Abstract
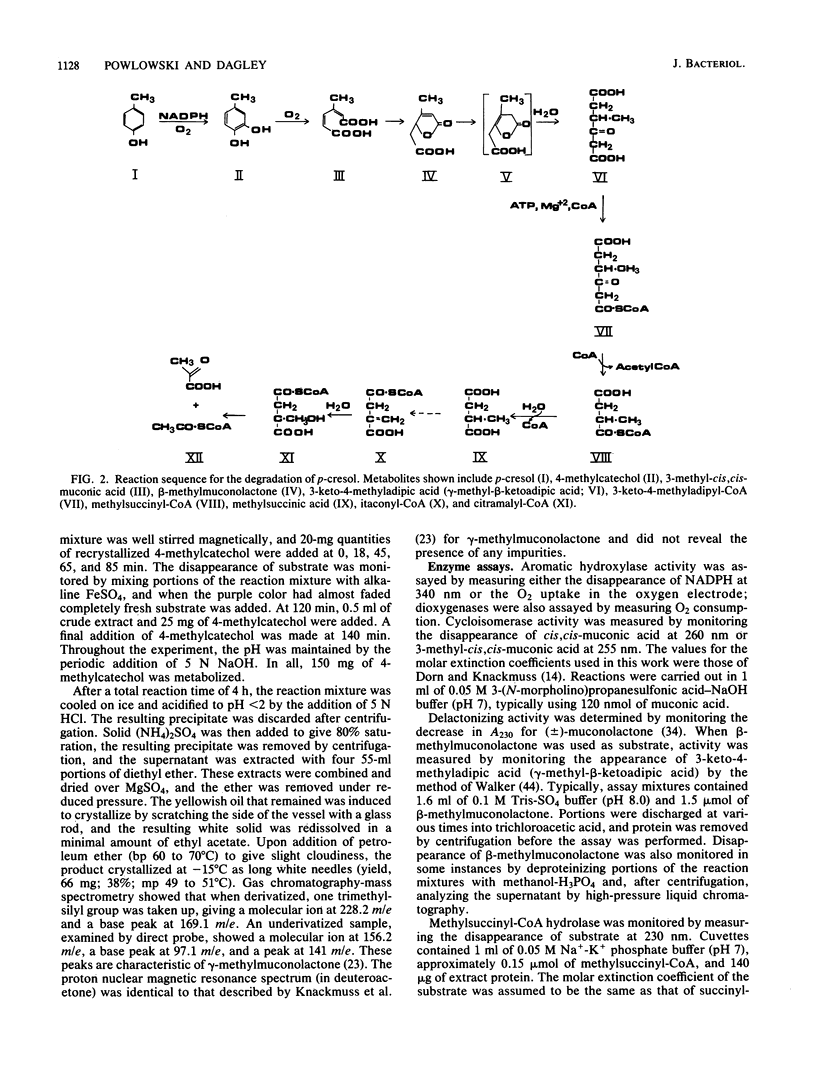
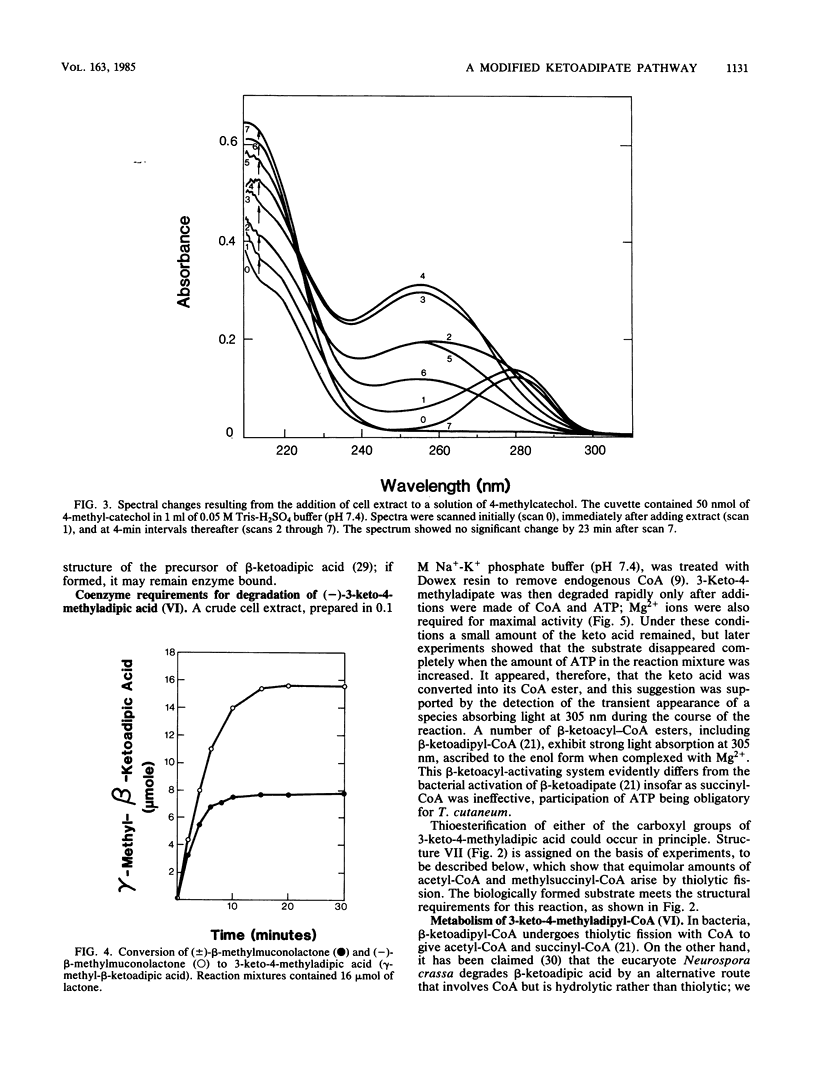
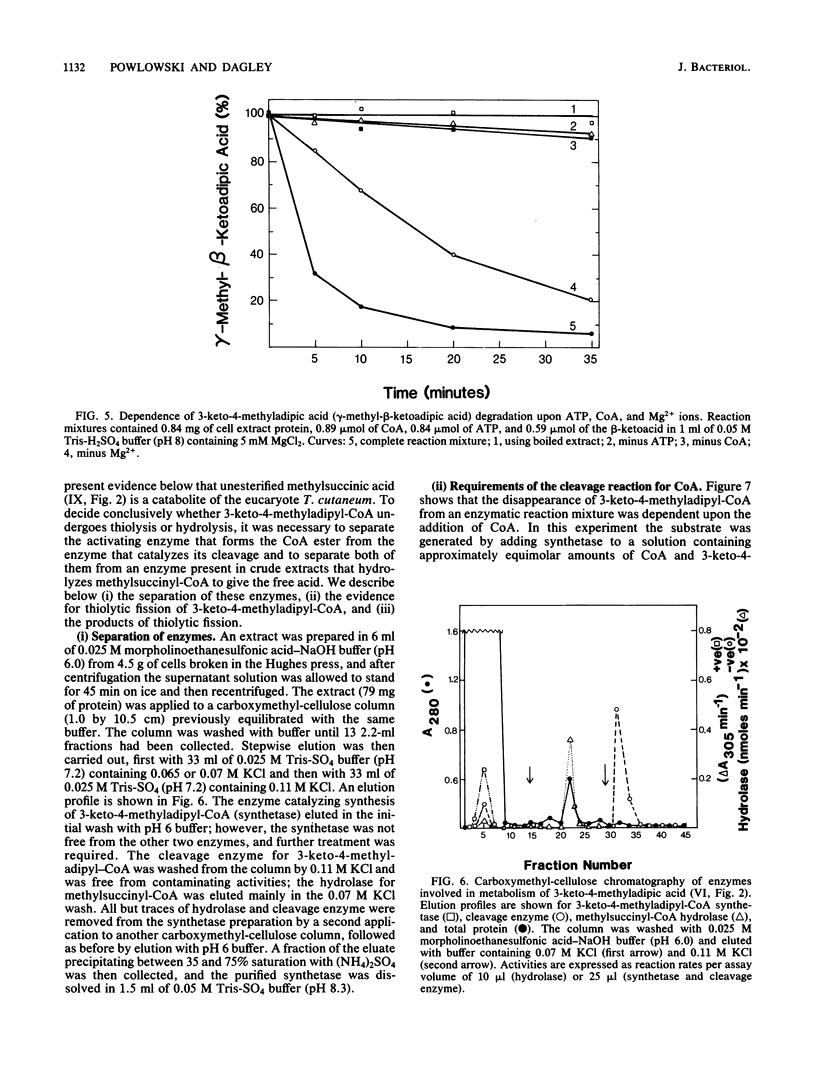
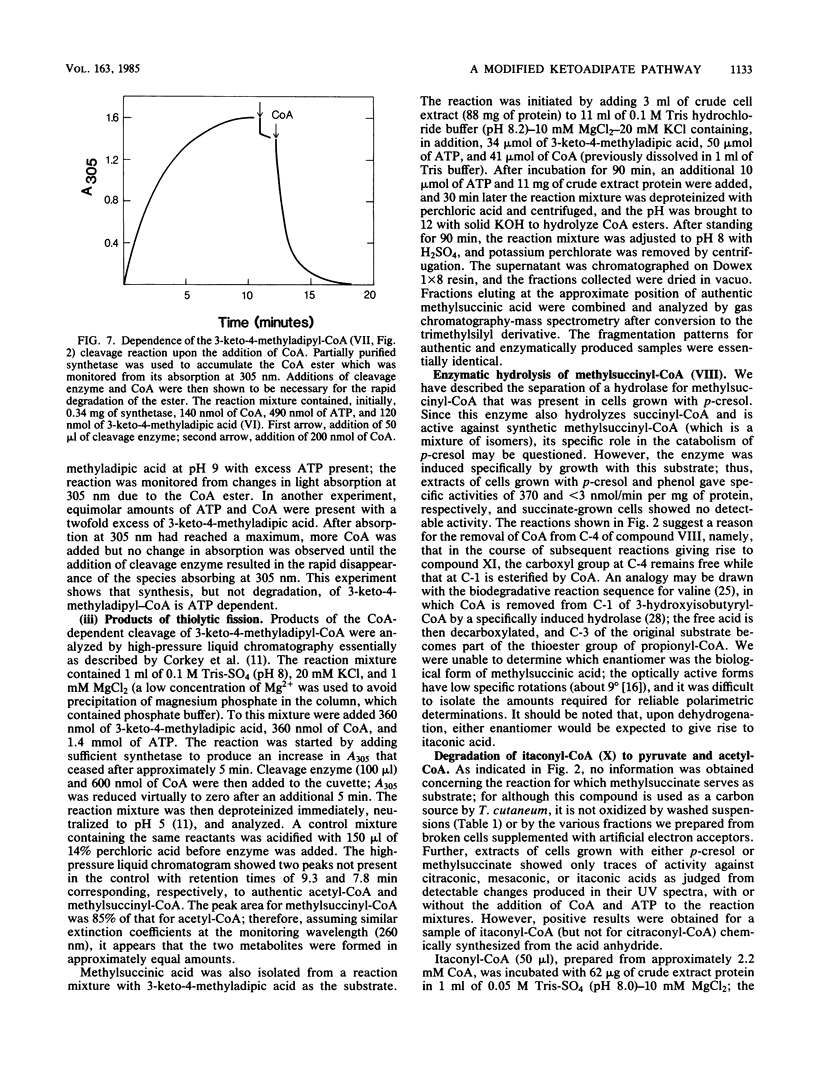
Trichosporon cutaneum, when grown with p-cresol, catalyzed intradiol fission of the benzene nucleus of 4-methylcatechol before the complete catabolism of these two substrates. Steps in their conversion to pyruvate and acetyl coenzyme A were investigated by using cell extracts, and some properties of various new microbial catabolites are also described. These included (-)-2,5-dihydro-3-methyl-5-oxofuran-2-acetic acid (beta-methylmuconolactone) and (-)-3-keto-4-methyladipic acid and its coenzyme A ester; the latter was degraded by an enzymatic reaction sequence that included the coenzyme A esters of methylsuccinic, itaconic, and citramalic acids. A notable feature of this sequence is the formation of beta-methylmuconolactone which can be readily metabolized, in contrast to the analogous reaction in bacteria that gives the "dead-end" compound gamma-methylmuconolactone; this compound cannot be enzymatically degraded and so renders the beta-ketoadipate pathway unavailable for methyl-substituted bacterial sources of carbon that are catabolized by way of 4-methylcatechol.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER J., WANG S. F., LARDY H. A. The metabolism of itaconic acid by liver mitochondria. J Biol Chem. 1957 Dec;229(2):865–879. [PubMed] [Google Scholar]
- ALLRED J. B. INTERFERENCE BY THIOLS IN ACETOACETATE DETERMINATION. Anal Biochem. 1965 Apr;11:138–143. doi: 10.1016/0003-2697(65)90053-9. [DOI] [PubMed] [Google Scholar]
- Anderson J. J., Dagley S. Catabolism of aromatic acids in Trichosporon cutaneum. J Bacteriol. 1980 Feb;141(2):534–543. doi: 10.1128/jb.141.2.534-543.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. J., Dagley S. Catabolism of tryptophan, anthranilate, and 2,3-dihydroxybenzoate in Trichosporon cutaneum. J Bacteriol. 1981 Apr;146(1):291–297. doi: 10.1128/jb.146.1.291-297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSCH H., HURLBERT R. B., POTTER V. R. Anion exchange chromatography of acids of the citric acid cycle. J Biol Chem. 1952 May;196(2):717–727. [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANTRENNE H., LIPMANN F. Coenzyme A dependence and acetyl donor function of the pyruvate-formate exchange system. J Biol Chem. 1950 Dec;187(2):757–767. [PubMed] [Google Scholar]
- Catelani D., Fiecchi A., Galli E. Dextro-gamma-carboxymethyl-gamma-methyl-delta-alpha-butenolide. A 1,2-ring-fission product of 4-methylcatechol by Pseudomonas desmolyticum. Biochem J. 1971 Jan;121(1):89–92. doi: 10.1042/bj1210089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The utilization of itaconate by Pseudomonas sp. Biochem J. 1964 Apr;91(1):82–91. doi: 10.1042/bj0910082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkey B. E., Brandt M., Williams R. J., Williamson J. R. Assay of short-chain acyl coenzyme A intermediates in tissue extracts by high-pressure liquid chromatography. Anal Biochem. 1981 Nov 15;118(1):30–41. doi: 10.1016/0003-2697(81)90152-4. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., PATEL M. D. Oxidation of p-cresol and related compounds by a Pseudomonas. Biochem J. 1957 Jun;66(2):227–233. doi: 10.1042/bj0660227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley S., Geary P. J., Wood J. M. The metabolism of protocatechuate by Pseudomonas testosteroni. Biochem J. 1968 Oct;109(4):559–568. doi: 10.1042/bj1090559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hareland W. A., Crawford R. L., Chapman P. J., Dagley S. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol. 1975 Jan;121(1):272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. The enzymic degradation of alkyl-substituted gentisates, maleates and malates. Biochem J. 1971 Mar;122(1):29–40. doi: 10.1042/bj1220029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATAGIRI M., HAYAISHI O. Enzymatic degradation of beta-ketoadipic acid. J Biol Chem. 1957 May;226(1):439–448. [PubMed] [Google Scholar]
- Keat M. J., Hopper D. J. P-cresol and 3,5-xylenol methylhydroxylases in Pseudomonas putida N.C.I.B. 9896. Biochem J. 1978 Nov 1;175(2):649–658. doi: 10.1042/bj1750649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Fujisawa H., Nakazawa A., Nakazawa T., Kanetsuna F., Taniuchi H., Nozaki M., Hayaishi O. Studies on pyrocatechase. I. Purification and spectral properties. J Biol Chem. 1967 Jul 25;242(14):3270–3278. [PubMed] [Google Scholar]
- Massey L. K., Sokatch J. R., Conrad R. S. Branched-chain amino acid catabolism in bacteria. Bacteriol Rev. 1976 Mar;40(1):42–54. doi: 10.1128/br.40.1.42-54.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAGAWA H., INOUE H., TAKEDA Y. CHARACTERISTICS OF CATECHOL OXYGENASE FROM BREVIBACTERIUM FUSCUM. J Biochem. 1963 Jul;54:65–74. doi: 10.1093/oxfordjournals.jbchem.a127748. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Varga J. M. Degradation of phenols by intact cells and cell-free preparations of Trichosporon cutaneum. Eur J Biochem. 1970 Mar 1;13(1):37–44. doi: 10.1111/j.1432-1033.1970.tb00896.x. [DOI] [PubMed] [Google Scholar]
- OTTEY L., TATUM E. L. The cleavage of beta-ketoadipic acie by Neurospora crassa. J Biol Chem. 1957 Nov;229(1):77–83. [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Felix A., Lillard M. O. Catechol 1,2-dioxygenase from Acinetobacter calcoaceticus: purification and properties. J Bacteriol. 1976 Jul;127(1):536–544. doi: 10.1128/jb.127.1.536-544.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powlowski J. B., Ingebrand J., Dagley S. Enzymology of the beta-ketoadipate pathway in Trichosporon cutaneum. J Bacteriol. 1985 Sep;163(3):1136–1141. doi: 10.1128/jb.163.3.1136-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R., STANIER R. Y. The mechanism of formation of beta-ketoadipic acid by bacteria. J Biol Chem. 1954 Oct;210(2):821–836. [PubMed] [Google Scholar]
- STANIER R. Y., SLEEPER B. P., TSUCHIDA M., MACDONALD D. L. The bacterial oxidation of aromatic compounds; III. The enzymatic oxidation of catechol and protocatechuic acid to beta-ketoadipic acid. J Bacteriol. 1950 Feb;59(2):137–151. doi: 10.1128/jb.59.2.137-151.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Burbee D. G., Dagley S. Catabolism of L-tyrosine in Trichosporon cutaneum. J Bacteriol. 1979 May;138(2):425–430. doi: 10.1128/jb.138.2.425-430.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra S. F. Degradation of tyrosine in anaerobically stored piggery wastes and in pig feces. Appl Environ Microbiol. 1978 Nov;36(5):631–638. doi: 10.1128/aem.36.5.631-638.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Neujahr H. Y. Purification and properties of catechol 1,2-oxygenase from Trichosporon cutaneum. Eur J Biochem. 1970 Feb;12(3):427–434. doi: 10.1111/j.1432-1033.1970.tb00869.x. [DOI] [PubMed] [Google Scholar]
- WALKER P. G. A colorimetric method for the estimation of acetoacetate. Biochem J. 1954 Dec;58(4):699–704. doi: 10.1042/bj0580699. [DOI] [PMC free article] [PubMed] [Google Scholar]



