Abstract
The Kaposi’s sarcoma (KS)-associated herpesvirus is a lymphotropic virus strongly implicated in the pathogenesis of KS and several lymphoproliferative disorders. The KS-associated herpesvirus K1 gene encodes a transmembrane protein bearing a functional immunoreceptor tyrosine-based activation motif (ITAM)-like sequence; it previously has been proposed to be important in viral tumorigenesis because its expression can trigger cell proliferation in vitro and in vivo. Here we show that expression of the full-length K1 protein can initiate calcium-dependent signal transduction in B cells; however, unlike other ITAM-based signal transduction events, K1 signaling occurs constitutively, in the absence of exogenous crosslinking ligands. This property is caused by its cysteine-rich ectodomain, which when transferred to other consensus ITAMs induces constitutive signaling. Although ITAM-based signaling by K1 involves classical syk and phospholipase C γ2 activation, both ITAM- and syk-independent signaling pathways are activated by K1 expression. These studies indicate that K1 is a deregulated signaling molecule with pleitropic effects that may explain its known growth deregulatory properties.
Kaposi’s sarcoma (KS)-associated herpesvirus (KSHV; also known as human herpesvirus 8, HHV8) is a human herpesvirus whose genome was first identified in biopsies of KS, the leading neoplasm of HIV-infected patients (1). Epidemiologic studies point strongly to a central role for KSHV infection in the development of KS (2–6), and viral DNA and RNA are present in the majority of KS spindle cells (7, 8). KSHV is also present in the B cell population in peripheral blood and is tightly associated with two lymphoproliferative disorders, primary effusion lymphoma (9, 10) and multicentric Castleman’s disease (11).
One approach to the identification of KSHV genes important in tumorigenesis is to examine viral genomic regions syntenic with known oncogenes in related viruses. Herpesvirus saimiri (HVS) is a closely related virus that induces lymphoma upon experimental transmission to certain primate hosts (12). Tumorigenesis in the best-studied HVS strain, strain C, depends on two genes, termed STP and Tip, both of which are modifiers of host cell signal transduction that map to the left-hand end of the genome (13). When we examined the coding organization of the left-hand end of the KSHV genome, we found that KSHV encodes no homolog of either STP or Tip; in their stead is an ORF, K1, whose coding region predicts a type I membrane protein with an N-terminal signal sequence, a single transmembrane region, and a small, 37-aa C-terminal cytoplasmic tail (14, 15). Although these properties might suggest a role as an envelope protein, we previously showed that the regulation of the gene was not consistent with a structural gene and proposed that the protein might play a nonstructural or regulatory role, e.g., in signal transduction or other processes (14). Strong evidence for a role in signaling recently has emerged from studies by Lee et al. (16) showing that K1 overexpression can transform Rat-1 fibroblasts in vitro, and that recombinant HVS strains bearing the KSHV K1 gene in place of that for STP can induce lymphocyte transformation in vitro and lymphoma in vivo. Moreover, they noted that the highly conserved C-terminal tail of K1 contains a sequence [YxxL (X)7 YxxP] that resembles the immunoreceptor tyrosine-based activation motif (ITAM; consensus sequence YxxL (X)7–8 YxxL/I (17, 18).
ITAMs are found in a variety of immune receptor complexes, including invariant chains associated with the B and T cell antigen receptors, and play central roles in signal transduction events leading to cellular activation after engagement of these receptors (19–21). After receptor engagement, both tyrosines in the ITAM are phosphorylated, presumably by src family kinases (22). Subsequently, Syk (in B cells) or Zap-70 (in T cells) bind the doubly phosphorylated ITAM through tandem SH2 domains and undergo tyrosine phosphorylation (23, 24). The ensuing activation of their kinase activities initiates a number of downstream signal transduction pathways, for example, activation of the Ras pathway and of phospholipase C, leading to an increase in intracellular Ca2+ and activation of nuclear factor of activated T cell (NFAT)-dependent transcription (21, 25, 26). Importantly, all previously identified ITAMs are tightly regulated: they require receptor engagement to initiate signaling and are inactive in the absence of ligand (19, 21).
Although the K1 sequence bears a substitution at one conserved position in the ITAM consensus sequence, this variant ITAM was shown to have the potential to be functional, because chimeric molecules in which K1 was artificially fused to CD8 can stimulate calcium mobilization when stimulated by crosslinking with anti-CD8 antibody (18). However, this experiment does not allow accurate prediction of the signaling properties of the wild-type (WT) K1 protein. For example, Epstein–Barr virus encodes an ITAM-containing membrane protein, LMP2, whose ITAM domain also was shown to stimulate calcium mobilization when fused to CD8 (after crosslinking with anti-CD8 mAb). However, expression of the full-length LMP2 molecule inhibits calcium mobilization by the B cell receptor, illustrating that chimeric ITAM-bearing polypeptides may not faithfully reproduce the biology of the native molecule (27–30).
Here we show that the full-length K1 gene product efficiently induces signal transduction in B cells. However, unlike other known ITAM-mediated reactions, K1 induces signal transduction in a constitutive fashion, independent of exogenous crosslinking ligand; this ligand-independent activation is attributable to the cysteine-rich extracellular domain of K1. Thus, K1 is a constitutively activated ITAM-bearing signaling molecule, whose pleitropic and deregulated output likely explain its potent biological activities.
MATERIALS AND METHODS
Cell Lines.
DT-40, a chicken B cell line, and its Syk-deficient derivative have been described (31). Raji cells, a human Burkitt’s Lymphoma cell line, were obtained from E. Kieff (Harvard University).
Antibodies.
Murine mAb to the hemagglutinin (HA) tag, YPYDVPDYA, was purchased from Babco. Antiphosphotyrosine mouse mAb (4G10) was purchased from Upstate Biotechnology (Lake Placid, NY). IL-2 receptor (IL-2R) crosslinking antibody was obtained from Warner Greene (University of California, San Francisco). Rabbit polyclonal anti-Syk antibody (1373) and murine mAb (4D10.1) were a generous gift of D. Chu (University of California, San Francisco). M4 antibody to the avian B cell antigen receptor was obtained from M. Cooper and C. L. Chen (University of Alabama, Birmingham).
Plasmids.
The WT K1 sequence were amplified from pML3 (14) by PCR and inserted into pCDEF3 under the ef-1α promoter (32). The HA epitope tag was inserted 20 aa downstream of the predicted signal sequence cleavage site to generate HA-K1. The Y>F mutants were generated by using a 3′ oligo containing the desired mutation for PCR. The signal sequence from CD8 fused to the Flag tag was linked in-frame to K1 at amino acid 18 (immediately after the K1 signal sequence) to create FlagK1 and also FlagK1 Y>F1,2. The 3× NFAT-luc plasmid contains three reiterated NFAT sites from the IL-2 promoter driving expression of luciferase (33). The ecto and cyto domains were predicted previously and were generated by PCR (14). All epsilon and Zeta chain plasmids were generated by PCR by using previously isolated plasmids containing those genes. All inserts were verified by sequencing.
Antibody Stimulation and Luciferase Assay.
Transfections and luciferase assays were done as described (33). All chimeric IL-2R constructs were checked for expression by FACS analysis, and all HA epitope-tagged molecules were tested for expression by Western blot. Positive controls for maximum luciferase stimulation, phorbol 12-myristate 13-acetate and ionomycin, and the M4 antibody to the B cell receptor, were done for each transfection. Each luciferase assay was done in triplicate on at least three different transfections with the exception of the IL-2R-ɛ transfectants, which were assayed in triplicate on two different transfections.
Western Blot.
Sonicated whole-cell lysates from approximately 1 × 106 cells, 1/10 of the supernatant from an immunoprecipitation, or the entire immunoprecipitation from 15–25 × 106 cells were separated on a 10% SDS/polyacrylamide gel. Proteins were transferred to membrane and probed with antibody according to the ECL manual (Amersham Pharmacia).
Immunoprecipitations.
Forty (Syk immunoprecipitations) or 60 (4G10 immunoprecipitations) × 106 Raji cells were electroporated as described above. Sixteen to 20 hours after transfection cells were harvested, and immunoprecipitations were done as described (34).
For peptide pull-downs, 15 × 106 Raji cells were lysed, and 1 μg of the relevant peptide was added with mixing for 1–2 hr, followed by mixing with 30 μl of a 50% slurry of avidin-agarose (Vector Laboratories) for 2 hr and subsequent washing. Unphosphorylated, singly phosphorylated, and doubly phosphorylated 19-aa peptides containing the K1 ITAM and flanking sequences and the Zeta doubly phosphorylated and unphosphorylated ITAM peptide were made by C. Turck (University of California, San Francisco).
RESULTS
Full-Length K1 Induces Signal Transduction in a Constitutive Fashion.
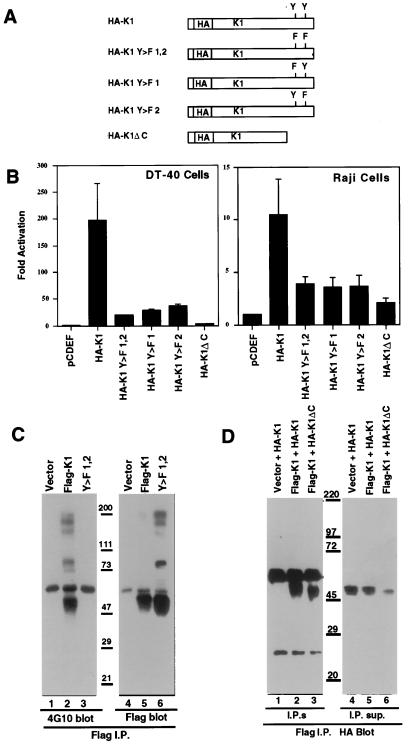
To test the ability of WT K1 to induce signal transduction, plasmids were constructed that contained WT or mutant K1 genes downstream of the strong eukaryotic EF2 promoter; in the mutant K1 genes, the critical tyrosine codons of the ITAM were changed either singly or doubly to phenylalanine codons. Each K1 gene product was tagged in its ectodomain with an HA epitope near the N terminus (to generate the clones HA-K1, HA-K1 Y>F1, HA-K1 Y>F2, and HA-K1 Y>F1, 2; see Fig. 1A). We also constructed a mutant (HA-K1ΔC) in which 23 aa of the cytoplasmic tail of K1, including the entire ITAM, were deleted. Signaling was assayed by assessing the activation of an NFAT and Ap-1 composite transcription reporter. ITAM-mediated signaling raises intracellular calcium, which in turn activates calcineurin, a phosphatase that activates NFAT; ITAM signaling also activates the Ras/mitogen-activated protein kinase pathway, which activates AP-1 (21, 35). DT40 cells were cotransfected with each of the K1 expression plasmids of Fig. 1A, together with the luciferase reporter construct (3xNFAT-LUC). Twenty to 24 hours after transfection, cells were lysed and assayed for luciferase activity. Expression of the full-length, WT HA-K1 molecule gives nearly a 200-fold stimulation of luciferase activity over that induced by the vector alone (Fig. 1B). Notably, no crosslinking reagents are necessary to achieve this large enhancement in luciferase expression. (Similar results were obtained with WT K1 protein without an HA tag and with the FlagK1 construct; data not shown). Expression of the HA-K1 Y>F1,2 double mutant strongly reduces luciferase expression (ca. 10-fold) but, interestingly, does not abolish it altogether (Fig. 1B). A substantial level of luciferase activity remains, 20-fold above that of the negative control. Each of the single mutants behave similarly, with 8- to 9-fold reductions compared with WT HA-K1 but still 25- to 30-fold above empty vector (Fig. 1B). The effects of these tyrosine mutations on luciferase activity are highly characteristic of an ITAM-dependent signaling pathway. However, the residual signal transduction in the absence of the tyrosines indicates that there is also involvement of an ITAM-independent pathway for signal transduction (see below). All luciferase induction is abrogated by expression of HA-K1ΔC, in which the entire ITAM and flanking sequences of the K1 cytoplasmic tail are deleted.
Figure 1.
K1 induces signal transduction in the absence of exogenous ligand and is phosphorylated on the tyrosines in the ITAM. (A) Schematic diagram of the constructs used. (B) Histograms of the fold activation of luciferase in DT-40 or Raji cells cotransfected with the indicated plasmid and a plasmid containing luciferase under the control of a triple NFAT site versus activity in cells transfected with empty vector. (C) Antiphosphotyrosine (4G10) antibody Western blot of cells transfected with the indicated plasmid, lysed, and immunoprecipitated with anti-Flag tag antibody (lanes 1–3). Lanes 4–6 are the same blot stripped and reprobed with the Flag antibody. (D) Anti-HA tag antibody Western blots of cells transfected with the indicated plasmids, lysed, and immunoprecipitated with anti-Flag tag antibody (lanes 1–3). The supernatants from the immunoprecitates also were blotted to show expression of HA-K1 or ΔC in all transfections (lanes 4–6).
To be certain that these results apply to human B cells as well, similar experiments were done in Raji cells (Fig. 1B, Right) and in BJAB cells (data not shown). Although less active than in DT40 cells, K1 leads to a large stimulation (11-fold) of luciferase activity in Raji cells over cells transfected with vector alone. As in DT40 cells, this activation is strongly reduced but not eliminated by mutation of one or both tyrosine residues in the putative ITAM (Fig. 1B) and is further reduced by the ΔC deletion. Similar levels of stimulation were found in the Epstein–Barr virus negative BJAB cell line (data not shown).
K1 Requires Both Tyrosines in the ITAM for Stable Tyrosine Phosphorylation.
Because ITAM tyrosine phosphorylation by src family kinases is crucial for signaling in the B and T cell antigen receptor systems, and as seen in Fig. 1B, tyrosines are important for high-level signaling by K1, we examined the phosphorylation of the K1 ITAM. Twenty hours after transfection of Raji cells with tagged WT or mutant K1 expression vectors, cells were lysed and extracts were prepared for immune precipitation. We found that HA-tagged K1 protein, although efficiently recognized by anti-HA on Western blots, cannot be efficiently precipitated by the same antibody (not shown). Therefore, we generated a plasmid containing the WT K1 sequence or the Y>F double mutant with the signal sequences replaced by the CD8 signal sequence and a Flag tag in-frame with K1. The Flag-tagged K1 activated the NFAT-luciferase reporter similarly to WT and HA-tagged K1, and by flow cytometry was shown to be expressed on the cell surface (data not shown). The Flag antibody (IBI Kodak, New York) was used to immunoprecipitate K1, and the immunoprecipitates were separated by SDS/PAGE, blotted to membranes, and probed with antiphosphotyrosine antibody (4G10). As shown in Fig. 1C, cells transfected with vector alone or with the FlagK1 Y>F1,2 mutant do not contain any species recognized by the antiphosphotyrosine antibody in the immunopreciptate. Cells expressing Flag-K1, however, have a strong band migrating the size of Flag-K1 in the immunoprecipitate (Fig. 1C, lane 2). No antiphosphotyrosine-reactive bands are seen in the immunoprecipitates from the Y>F double mutant, though it is well expressed and immunoprecipitated (Fig. 1C, lane 6). This experiment also shows that neither of the two tyrosines present in the K1 cytoplasmic tail outside of the ITAM are stably phosphorylated.
The single tyrosine mutants were assayed for phosphorylation in the HA-tagged K1 constructs by using antiphosphotyrosine antibody (4G10) for immunoprecipitation and antibody to the HA tag for immunoblotting. In this experiment the HA-tagged K1 is immunoprecipitated but neither of the single Y>F mutant K1 proteins is immunoprecipitated (data not shown), a result that is also in accord with findings in other ITAM-bearing proteins. This finding suggests that either the phosphorylations at this site are highly and reciprocally cooperative, or, more likely, that lesions that impair SH2 recognition of the ITAM (e.g., by syk family members) leave the remaining phosphotyrosines unshielded from the action of cellular phosphatases (36).
K1 Forms Homo-Multimers.
To examine K1-K1 interactions we took advantage of the two tagged forms of K1. Raji cells were cotransfected with HA-K1 or HA-K1DC and either empty vector or Flag-K1, and the anti-Flag antibody was used to immunoprecipitate the transfected cell lysates. The anti-Flag antibody was capable of immunoprecipitating HA-K1 or HA-K1DC only in presence of Flag-K1, indicating that Flag-K1 and HA-K1 multimerize (Fig. 1D).
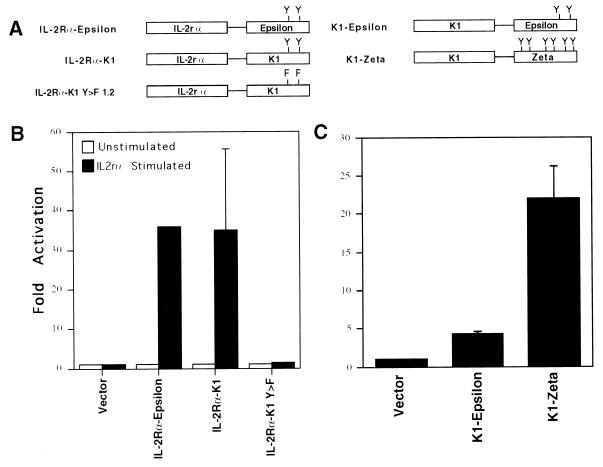
The Extracellular Domain of K1 Imparts Constitutive Activity to Consensus ITAMs.
A striking feature of the above experiments is that K1 signaling is independent of exogenous crosslinking reagents. To better understand the nature of this phenomenon, we constructed two sets of reciprocal chimeras (Fig. 2A). In one set, the K1 cytoplasmic tail was fused to the external and transmembrane domains of the IL-2R α chain (IL-2Rα). In another set, canonical ITAM-containing cytoplasmic tails (from the zeta and epsilon chains of the T cell antigen receptor) were fused to the K1 ectodomain and transmembrane region (see Fig. 2A). If ligand-independent signaling was caused by complexes assembled constitutively on the variant K1 ITAM, then such signaling should occur in the IL-2Rα chimeras in the absence of crosslinking anti-IL-2Rα antibodies. As shown in Fig. 2B, signaling did not occur: expression of such chimeras activates NFAT-driven luciferase expression only in the presence of anti-IL-2Rα antibody, a result that agrees well with earlier studies of CD8/K1 ITAM chimeras (18). By contrast, when K1’s ectodomain is fused to either of the indicated classical ITAMs, signaling is observed in the absence of added crosslinking reagents (Fig. 2C). Thus, the constitutive activity that differentiates K1 signaling from that of other ITAM-based molecules can be imparted to other ITAMs by the K1 ectodomain.
Figure 2.
The ectodomain of K1 is responsible for the constitutive signal transduction and can impart constitutive signal transduction to other ITAMs. (A) Schematic diagram of the constructs used. (B) Histograms of the fold activation of luciferase in DT-40 cells, cotransfected with the indicated plasmid and the NFAT-luc plasmid stimulated with a crosslinking antibody versus activity in cells without antibody stimulation. (C) Histogram of the fold activation of luciferase in DT-40 cells cotransfected with the indicated plasmid and the NFAT-luc plasmid without antibody stimulation.
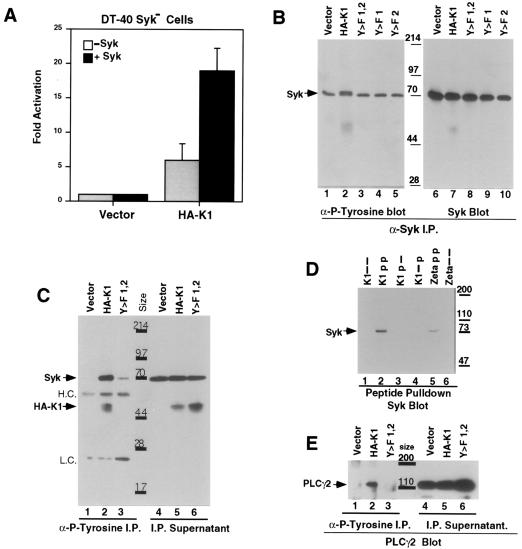
K1 Signal Transduction Involves Syk Phosphorylation.
Activation of NFAT by ITAM-mediated signaling by the B cell receptor is known to involve the tyrosine kinase syk, which is thought to directly engage the phosphorylated tyrosines of the ITAM via its tandem SH2 domains (37, 38). To examine the potential involvement of syk in K1-mediated signaling, we capitalized on the availability of a DT40 derivative in which both copies of the cellular syk gene have been inactivated by homologous recombination (31). As shown in Fig. 3A, syk-deficient DT40 cells display only modest activation of luciferase activity in response to expression of HA-K1 (5- to 7-fold). When WT human syk is restored by transient cotransfection, a significant increase in luciferase induction is observed (to ca. 20-fold). No activation is seen when the vector alone was cotransfected with the human syk construct (Fig. 3A). Both K1 and syk proteins were expressed similarly in all of these transfections as judged by Western blotting (data not shown). These data indicate that syk is important for full activation of K1 signaling. However, the presence of significant residual levels of K1 signaling in the absence of syk expression again reveals that K1 signaling can be mediated by other molecules or pathways as well.
Figure 3.
K1 requires syk for high-level signal transduction and induces phosphorylation of syk and PLCγ2. (A) Histograms of the fold activation of luciferase in DT-40 syk negative cells cotransfected with the indicated plasmids and the NFAT-luc plasmid, with or without an expression plasmid containing human syk, versus activity in cells transfected with the empty vector. (B) An antiphosphotyrosine (4G10) Western blot of Raji cells transfected with the indicated plasmid and a syk expression construct and precipitated with a human syk antibody. The right side is the same blot stripped and reprobed with an anti-syk antibody. (C) A Western blot of Raji cells transfected with the indicated plasmid, immunoprecipitated with 4G10, and blotted first with an anti-syk antibody followed by an anti-HA antibody. (D) Anti-syk Western blot of Raji cell lysates incubated with the indicated unphosphorylated, singly phosphorylated, or doubly phosphorylated biotin-labeled peptide and precipitated with avidin-agarose. (E) Anti-PLCγ2 Western blot of Raji cells transfected with the indicated plasmid and immunoprecipitated with 4G10. The right side contains the supernatant from the immunoprecipitates.
To examine the phosphorylation state of syk in K1-expressing cells, Raji cells were cotransfected with a human syk expression construct and expression vectors for either WT HA-K1 or its single or double Y>F mutants. Cells were harvested 18 hr posttransfection, and syk was precipitated with anti-syk antibody (Fig. 3B) or with an antiphosphotyrosine antibody (4G10) (Fig. 3C). The immunoprecipitates were run on SDS/PAGE and blotted to a membrane; the syk immunoprecipitates were probed with an antiphosphotyrosine mAb (4G10), while the 4G10 immunoprecipitates were probed with the anti-syk antibody. As seen in Fig. 3B, a basal level of phosphorylated Syk is detectable in the cells (lanes 1, 3, 4, and 5) that were transfected with the empty vector or the Y>F mutants. However, the cells expressing WT HA-K1 display an elevated amount of tyrosine-phosphorylated syk, a substantial fraction of which displayed a retarded electrophoretic mobility (Fig. 3B, lane 2). The blots were reprobed with an anti-syk mAb to show that there are similar amounts of total syk protein in all the lanes (Fig. 3B). The same enhanced phosphorylation of syk was seen in four separate experiments (data not shown). These findings were confirmed by examination of the antiphosphotyrosine immune precipitates with anti-syk antibody (Fig. 3C). The immunoprecipitates from cells transfected with the empty vector contain very little syk, whereas immunoprecipitates from cells transfected with K1 harbor much larger amounts of syk, in accord with the syk immunoprecipitation results (Fig. 3C). The Y>F mutants immunoprecipitate only a very small amount of syk, showing that both K1 ITAM tyrosines are necessary for the prominent syk phosphorylation (Fig. 3C and data not shown). Western blots of the supernatants show the presence of similar amounts of Syk in all extracts (Fig. 3C, lanes 4–6). This same blot subsequently was probed with the anti-HA antibody to verify that, as in Fig. 1C, only WT K1 is immunoprecipitated by 4G10 (Fig. 3C).
Syk Interacts with the Doubly Phosphorylated K1 ITAM Peptide.
In other well-studied cases, syk phosphorylation and activation is the result of direct interaction with the ITAM (21, 38). To examine the interaction of K1 and syk, we first attempted coimmunoprecipitation. WT or mutant HA-K1 genes were transfected into Raji cells, and lysates were prepared. After precipitation with anti-syk, precipitates were examined by immunoblotting with anti-HA mAb. We were unable to consistently observe an interaction between syk and K1 in this fashion (not shown); perhaps the mutation in the K1 ITAM lowers the affinity of syk binding to K1. In another approach, we used biotinylated synthetic peptides containing the K1 ITAM and flanking sequences, either unphosphorylated, doubly, or singly phosphorylated at the ITAM tyrosine residues. Raji cell lysates were incubated with these peptides, and peptide-bound proteins were collected on avidin-agarose beads. After elution and separation by SDS/PAGE, the eluates were examined by immunoblotting with anti-syk antibody. The doubly phosphorylated peptide is able to precipitate high levels of syk (Fig. 3D), whereas the unphosphorylated (Fig. 3D) or singly phosphorylated peptides displayed substantially reduced binding. Thus, the K1 ITAM can enter into complexes with syk, though our experiments cannot discern whether such interactions are direct or indirect.
Expression of K1 Leads to Phosphorylation of Phospholipase C γ2 (PLCγ2) and Other Proteins.
Because activated syk is known to lead to phosphorylation of PLCγ, leading to an increase in intracellular Ca2+ levels required for calcineurin-mediated NFAT activation (35), we also examined the phosphorylation state of PLCγ2 in K1 transfectants. Extracts from cells transfected with plasmids expressing WT or mutant K1 genes were immunoprecipitated with antiphosphotyrosine antibody, and the resulting precipitates were examined by SDS/PAGE and immunoblotting with antibodies to PLCγ2. PLCγ2 is phosphorylated in the presence of WT K1 (Fig. 3E) but not in the presence of the double (Fig. 3E) or single (not shown) tyrosine mutants of K1. In parallel, a blot containing similar antiphosphotyrosine immunoprecipitates to the ones just described also was probed with the antiphosphotyrosine antibody. This experiment revealed that, besides syk and PLCγ2, two other cellular phosphoproteins, could be detected in the K1-transfected lysates but not vector or the double Y>F mutant (data not shown).
DISCUSSION
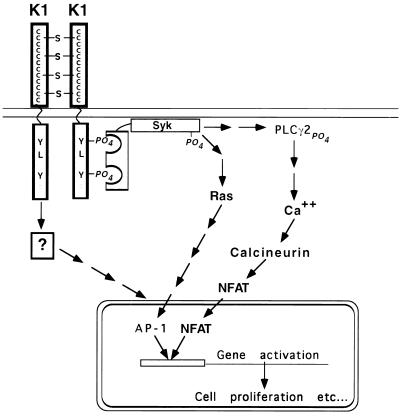
These findings establish that the K1 gene product of KSHV is a potent deregulated signaling molecule that can initiate signaling by both ITAM-dependent and ITAM-independent pathways in B cells in the absence of exogenous crosslinking ligands. Two possible explanations can be envisioned for the apparent ligand independence of K1 signaling. One possibility is that the K1 homo-oligomerization (Fig. 1D) drives the signaling process; Fig. 4 depicts a model for K1 signaling based on this view. In this model the 12 conserved cysteines in the extracellular domain lead to homo-multimerization, resulting in phosphorylation of the K1 ITAM, presumably by src family kinases. Syk then can bind the doubly phosphorylated ITAM and is itself then phosphorylated at a distal site. Syk phosphorylation leads to a cascade of further downstream signaling events including phosphorylation of PLCγ2, calcium mobilization, and activation of NFATc, as well as activation of the Ras/MEK pathway activating AP-1, which combined with NFATc up-regulates NFAT-dependent promoters.
Figure 4.
Pathways of K1-mediated signal transduction. K1 homo-multimerizes, possibly through the 12 cysteines in the cytoplasmic domain, thereby inducing phosphorylation of the tyrosines in the variant ITAM. Syk binds the doubly phosphorylated ITAM and is itself phosphorylated, leading to activation of the RAS pathway and also phosphorylation of PLCγ2, calcium mobilization, and nuclear localization of NFAT. NFAT in concert with AP-1 bind NFAT binding sites and in these experiments activate luciferase expression. There is also an ITAM and syk-independent signal transduction pathway activating gene expression.
An alternative model is that K1 multimerization may be driven by endogenous ligands constitutively present on the cell surface. In either case, the biological result is the same: constitutive activation of several pathways of signal transduction, including a signaling pathway that does not require tyrosine phosphorylation of the ITAM or the presence of syk. This pathway may merge with one of the other depicted pathways at a later stage to activate luciferase expression or may activate expression through an entirely different pathway. One or more of these pathways surely underlies the growth deregulation induced by K1 expression in the experimental settings previously described (16). Because many of the molecules (e.g., lck, lyn, syk, and ZAP70) traditionally implicated in ITAM-based signaling are absent in nonlymphoid cells (e.g., Rat-1 fibroblast cells), it is possible that the ITAM-independent pathways activated by K1 may be especially important in such contexts.
How might these results relate to the pathogenesis of KSHV-associated diseases in vivo? Several possibilities can be envisioned. First, if K1 is expressed in viral latency, then it could act directly (in concert with other latent products like v-cyclin) to stimulate cell proliferation in latently infected B cells (e.g., in primary effusion lymphoma) or in endothelial cells (in KS). Activation of ITAM-dependent signaling has been shown to deregulate cyclin D2 and other proteins involved in cell cycle control in B cells (39). K1 is expressed at low levels in untreated primary effusion lymphoma cell cultures and is up-regulated after chemical induction of lytic replication, a result compatible with identification of K1 as either a latent or a lytic function (14). K1 expression in lytic infection also could play an important role in KSHV pathogenesis. For example, activation of cellular gene expression by K1 signaling pathways could provide paracrine signals that stimulate proliferation or angiogenesis in neighboring cells; such paracrine loops have long been postulated to play an important part in KS histogenesis (40, 41). Enhancement of ITAM signaling in B cells also may be involved in viral gene activation or the facilitation of other steps in virus reactivation from latency. KSHV reactivation in cultured cells is known to be up-regulated by molecules (e.g., ionomycin) that up-regulate intracellular calcium as does K1 (J.L. Chang, R. Renne, and D.G., unpublished work). Such activities may have particular relevance to KS pathogenesis. Taxonomically, KSHV is a member of the lymphotropic subfamily of herpesviruses, and considerable evidence supports the notion that the primary reservoir of infection is the lymphoid system, especially latently infected B cells. However, KS is an endothelial, not a lymphoid, neoplasm. Because spread of KSHV from its primary lymphoid reservoir to endothelial targets requires lytic reactivation, K1-mediated signaling in that context may contribute indirectly as well as directly to KS pathogenesis.
Acknowledgments
We thank V. S. Shapiro for invaluable advice and D. Chu and N.S.C. van Oers for reagents. This work was supported by National Institutes of Health Grant CA73506 (to D.G.) and the Howard Hughes Medical Institute (to A.W.). R.M. is supported by a National Institutes of Health medical scientist training grant.
ABBREVIATIONS
- KS
Kaposi’s sarcoma
- KSHV
KS-associated herpesvirus
- ITAM
immunoreceptor tyrosine-based activation motif
- HA
hemagglutinin
- NFAT
nuclear factor of activated T cells
- IL-2R
IL-2 receptor
- PLCγ2
phospholipase C γ-2
- WT
wild type
References
- 1.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Whitby D, Howard M R, Tenant-Flowers M, Brink N S, Copas A, Boshoff C, Hatzioannou T, Suggett F E, Aldam D M, Denton A S, et al. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 3.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 4.Gao S J, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo C R, Saah A, Phair J, et al. Nat Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 5.Gao S J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 6.Martin J N, Ganem D, Osmond D H, Page-Shafer K A, Macrae D, Kedes D H. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 7.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O’Leary J J. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 9.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 10.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 11.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay M F, Clauvel J P, Raphael M, Degos L, et al. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 12.Fleckenstein B, Desrosier R C. In: Herpesvirus Saimiri and Herpesvirus Ateles. Roizman B, editor. Vol. 1. New York: Plenum; 1982. pp. 253–332. [Google Scholar]
- 13.Jung J U, Trimble J J, King N W, Biesinger B, Fleckenstein B W, Desrosiers R C. Proc Natl Acad Sci USA. 1991;88:7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagunoff M, Ganem D. Virology. 1997;236:147–154. doi: 10.1006/viro.1997.8713. [DOI] [PubMed] [Google Scholar]
- 15.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Fleckenstein B, Lackner A, Desrosiers R C, Jung J U. Nat Med. 1998;4:435–440. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- 17.Reth M. Nature (London) 1989;338:383–384. [Google Scholar]
- 18.Lee H, Guo J, Li M, Choi J-K, DeMaria M, Rosenzweig M, Jung J U. Mol Cell Biol. 1998;18:5219–5228. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irving B, Weiss A. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 20.Romeo C, Seed B. Cell. 1991;64:1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- 21.Weiss A, Littman D R. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 22.Irving B A, Chan A C, Weiss A. J Exp Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark M R, Campbell K S, Kazlauskas A, Johnson S A, Hertz M, Potter T A, Pleiman C, Cambier J C. Science. 1992;258:123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 24.Iwashima M, Irving B A, van Oers N S C, Chan A C, Weiss A. Science. 1994;263:1136–1139. [PubMed] [Google Scholar]
- 25.Jain J, McCaffrey P G, Valge-Archer V E, Rao A. Nature (London) 1992;356:801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- 26.Northrup J P, Ullman K S, Crabtree G R. J Biol Chem. 1993;268:2917–2923. [PubMed] [Google Scholar]
- 27.Alber G, Kim K-M, Weiser P, Riesterer C, Carsetti R, Reth M. Curr Biol. 1993;3:333–339. doi: 10.1016/0960-9822(93)90196-u. [DOI] [PubMed] [Google Scholar]
- 28.Beaufils P, Choquet D, Mamoun R Z, Malissen B. EMBO J. 1993;12:5105–5112. doi: 10.1002/j.1460-2075.1993.tb06205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller C L, Longnecker R, Kieff E. J Virol. 1993;67:3087–3094. doi: 10.1128/jvi.67.6.3087-3094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller C L, Burkhardt A L, Lee J H, Stealey B, Longnecker R, Bolen J B, Kieff E. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 31.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman L A, Cutrone E C, Kotenko S V, Krause C D, Langer J A. BioTechniques. 1996;21:1013–1015. doi: 10.2144/96216bm10. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro V S, Mollenauer M N, Greene W C, Weiss A. J Exp Med. 1996;184:1663–1669. doi: 10.1084/jem.184.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai D M, Sap J, Silvennoinen O, Schlessinger J, Weiss A. EMBO J. 1994;13:4002–4010. doi: 10.1002/j.1460-2075.1994.tb06716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeFranco A L. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 36.Isakov N. J Leukocyte Biol. 1997;61:6–16. doi: 10.1002/jlb.61.1.6. [DOI] [PubMed] [Google Scholar]
- 37.Kurosaki T, Johnson S A, Pao L, Sada K, Yamamura H, Cambier J C. J Exp Med. 1995;182:1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutchcroft J E, Harrison M L, Geahlen R L. J Biol Chem. 1991;266:14846–14849. [PubMed] [Google Scholar]
- 39.Solvason N, Wu W, Nisha K, Wu X, Lees E, Howard M. J Exp Med. 1996;184:407–417. doi: 10.1084/jem.184.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ensoli B, Nakamura S, Salahuddin S, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo R. Science. 1989;243:223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- 41.Ensoli B, Barillari G, Gallo R. Immunol Rev. 1992;127:147–155. doi: 10.1111/j.1600-065x.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]