.1997.3618. [DOI] [PubMed] [Google Scholar]
- Carmena M., Gonzalez C., Casal J., Ripoll P. Dosage dependence of maternal contribution to somatic cell division in Drosophila melanogaster . Development. 1991;113:1357–1364. doi: 10.1242/dev.113.4.1357. [DOI] [PubMed] [Google Scholar]
- Casal J., Gonzalez C., Wandosell F., Avila J., Ripoll P. Abnormal meiotic spindles cause a cascade of defects during spermatogenesis in asp males of Drosophila . Development. 1990;108:251–260. doi: 10.1242/dev.108.2.251. [DOI] [PubMed] [Google Scholar]
- Cenci G., Bonaccorsi S., Pisano C., Verni F., Gatti M. Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J. Cell Sci. 1994;107:3521–3534. doi: 10.1242/jcs.107.12.3521. [DOI] [PubMed] [Google Scholar]
- Compton D.A. Focusing on spindle poles. J. Cell Sci. 1998;111:1477–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- Echeverri C.J., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow S.A., Komma D.J. Spindle dynamics during meiosis in Drosophila oocytes. J. Cell Biol. 1997;137:1321–1336. doi: 10.1083/jcb.137.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M.T. Spermatogenesis. In: Bate M., Arias A.M., editors. The Development of Drosophila Melanogaster. Vol. I. Cold Spring Harbor Laboratory Press; Plainview, NY: 1993. pp. 71–147. [Google Scholar]
- Gaglio T., Saredi A., Compton D.A. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J. Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T., Saredi A., Bingham J.B., Hasbani M.J., Gill S.R., Schroer T.A., Compton D.A. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J. Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T., Dionne M.A., Compton D.A. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M., Giansanti M.G., Bonaccorsi S. Relationships between the central spindle and the contractile ring during cytokinesis in animal cells. Microsc. Res. Tech. 2000;49:202–208. doi: 10.1002/(SICI)1097-0029(20000415)49:2<202::AID-JEMT13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Gergely F., Kidd D., Jeffers K., Wakefield J.G., Raff J.W. D-TACCa novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:241–252. doi: 10.1093/emboj/19.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti M.G., Bonaccorsi S., Williams B., Williams E.V., Santolamazza C., Goldberg M.L., Gatti M. Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev. 1998;12:396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti M.G., Bonaccorsi S., Gatti M. The role of anillin in meiotic cytokinesis of Drosophila males. J. Cell Sci. 1999;112:2323–2334. doi: 10.1242/jcs.112.14.2323. [DOI] [PubMed] [Google Scholar]
- Giansanti M.G., Gatti M., Bonaccorsi S. The role of centrosomes and astral microtubules during asymmetric division of Drosophila neuroblasts. Development. 2001;128:1137–1145. doi: 10.1242/dev.128.7.1137. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Casal J., Ripoll P. Relationship between chromosome content and nuclear diameter in early spermatids of Drosophila melanogaster Genet. Res. 54 1989. 205 212a [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Molina I., Casal J., Ripoll P. Gross genetic dissection and interaction of the chromosomal region 95E;96F of Drosophila melanogaster Genetics 123 1989. 371 377b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Saunders D.C., Casal J., Molina I., Carmena M., Ripoll P., Glover D.M. Mutations at the asp locus of Drosophila lead to multiple free centrosomes in syncytial embryos, but restrict centrosome duplication in larval neuroblasts. J. Cell Sci. 1990;96:605–616. doi: 10.1242/jcs.96.4.605. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Glover D.M. Techniques for studying mitosis in Drosophila . In: Fantes P., Brooks R., editors. The Cell Cycle. A Practical Approach. IRL Press; Oxford: 1993. pp. 143–175. [Google Scholar]
- Gunawardane R.N., Lizarraga S.B., Wiese C., Wilde A., Zheng Y. Gamma-tubulin complexes and their role in microtubule nucleation. Curr. Top. Dev. Biol. 2000;49:55–73. doi: 10.1016/s0070-2153(99)49004-0. [DOI] [PubMed] [Google Scholar]
- Gunsalus K.C., Bonaccorsi S., Williams E., Verni F., Gatti M., Goldberg M.L. Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J. Cell Biol. 1995;131:1243–1259. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Becker P., Hyman A., Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., Hyman A. Spindle assembly in Xenopus egg extractsrespective roles of centrosomes and microtubule self-organization. J. Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian M., Tollon Y., Lajoie-Mazenc I., Moisand A., Mazarguil H., Puget A., Wright M. Gamma-tubulin participates in the formation of the midbody during cytokinesis in mammalian cells. J. Cell Sci. 1993;105:145–156. doi: 10.1242/jcs.105.1.145. [DOI] [PubMed] [Google Scholar]
- Kellogg D.R., Field C.M., Alberts B.M. Identification of microtubule-associated proteins in the centrosome, spindle and kinetochore of the early Drosophila embryo. J. Cell Biol. 1989;109:2977–2991. doi: 10.1083/jcb.109.6.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Kaufman T.C. The homeotic target gene centrosomin encodes an essential centrosomal component. Cell. 1996;85:585–596. doi: 10.1016/s0092-8674(00)81258-1. [DOI] [PubMed] [Google Scholar]
- Lindsley D.L., Zimm G.G. The Genome of Drosophila Melanogaster 1992. Academic Press; San Diego, CA: pp. 1133 [Google Scholar]
- Matthies H.J., McDonald H.B., Goldstein L.S., Theurkauf W.E. Anastral meiotic spindle morphogenesisrole of the non-claret disjunctional kinesin-like protein. J. Cell Biol. 1996;134:455–464. doi: 10.1083/jcb.134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Cleveland D.W. Pathways of spindle pole formationdifferent mechanisms, conserved components. J. Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Heald R., Samejima K., Earnshaw W., Cleveland D.W. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 2000;149:851–861. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Ramyar K., Vechio J.D., Cleveland D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Quintyne N.J., Gill S.R., Eckley D.M., Crego C.L., Compton D.A., Schroer T.A. Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff J.W., Kellogg D.R., Alberts B.M. Drosophila gamma-tubulin is part of a complex containing two previously identified centrosomal MAPs. J. Cell Biol. 1993;121:823–835. doi: 10.1083/jcb.121.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo E., Gonzalez C. Visualizing the spindle checkpoint in Drosophila spermatocytes. EMBO (Eur. Mol. Biol. Organ.) Reports. 2000;1:65–70. doi: 10.1093/embo-reports/kvd011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli M.G., Callaini G. Meiotic spindle organization in fertilized Drosophila oocytepresence of centrosomal components in the meiotic apparatus. J. Cell Sci. 1996;109:911–918. doi: 10.1242/jcs.109.5.911. [DOI] [PubMed] [Google Scholar]
- Riparbelli M.G., Callaini G. Gamma-Tubulin is transiently associated with the Drosophila oocyte meiotic apparatus. Eur. J. Cell Biol. 1998;75:21–28. doi: 10.1016/s0171-9335(98)80042-3. [DOI] [PubMed] [Google Scholar]
- Ripoll P., Pimpinelli S., Valdivia M.M., Avila J. A cell division mutant of Drosophila with a functionally abnormal spindle. Cell. 1985;41:907–912. doi: 10.1016/s0092-8674(85)80071-4. [DOI] [PubMed] [Google Scholar]
- Saunders R.D., Avides M.C., Howard T., Gonzalez C., Glover D.M. The Drosophila gene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J. Cell Biol. 1997;137:881–890. doi: 10.1083/jcb.137.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K.E., LeGuellec K., Philippe M., Mitchison T.J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., Yu K.R., Sisson J.C., Sullivan W., Scholey J.M. Antagonistic microtubule-sliding motors position mitotic centrosomes in Drosophila early embryos. Nat. Cell Biol. 1999;1:51–54. doi: 10.1038/9025. [DOI] [PubMed] [Google Scholar]
- Shu H.B., Li Z., Palacios M.J., Li Q., Joshi H.C. A transient association of gamma-tubulin at the midbody is required for the completion of cytokinesis during the mammalian cell division. J. Cell Sci. 1995;108:2955–2962. doi: 10.1242/jcs.108.9.2955. [DOI] [PubMed] [Google Scholar]
- Theurkauf W.E., Hawley R.S. Meiotic spindle assembly in Drosophila femalesbehavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Cooper H., Carmena M., Gonzalez C., Glover D.M. Mutations in new cell cycle genes that fail to complement a multiply mutant third chromosome of Drosophila . Genetics. 1996;144:1097–1105. doi: 10.1534/genetics.112.1097.test. [DOI] [PubMed] [Google Scholar]
- Williams B.C., Riedy M.F., Williams E.V., Gatti M., Goldberg M.L. The Drosophila kinesin-like protein KLP3A is a midbody component required for central spindle assembly and initiation of cytokinesis. J. Cell Biol. 1995;129:709–723. doi: 10.1083/jcb.129.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman W., Sparks C.A., Doxsey S.J. Amorphous no longerthe centrosome comes into focus. Curr. Opin. Cell. Biol. 1999;11:122–128. doi: 10.1016/s0955-0674(99)80015-5. [DOI] [PubMed] [Google Scholar]