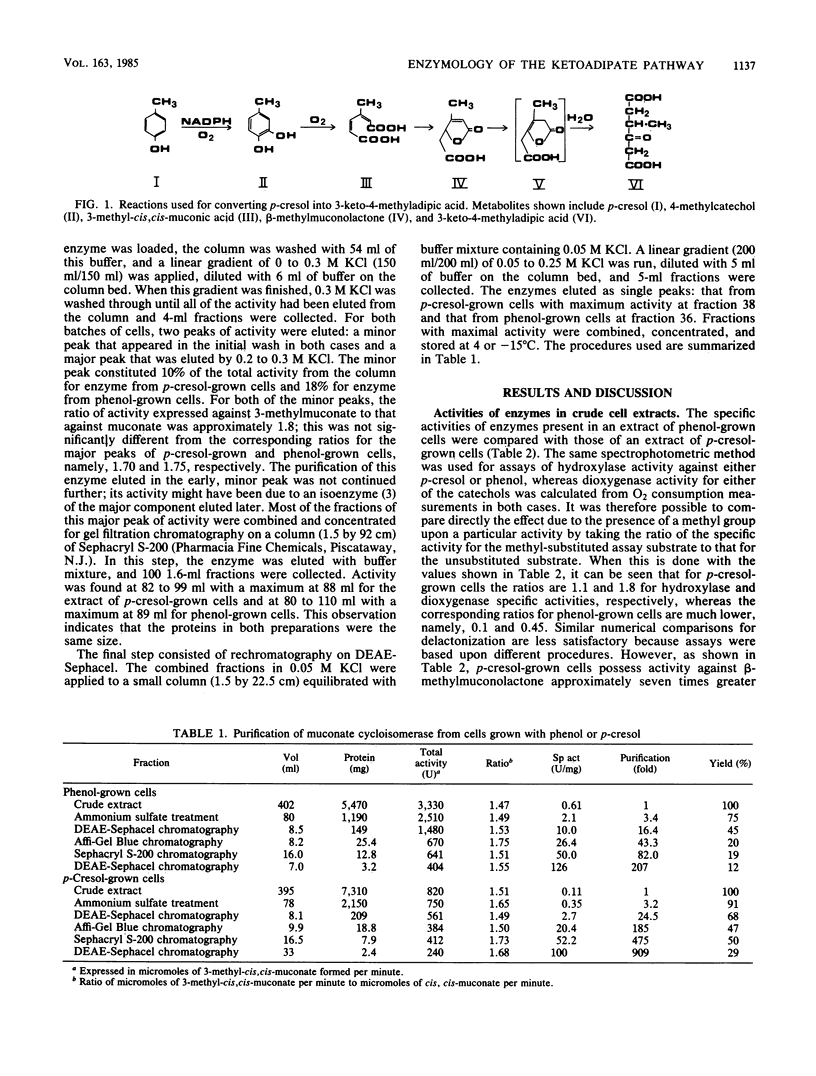
Abstract
Cell extracts were prepared from Trichosporon cutaneum grown with phenol or p-cresol, and activities were assayed for enzymes catalyzing conversion of these two carbon sources into 3-ketoadipate (beta-ketoadipate) and 3-keto-4-methyladipate, respectively. When activities of each enzyme were expressed as a ratio, the rate for methyl-substituted substrate being divided by that for the unsubstituted substrate, it was apparent that p-cresol-grown cells elaborated pairs of enzymes for hydroxylation, dioxygenation, and delactonization. One enzyme of each pair was more active against its methyl-substituted substrate, and the other was more active against its unsubstituted substrate. Column chromatography was used to separate two hydroxylase activities and also 1,2-dioxygenase activities; the catechol 1,2-dioxygenases were further purified to electrophoretic homogeneity. Extracts of phenol-grown cells contained only those enzymes in this group that were more active against unsubstituted substrates. In contrast, whether cells were grown with phenol or p-cresol, only one muconate cycloisomerase (lactonizing enzyme) was elaborated which was more active against 3-methyl-cis,cis-muconate than against cis,cis-muconate; in this respect it differed from a cycloisomerase of another strain of T. cutaneum which has been characterized. The cycloisomerase was purified from both phenol-grown and p-cresol-grown cells, and some characteristics were determined.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal A., Neujahr H. Y. cis,cis-Muconate cyclase from Trichosporon cutaneum. Biochem J. 1980 Oct 1;191(1):37–43. doi: 10.1042/bj1910037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATAGIRI M., HAYAISHI O. Enzymatic degradation of beta-ketoadipic acid. J Biol Chem. 1957 May;226(1):439–448. [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. I. Properties of cis,cis-muconate-lactonizing enzyme and muconolactone isomerase from Pseudomonas putida. Biochemistry. 1973 Aug 28;12(18):3523–3530. doi: 10.1021/bi00742a027. [DOI] [PubMed] [Google Scholar]
- Nakai C., Kagamiyama H., Saeki Y., Nozaki M. Nonidentical subunits of pyrocatechase from Pseudomonas arvilla C-1. Arch Biochem Biophys. 1979 Jun;195(1):12–22. doi: 10.1016/0003-9861(79)90322-9. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Gaal A. Phenol hydroxylase from yeast. Purification and properties of the enzyme from Trichosporon cutaneum. Eur J Biochem. 1973 Jun;35(2):386–400. doi: 10.1111/j.1432-1033.1973.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Kjellén K. G. Phenol hydroxylase from yeast. Reaction with phenol derivatives. J Biol Chem. 1978 Dec 25;253(24):8835–8841. [PubMed] [Google Scholar]
- Neujahr H. Y., Kjellén K. G. Phenol hydroxylase from yeast: a lysyl residue essential for binding of reduced nicotinamide adenine dinucleotide phosphate. Biochemistry. 1980 Oct 28;19(22):4967–4972. doi: 10.1021/bi00563a005. [DOI] [PubMed] [Google Scholar]
- Powlowski J. B., Dagley S. beta-Ketoadipate pathway in Trichosporon cutaneum modified for methyl-substituted metabolites. J Bacteriol. 1985 Sep;163(3):1126–1135. doi: 10.1128/jb.163.3.1126-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R., STANIER R. Y. The mechanism of formation of beta-ketoadipic acid by bacteria. J Biol Chem. 1954 Oct;210(2):821–836. [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Sze I. S., Dagley S. Properties of salicylate hydroxylase and hydroxyquinol 1,2-dioxygenase purified from Trichosporon cutaneum. J Bacteriol. 1984 Jul;159(1):353–359. doi: 10.1128/jb.159.1.353-359.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher D. R., Cain R. B. Metabolism of aromatic compounds by fungi. 1. Purification and physical properties of 3-carboxy-cis-cis-muconate cyclase from Aspergillus niger. Eur J Biochem. 1974 Oct 2;48(2):549–556. doi: 10.1111/j.1432-1033.1974.tb03796.x. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Neujahr H. Y. Purification and properties of catechol 1,2-oxygenase from Trichosporon cutaneum. Eur J Biochem. 1970 Feb;12(3):427–434. doi: 10.1111/j.1432-1033.1970.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Hori K., Fujiwara M., Saeki Y., Kagamiyama H. Nonidentical subunits of protocatechuate 3,4-dioxygenase. Biochemistry. 1976 Sep 7;15(18):4048–4053. doi: 10.1021/bi00663a020. [DOI] [PubMed] [Google Scholar]



