Abstract
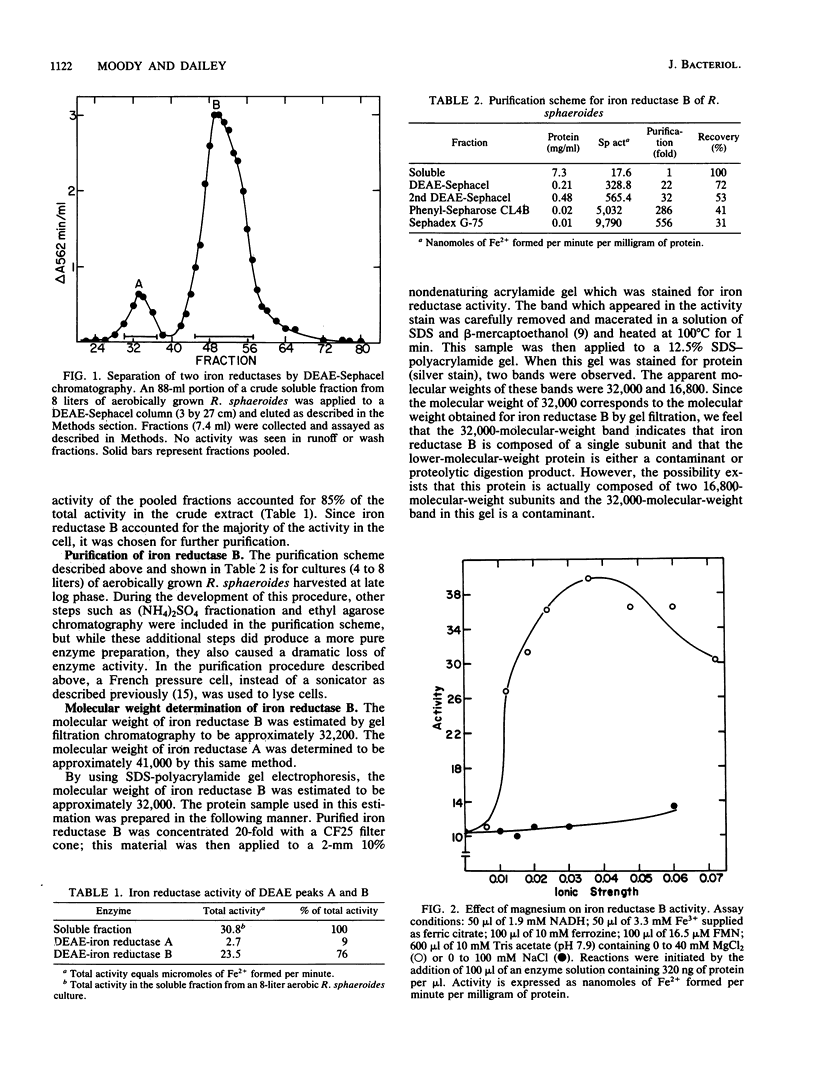
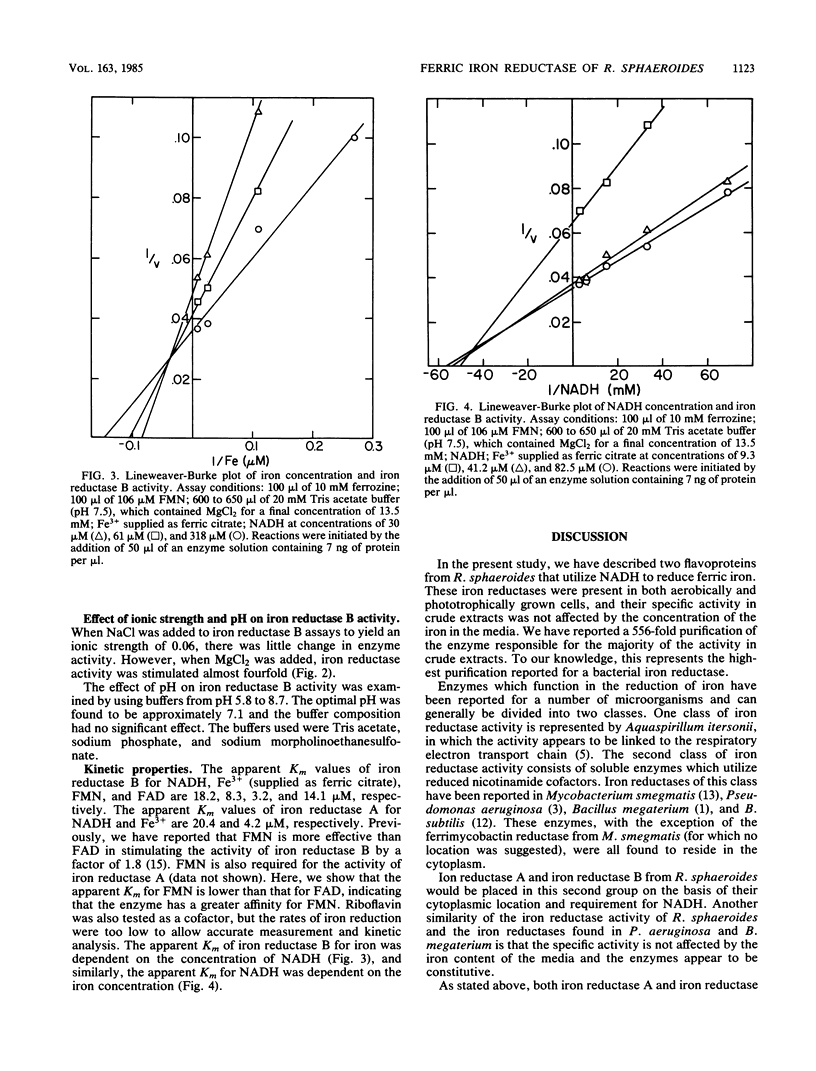
Ferric iron reductase activity was examined in the facultative photosynthetic bacterium Rhodopseudomonas sphaeroides. The specific activities of extracts from cells grown under phototrophic and aerobic conditions were similar and not affected by the concentration of iron in the growth media. The activity was resolved by ion-exchange column chromatography into two fractions, designated iron reductase A and iron reductase B, with molecular weights of 41,000 and 32,000, respectively. Both of these soluble cytoplasmic enzymes required the presence of flavin mononucleotide for activity and utilized NADH to reduce iron supplied as ferric citrate. Iron reductase B was responsible for the majority of activity in crude extracts and was purified 556-fold by conventional protein purification techniques. The apparent Km values of iron reductase B for NADH, Fe3+, and flavin mononucleotide were determined to be 18.2, 8.3, and 3.2 microM, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceneaux J. E., Byers B. R. Ferrisiderophore reductase activity in Bacillus megaterium. J Bacteriol. 1980 Feb;141(2):715–721. doi: 10.1128/jb.141.2.715-721.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron reductases from Pseudomonas aeruginosa. J Bacteriol. 1980 Jan;141(1):199–204. doi: 10.1128/jb.141.1.199-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977 Feb;129(2):815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A. Purification and characterization of membrane-bound ferrochelatase from Rhodopseudomonas sphaeroides. J Biol Chem. 1982 Dec 25;257(24):14714–14718. [PubMed] [Google Scholar]
- Ecker D. J., Lancaster J. R., Jr, Emery T. Siderophore iron transport followed by electron paramagnetic resonance spectroscopy. J Biol Chem. 1982 Aug 10;257(15):8623–8626. [PubMed] [Google Scholar]
- Horio T., Bartsch R. G., Kakuno T., Kamen M. D. Two reduced nicotinamide adenine dinucleotide dehydrogenases from the photosynthetic bacterium, Rhodospirillum rubrum. J Biol Chem. 1969 Nov 10;244(21):5899–5909. [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodge J. S., Gaines C. G., Arceneaux J. E., Byers B. R. Ferrisiderophore reductase activity in Agrobacterium tumefaciens. J Bacteriol. 1982 Feb;149(2):771–774. doi: 10.1128/jb.149.2.771-774.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge J. S., Gaines C. G., Arceneaux J. E., Byers B. R. Heme inhibition of ferrisiderophore reductase in Bacillus subtilis. J Bacteriol. 1982 Nov;152(2):943–945. doi: 10.1128/jb.152.2.943-945.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Aerobic ferrisiderophore reductase assay and activity stain for native polyacrylamide gels. Anal Biochem. 1983 Oct 1;134(1):235–239. doi: 10.1016/0003-2697(83)90290-7. [DOI] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Iron transport and its relation to heme biosynthesis in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Mar;161(3):1074–1079. doi: 10.1128/jb.161.3.1074-1079.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Siderophore utilization and iron uptake by Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1984 Oct;234(1):178–186. doi: 10.1016/0003-9861(84)90339-4. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Page W. J., Huyer M. Derepression of the Azotobacter vinelandii siderophore system, using iron-containing minerals to limit iron repletion. J Bacteriol. 1984 May;158(2):496–502. doi: 10.1128/jb.158.2.496-502.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait G. H. The identification and biosynthesis of siderochromes formed by Micrococcus denitrificans. Biochem J. 1975 Jan;146(1):191–204. doi: 10.1042/bj1460191. [DOI] [PMC free article] [PubMed] [Google Scholar]



