Abstract
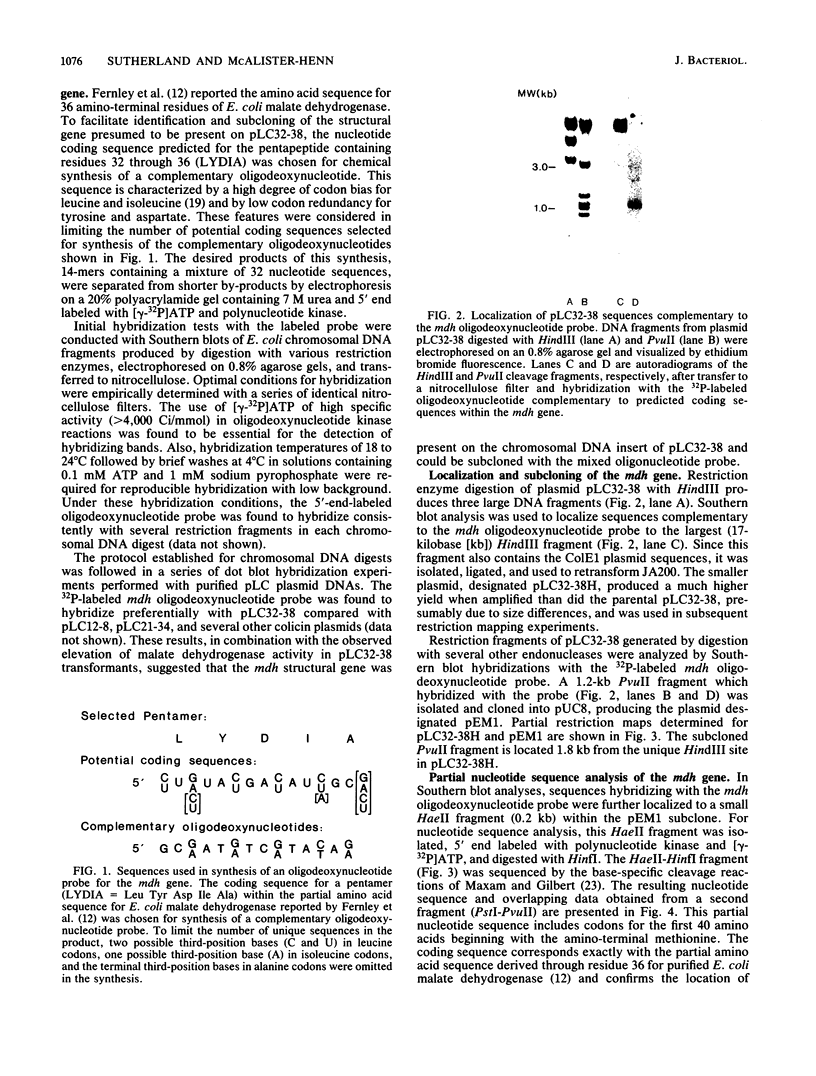
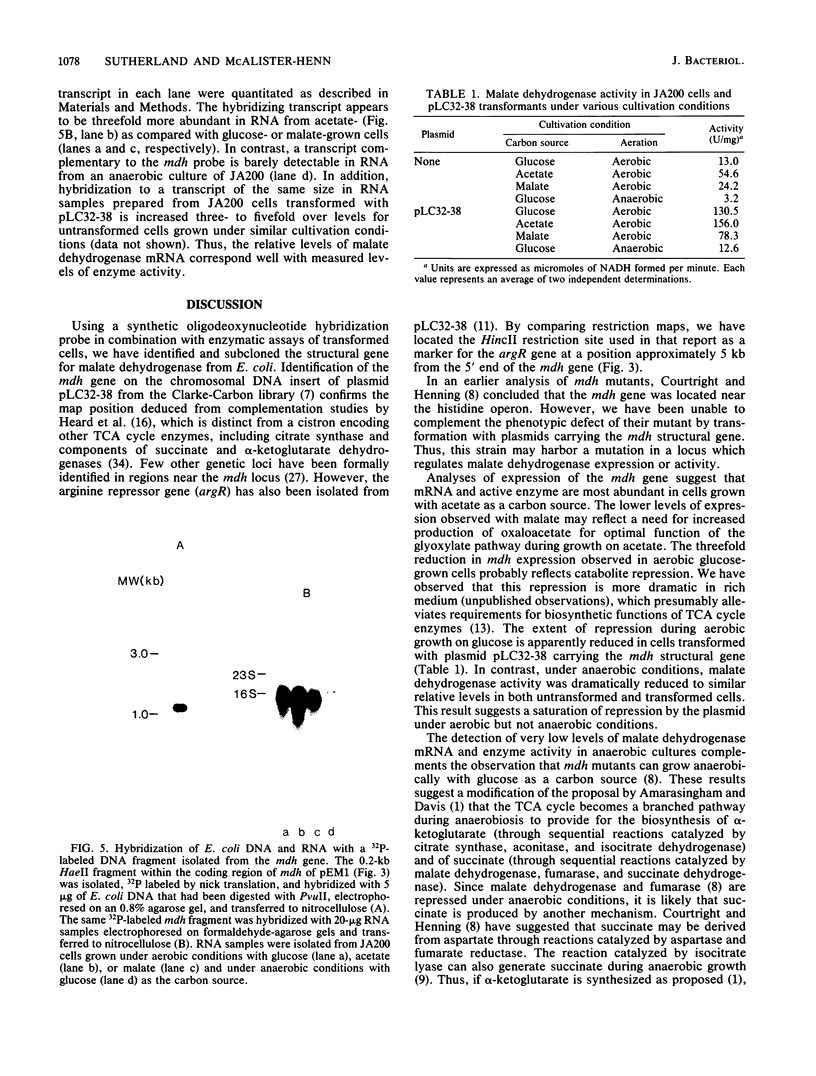
An oligodeoxynucleotide specific for a pentapeptide sequence corresponding to amino acid residues 32 through 36 of Escherichia coli malate dehydrogenase was chemically synthesized and used to identify the mdh gene on plasmid pLC32-38 from the Clarke-Carbon recombinant library. Cells transformed with this plasmid exhibited a 10-fold increase in malate dehydrogenase activity. A 1.2-kilobase PvuII fragment which hybridized with the oligodeoxynucleotide probe was subcloned, and the identity of the mdh structural gene was confirmed by partial nucleotide sequence analysis. The expression of the mdh gene, as measured by both Northern blotting and enzyme assays, was found to vary over a 20-fold range with different culture conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Birktoft J. J., Banaszak L. J. The presence of a histidine-aspartic acid pair in the active site of 2-hydroxyacid dehydrogenases. X-ray refinement of cytoplasmic malate dehydrogenase. J Biol Chem. 1983 Jan 10;258(1):472–482. doi: 10.2210/pdb2mdh/pdb. [DOI] [PubMed] [Google Scholar]
- Birktoft J. J., Fernley R. T., Bradshaw R. A., Banaszak L. J. Amino acid sequence homology among the 2-hydroxy acid dehydrogenases: mitochondrial and cytoplasmic malate dehydrogenases form a homologous system with lactate dehydrogenase. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6166–6170. doi: 10.1073/pnas.79.20.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Courtright J. B., Henning U. Malate dehydrogenase mutants in Escherichia coli K-12. J Bacteriol. 1970 Jun;102(3):722–728. doi: 10.1128/jb.102.3.722-728.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creaghan I. T., Guest J. R. Succinate dehydrogenase-dependent nutritional requirement for succinate in mutants of Escherichia coli K12. J Gen Microbiol. 1978 Jul;107(1):1–13. doi: 10.1099/00221287-107-1-1. [DOI] [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. Isolation of plasmids carrying the arginine repressor gene argR of Escherichia coli K12. Mol Gen Genet. 1980;178(2):447–452. doi: 10.1007/BF00270498. [DOI] [PubMed] [Google Scholar]
- Fernley R. T., Lentz S. R., Bradshaw R. A. Malate dehydrogenase: isolation from E. coli and comparison with the eukaryotic mitochondrial and cytoplasmic forms. Biosci Rep. 1981 Jun;1(6):497–507. doi: 10.1007/BF01121583. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Mossman M. R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Roberts R. E. Cloning, mapping, and expression of the fumarase gene of Escherichia coli K-12. J Bacteriol. 1983 Feb;153(2):588–596. doi: 10.1128/jb.153.2.588-596.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Juni E. Properties of mutants of Escherichia coli lacking malic dehydrogenase and their revertants. J Biol Chem. 1979 May 10;254(9):3570–3575. [PubMed] [Google Scholar]
- Heard J. T., Jr, Butler M. A., Baptist J. N., Matney T. S. Chromosomal location of mutations affecting the electrophoretic mobility of malate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1975 Apr;122(1):329–331. doi: 10.1128/jb.122.1.329-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M., Fritsch M. K., Gorski J. Probable nuclear precursors of preprolactin mRNA in rat pituitary cells. J Biol Chem. 1981 Mar 25;256(6):2597–2600. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. The role and maintenance of the tricarboxylic acid cycle in Escherichia coli. Biochem Soc Symp. 1970;30:155–171. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miles J. S., Guest J. R. Complete nucleotide sequence of the fumarase gene fumA, of Escherichia coli. Nucleic Acids Res. 1984 Apr 25;12(8):3631–3642. doi: 10.1093/nar/12.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Vaughn V., Phillips T. A., Bloch P. L. Gene-protein index of Escherichia coli K-12. Microbiol Rev. 1983 Jun;47(2):231–284. doi: 10.1128/mr.47.2.231-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Shoyab M., Sen A. The isolation of extrachromosomal DNA by hydroxyapatite chromatography. Methods Enzymol. 1979;68:199–206. doi: 10.1016/0076-6879(79)68015-1. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Molecular cloning of four tricarboxylic acid cyclic genes of Escherichia coli. J Bacteriol. 1982 Aug;151(2):542–552. doi: 10.1128/jb.151.2.542-552.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]