Abstract
The complex carbohydrate molecule globo H hexasaccharide has been synthesized, conjugated to keyhole limpet hemocyanin, and administered with the immunologic adjuvant QS-21 as a vaccine for patients with prostate cancer who have relapsed after primary therapies such as radiation or surgery. Globo H is one of several candidate antigens present on prostate cancer cells that can serve as targets for immune recognition and treatment strategies. The vaccine, given as five subcutaneous vaccinations over 26 weeks, has been shown to be safe and capable of inducing specific high-titer IgM antibodies against globo H. Its immunogenicity was confirmed in prostate cancer patients with a broad range of stages and tumor burdens. Observations of several patients who had evidence of disease relapse restricted to a rising biochemical marker, prostate-specific antigen (PSA), indicated that a treatment effect could occur within 3 months after completion of the vaccine therapy. This effect was manifested as a decline of the slope of the log of PSA concentration vs. time plot after treatment compared with values before treatment. Five patients continue to have stable PSA slope profiles in the absence of any radiographic evidence of disease for more than 2 years. The concept of using PSA slope profiles in assessing early treatment effects in biological therapies such as vaccines awaits further validation in phase II and III trials. The use of a variety of lesser known candidate glycoprotein and carbohydrate antigens in prostate cancer serves as a focus for the development of a multivalent vaccine of the treatment of relapsed prostate cancer in patients with minimal tumor burden.
Oncogenesis is often associated with changes in the expression of cell surface carbohydrates. In some instances, the carbohydrate pattern may be specific to the disease type. In others, the expression level may be markedly enhanced by the onset of disease. Several of these tumor-associated antigens, including GM2, GD2, GD3, fucosyl GM1, STn and Lewisy, have now been purified and conjugated to a protein carrier such as keyhole limpet hemocyanin (KLH) (1–6). After vaccination of mice and co-administration with the immunologic adjuvant QS-21, consistent induction of IgM and IgG antibodies, reactive with tumor cells, has been noted. The vaccine-induced antibody response against GM2 has been associated with an improved disease-free survival (7, 8). In this report we focus on the globo H tumor-associated constellation.
Globo H (Fucα1–2Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4Glc) (9, 10) is a hexasaccharide, originally isolated as a ceramide-linked glycolipid by Hakomori and co-workers (9), from the human breast cancer cell line MCF-7. It is expressed at the cancer cell surface as a glycolipid, and possibly as a glycoprotein (11, 12). Globo H has been further characterized by several methods, including immunohistochemistry using the murine monoclonal antibody (mAb) MBr1 (9, 13). These studies demonstrated the expression of cell surface carbohydrates, assumed to be globo H, which react with the MBr1 antibody on many normal tissues (12, 13), including normal breast, pancreas, small bowel, and prostate tissue. The antigen in these tissues is, however, predominantly localized at the secretory borders where access to the immune system is restricted. However, there is enhanced expression of MBr1-positive antigens on both primary and metastatic prostate cancer specimens (14). These two findings provide the rationale for evaluating globo H as a candidate target antigen in clinical trials for patients with relapsed prostate cancer.
Bilodeau et al. (15) reported the total synthesis of globo H hexasaccharide by chemical means. Ragupathi et al. (16) demonstrated that whereas mAb MBr1 bound optimally to the terminal four sugars of the hexasaccharide, mice immunized with the hexasaccharide conjugate produced antibodies against all portions of the molecule. Because Helling et al. (17) had shown increased immunogenicity of GM2-KLH conjugates when QS-21 was used as an immunologic adjuvant, mice were immunized with globo H hexasaccharide conjugated to the carrier molecule KLH plus QS-21 (16). All mice made high-titer IgM and IgG responses against globo H antigen (median titers 1/128,000 and 1/2,560, respectively). These sera reacted with globo H-positive cancer cells by immune adherence and fluorescence-activated cell sorting assays (16), thereby suggesting that a glycoconjugate of globo H hexasaccharide might be useful in potential vaccine strategies.
On the basis of this experience in experimental animal models, which demonstrated the immunogenicity of synthetic globo H hexasaccharide conjugates, its expression on human prostate cancers, and its restricted expression on normal tissues, we selected this antigen as one of several well-defined candidates as a cell surface target on prostate cancer cells for immune stimulation. Accordingly, we have initiated a phase I dose-seeking and vaccine safety trial in prostate cancer patients who had relapsed after prostatectomy or radiation therapy. The carbohydrate moiety of the vaccine we would be studying would be the most complex such agent ever prepared by total synthesis and brought to the stage of clinical evaluation.
The results described herein demonstrate safe and successful induction of specific antibodies against globo H by using the synthetic carbohydrate-protein construct in patients with relapsed prostate cancer. This vaccine trial has also attempted to assess the implications of prostate-specific antigen (PSA) slope (slope of the natural log of PSA concentration vs. time plot) in monitoring potential treatment effects. This was attempted by comparing pre- with post-treatment PSA profiles. The detection of changes in the pre- versus posttreatment PSA slopes is suggestive of a treatment effect.
METHODS
Vaccine Preparation.
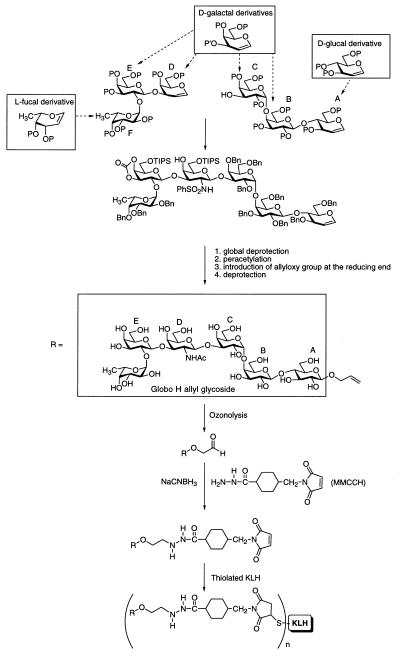
Globo H hexasaccharide was synthesized by using the glycal assembly approach to oligosaccharide synthesis. The construct was armed with a terminal allyl group (in place of ceramide) [16, 18 (a preliminary “proof of principle” study), 19]. The allyl group at the terminus of the fully synthetic construct was converted to an aldehyde group by ozonolysis and linked to the -NH2 group on the bifunctional cross-linker 4-(4-N-maleimidomethyl)cyclohexane-1-carbonylhydrazide [MMCCH; Pierce (20)]. The maleimide group of MMCCH was reacted with KLH (Intracel, Rockville, MD), as shown in Fig. 1, which had been thiolated by treatment with 2-iminothiolane, which converts -NH2 groups in KLH to sulfhydryl groups. The activated globo H was then mixed with thiolated KLH and incubated at room temperature for 2 hr. The globo H-KLH conjugate was washed, filtered to confirm sterility, and distributed into individual vials so that they contained 3, 10, 30, or 100 μg in saline. The globo H/KLH molar ratio was 716 determined by the high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) assay for carbohydrate after acid hydrolysis and Bio-Rad (20) assay for proteins. The vaccine conjugate was administered with 100 μg of QS-21 (Aquila Biopharmaceuticals, Framingham, MA) and used under an investigational new drug license with the Food and Drug Administration held by the Memorial Sloan–Kettering Cancer Center.
Figure 1.
Synthesis of globo H-KLH conjugate after ozone cleavage of globo H allyl glycoside and coupling to thiolated KLH by the cross-linker MMCCH method (n ≅ 720). P, protecting group; TIPS, triisopropylsilyl; Bn, benzyl.
Patient Eligibility.
Patients with relapsed prostate cancer were eligible for the study with the following restrictions: Karnofsky performance status >60%, white blood cell count ≥3,500/mm3, platelet count ≥100,000/mm3, bilirubin <2.0 mg/100 ml or serum glutamate–oxaloacetate transaminase < 3.0 times the upper limit of normal, and serum creatinine ≤2.0 mg/100 ml or creatinine clearance ≥40 ml/min. Patients had to have recovered from the toxicity of any prior therapy and not have received chemotherapy or radiation therapy for at least 4 weeks prior to entry into the trial. No history of an active secondary malignancy within the prior 5 years except for nonmelanoma skin cancer was permitted. All patients gave informed consent.
To be entered into the trial, patients must have exhibited evidence of three consecutively rising PSA status determined at a minimal interval of 2 weeks based on the following criteria: patients who had undergone prostatectomy must have had a minimal PSA entry value of 1.0 ng/ml with a 50% increase in range of values; patients who had undergone radiation therapy must have minimal PSA entry value of 2.0 ng/ml with a 50% increase in range of values; patients who were androgen independent must have a minimal PSA value of 10.00 with 25% increase in range of values.
Dose and Immunization Schedule.
Four sequential groups of 5 patients received globo H-KLH vaccine at the following doses of globo H-KLH per vaccination: 10, 30, 100, and 3 μg corresponding to dose levels 1, 2, 3, and 4, respectively. The fourth dose level was to be 3 or 300, depending on the antibody response seen with the first three dose levels. Because the three dose levels gave similar results, 3 μg was selected as the fourth dose level to find the lowest immunogenic dose. The globo H-KLH conjugate was administered subcutaneously to random sites on the upper arm and upper leg at weekly intervals for 3 weeks. This schedule was followed by an interval of 4 weeks until week 7, when a fourth vaccination was given, then, at week 19 (3 months later), a fifth vaccination was given. A sixth vaccination was given at week 50 (6 months after completion of therapy) in some cases if the patients’ antibody titers had declined by ≥50%. To safeguard against the possibility of autoimmune injury by antibodies induced to globo H, the renal function of patients was assessed by creatinine clearance, and gastrointestinal toxicity as manifested by occult blood was assessed by routine rectal examinations with stool guaiac.
Serologic Assays.
Serum samples were obtained at weeks 1, 2, 3, 7, 9, 13, 19, and 26 after immunization according to the protocol. Patients were monitored expectantly upon completion of the vaccine trial on a monthly basis for the first 6 months then at 2-month intervals, at which time routine blood samples were drawn for both immunologic studies and biochemical markers such as PSA and acid phosphatase. IgM and IgG antibody titers were measured by ELISA as described previously (17, 21), and IgG subclasses were determined by using IgG subclass-specific antibodies (Zymed). Briefly, 96-well flat-bottomed plates (Nunc) were precoated with globo H ceramide at 0.2 μg per well. Serially diluted sera in 1.5% human serum albumin (HSA) were added in triplicate along with sera from patients with known specific high-titer IgM and IgG antibodies or no antibodies, which served as positive and negative controls, respectively. Mouse anti-human IgM or IgG antibodies (Southern Biotechnology) and goat anti-human IgM or IgG conjugated to alkaline phosphatase (AP) (Southern Biotechnology) were used to complete the assay. Plates were read at 10–15 min for IgG and 25–30 min for IgM or until the positive control wells underwent colorimetric changes at 414 nm on an ELISA plate reader (Bio-Rad model 550 Microplate Reader). The titer was defined as the highest dilution yielding an optical density of ≥0.1.
Flow Cytometric Analysis.
Pre- and posttreatment sera were diluted 2- to 4-fold from their maximum antibody titer determined by ELISA and incubated with the MCF-7 cells for 1 hr on ice, washed, treated with goat anti-human IgM or IgG labeled with fluorescein isothiocyanate (FITC), and analyzed by flow cytometry for mean percent positive cells. Anti-globo H antibody VK-9 (22) served as a positive control.
Complement-Mediated Cytotoxicity Assays.
Sera at a dilution of 1:10 were evaluated for mediating lysis by complement in a 4-hr 51Cr release assay as described elsewhere (17, 21). Cells from the globo H−, GD3+ human melanoma cell line SKMEL28, globo H+ human breast cancer cell line MCF-7, and globo H+ human bladder cancer cell line MGH labeled with 100 μCi of Na251CrO4 (New England Nuclear; 1 μCi = 37 kBq) served as targets. Anti-GD3 mAb R24 (22) (provided by Paul Chapman, Memorial Sloan–Kettering Cancer Center), anti-globo H mAb VK-9, and mAb MBr1 (provided by Maria Colnaghi, Istituto Nazionale, Milan), and patient sera both before and after immunization were tested. Rabbit or human complement diluted 1:5 was added to the appropriate wells. Supernatants from plates were harvested by using the Skatron Collection System, and radioactivity on discs was counted for 1 min in a γ counter. Control wells included those for maximum release of isotope in 1% SDS and for spontaneous release in the absence of complement with medium alone. The percentage of specific lysis was calculated as follows: % cytotoxicity = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] ×100.
Pre- and Post-Therapy Evaluations.
Interval safety assessments included a patient diary. Antitumor assessments were performed during week 13. If the patient did not demonstrate radiographic progression of disease, the patient received a fifth vaccination on week 19. The patient was restaged at week 13, at the completion of the trial at week 26, and every 3 months thereafter. Patients were seen monthly for the first 6 months, then every 2–3 months thereafter. Blood samples for immune studies were drawn at the time of each clinic visit.
Biostatistical Assessment of PSA Slopes.
Twenty patients were accrued to the trial, of whom 18 were evaluatable. Of the two patients taken out of the study, one patient had an acute septic event unrelated to the vaccine, and the other had sudden symptomatic clinical progression of disease within 3 weeks of starting vaccine treatment. The six disease-free patients, who continue to be monitored expectantly after completion of the trial, were examined with respect to changes in the log of serum PSA concentration over time. Pre- and posttreatment PSA slopes, defined as the least squares lines calculated from a minimum of three PSA measurements recorded within a 6-month interval before treatment, were examined and compared with posttreatment log slopes. The 95% confidence intervals for both slope estimates were calculated by using bootstrap techniques (23).
RESULTS
Patient Demographics.
Twenty patients (age range 57–79), of whom 18 were evaluatable, were accrued to the trial. Nine of the 18 patients had no radiographic evidence of disease. Of these, 8 patients were hormone naive and 1 patient had received intermittent hormone treatment. The remaining 9 patients had evidence of metastatic disease in bone, and 1 patient had disease in a single retroperitoneal lymph node. Of these patients, only 2 of 9 had been on hormones intermittently.
Vaccine Safety.
All 18 patients completed the five immunizations. Twelve patients (66%) experienced a grade II local reaction lasting 2–4 days and consisting of erythema, edema, and swelling at the injection site, and 4 (22%) had grade I reactivity. One patient had grade II chills, and another grade II fatigue, while 2 patients had grade II fever. No renal or gastrointestinal toxicity was observed.
Humoral Response to Vaccine.
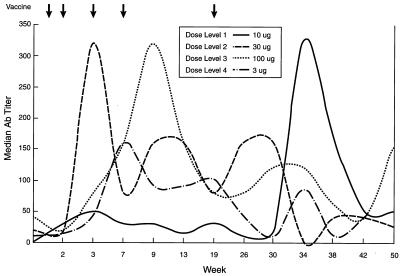
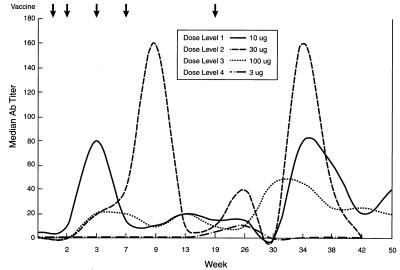
The median IgM and IgG antibody titers against globo H by ELISA prior to vaccination were 0 and 1:20, respectively. IgM and IgG antibodies developed as early as week 3 at all dose levels (Figs. 2 and 3). However, by week 9, median IgM and IgG antibody titers had reached their peak at all doses. IgM was the predominant antibody expressed with the exception of one patient, no. 10, who generated a high-titer IgG antibody response. Of five patients treated at the 30-μg dose level, one patient developed an IgM antibody titer of 1:10,240 by week 3, which declined to 1:320 by week 26. Another patient developed an IgG titer of 1:20,480 by week 3, which was maintained until week 9; this declined to 1:10,240 by week 26. Both patients had significant metastatic disease in bone. Although the median titers do not reflect these outlying values, the 30-μg dose level appeared to be optimal. As seen in Figs. 2 and 3, most antibody titers declined by week 19, the time of the fifth and final vaccination, and subsequently rose to close to peak titers between weeks 34 and 38. Most antibody titers, although still detectable at week 50, had clearly declined by at least 50% from the maximum titers. Antibodies were mainly of the IgG1 and IgG3 subtype as determined by ELISA.
Figure 2.
Time course of median IgM antibody titer as determined by ELISA with globo H ceramide after five vaccinations.
Figure 3.
Time course of median IgG antibody titer as determined by ELISA with globo H ceramide after five vaccinations. Note that antibody titers are on a different scale than in Fig. 2.
Nine patients received a booster immunization at week 50, with the same dosage as the primary vaccines. Two patients from dose level 1 received booster immunizations at 10 μg, four patients from dose level 2 received boosters at 30 μg, and three patients from dose level 3 received boosters at 100 μg.
Reactivity of Pre- and Posttreatment Sera with MCF-7 Cell Line.
Reactivity of pre- and posttreatment sera against MCF-7 cell line was assayed by fluorescence-activated cell sorting; pretreatment sera were gated to reactivity between 9% and 11% and compared with posttreatment reactivity (Table 1). The posttreatment sera of 10 of 18 patients demonstrated 2- to 9-fold increased IgM reactivity compared with pretreatment sera. No significant IgG reactivity was noted, with the exception of patients 8 and 9.
Table 1.
Flow cytometry on sera from 18 patients
| Patient no. | Dose level | μg | %
positive cells
|
|||
|---|---|---|---|---|---|---|
| IgM
|
IgG
|
|||||
| Pre- | Post- | Pre- | Post- | |||
| 1 | 1 | 10 | 9.6 | 12.8 | 9.1 | 9.9 |
| 2 | 1 | 10 | 10.9 | 30.7 | 10.1 | 10.8 |
| 3 | 1 | 10 | 9.9 | 25.2 | 9.2 | 4.9 |
| 4 | 1 | 10 | 10.8 | 26.4 | 12.7 | 10.4 |
| 6 | 2 | 30 | 10.8 | 52.2 | 10.5 | 10.4 |
| 7 | 2 | 30 | 9.0 | 11.2 | 10.5 | 10.3 |
| 8 | 2 | 30 | 10.5 | 96.9 | 9.5 | 34.9 |
| 9 | 2 | 30 | 11.1 | 47.5 | 11.7 | 33.3 |
| 10 | 2 | 30 | 9.5 | 16.3 | 9.7 | 14.2 |
| 11 | 3 | 100 | 10.5 | 23.2 | 10.4 | 8.2 |
| 12 | 3 | 100 | 9.9 | 32.4 | 10.1 | 13.5 |
| 13 | 3 | 100 | 10.9 | 63.0 | 9.2 | 12.9 |
| 14 | 3 | 100 | 10.6 | 19.8 | 9.4 | 15.9 |
| 15 | 3 | 100 | 9.6 | 33.6 | 11.4 | 12.7 |
| 16 | 4 | 3 | 11.4 | 19.9 | 10.6 | 5.9 |
| 17 | 4 | 3 | 9.9 | 4.7 | 9.9 | 13.2 |
| 18 | 4 | 3 | 9.1 | 7.1 | 10.3 | 2.2 |
| 19 | 4 | 3 | 8.5 | 8.9 | 11.3 | 6.3 |
| MBr1 mAb | 64.7 | — | ||||
| VK-9 mAb | — | 62.1 | ||||
| Unstained | 0.9 | 1.7 | ||||
Patients’ pre- and post-treatment sera were incubated with MCF-7 cells and then with FITC-conjugated anti-human IgM or IgG antibody. The percent fluorescent cells incubated with pretreatment sera were gated to have values of approximately 10% and were compared with posttreatment values. Positive controls included anti-globo H mAb VK9. Patient 5 was taken out of the study because of overwhelming infection. Patient 20 was taken out because of rapid progression of disease.
Complement-Mediated Lysis.
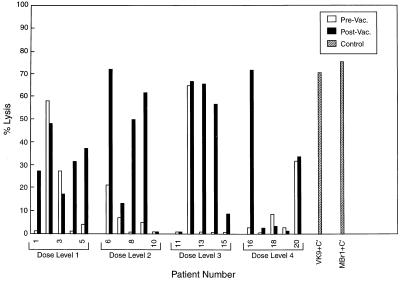
Pre- and postimmunization sera from 18 patients were evaluated for the ability to mediate lysis by complement. As seen in Fig. 4, sera from 9 patients showed clear increase in complement-mediated lysis after vaccination (patients 1, 4, 5, 6, 8, 9, 13, 14, and 16) against MCF-7 in the presence of either rabbit or human serum as complement source. MCF-7 cells did not bind complement alone or in concert with control antibody R24, whereas SK-MEL-28 showed consistent complement-mediated lysis with mAb R24 but not with complement alone or mAbs VK9 or MBr1. Both VK9 and MBr1 mediated ≥60% lysis of MCF-7 and MGH cell lines.
Figure 4.
Complement-mediated lysis of MCF-7 tumor cells by pre- and postimmunization sera from 18 evaluatable patients. Positive controls included MCF-7 cells plus mAb VK9 (IgG) and MBr1 (IgM) with complement.
Preliminary Evaluation of Treatment Effect: The PSA Criterion.
All patients showed PSA rises during the first 26 weeks of treatment. In some cases, especially in these patients without radiographic evidence of disease, PSA rate of rise appeared to slow during the course of the immunization. Patients who completed 26 weeks of the trial were monitored expectantly with blood work on a monthly basis and quarterly radiographic scans. Nine of 18 evaluatable patients had radiographic evidence of disease at the initiation of study. Of these, 8 clearly showed further progression (by radiographic criteria and continued increases of PSA at the same rate of rise as that before vaccination) within 6 months of the initiation of the study. The earliest patient in this group was taken out of the study after 9 weeks because of disease progression. One remaining patient with history of biopsy-confirmed metastatic prostate cancer within an internal iliac lymph node continues to be monitored and is now at 110 weeks since the initiation of the study with no evidence of radiographic progression in bone. The lymph node has diminished in size by 50%.
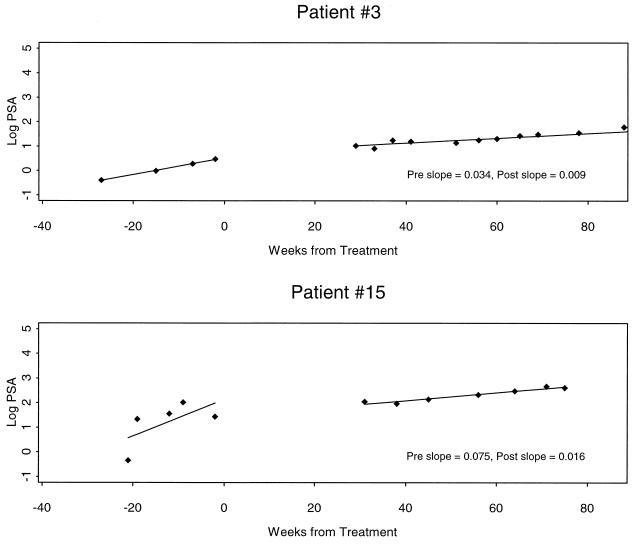
The 9 patients who had manifested no evidence of disease at the time of initiation of the study were monitored expectantly and remained radiographically free of disease. Nonetheless, three patients elected to begin hormonal therapy because of rising PSA levels, albeit in the monitoring phase. Five patients have expressed their preference not to receive any intervention and to continue expectant monitoring. PSA slope changes were determined in these 5 patients. In 2 of the 5 patients the PSA slope appeared to decrease. These 2 patients are presented in Fig. 5. Using log of PSA concentration, we determined a difference between pretreatment and posttreatment slopes with lower and upper confidence bounds. There was no overlap between confidence intervals, suggesting that a difference could be discerned between pre- and posttreatment effects.
Figure 5.
Decline of the slope of the log of PSA concentration (ng/ml) vs. time after treatment compared with values before treatment for patients 3 and 15. These patients continue to be radiographically free of disease with stable PSA slopes at greater than week 80 after treatment. Patient 3 shows an intermediate treatment effect and patient 15, a more significant change in post- compared with pretreatment slope.
DISCUSSION
We present here the full results of a clinical trial with a vaccine containing globo H-KLH conjugate plus the immunological adjuvant QS-21. The results establish the safety and the lowest optimally immunogenic dose of this large, complex synthetic carbohydrate antigen in patients with relapsed prostate cancer. All immunized patients exhibited good IgM responses against globo H, confirming its immunogenicity in prostate cancer patients with a broad range of stages and tumor burdens. Of the four doses tested, the 3-μg dose clearly resulted in lower IgM and IgG titers against globo H, but no significant difference was observed between the titers induced by the 10-, 30-, and 100-μg doses. The 30-μg dose appeared to be optimal and has been selected for use in future trials. Interestingly, the antibody responses in patients with more advanced and measurable prostate cancer appeared as good as (or better than) the responses in patients without measurable disease and only modest elevations in PSA. Inhibition studies and thin-layer immune studies (18) confirm the anti-globo H specificity of the antibodies generated. The decline in antibody titers by week 19 suggests that a dosing schedule with more frequent immunizations should be evaluated.
Antibodies passively administered or actively induced with vaccines in a variety of preclinical models have demonstrated the ability to eradicate circulating tumor cells and micrometastases (3). The mechanism of action is assumed to be complement-mediated lysis (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and inflammatory reactions. IgM antibodies induced in this trial were able to react with tumor cells as demonstrated by flow cytometry in 10 of 18 patients and in 9 patients induced complement-mediated lysis of globo H-expressing cell lines. The IgG antibodies induced were primarily IgG1, a subclass known to be able to mediate not only CDC but also ADCC. IgG3 antibodies were also found. The low reactivity by flow cytometry of these IgG antibodies may be a consequence of low affinity. This is a frequent finding in IgG antibodies against carbohydrate antigens that is partially overcome by the pentavalent nature of IgM antibodies.
As globo H is expressed at the epithelial surface of a variety of normal tissues, there was concern that antibodies induced against globo H might result in autoimmunity against some of these tissues. However, no evidence of local or systemic toxicity was detected. Hepatic and renal function tests were unchanged, and there was no evidence of diarrhea, salivary gland dysfunction, pancreatitis, or prostatitis. The only side effects detected were those seen in previous trials with the immunological adjuvant QS-21—i.e., local induration and inflammation at the vaccination site lasting several days, and in occasional patients low-grade fever and flu-like symptoms lasting 1–2 days. The safety profile was similar to that observed with other KLH conjugates plus QS-21 (4, 5, 17) despite antigen expression of globo H on a variety of normal tissues.
Prostate cancer is a unique disease in which to study vaccine strategies in that a highly specific biomarker, PSA, is available. This antigen can be used to monitor the disease at low tumor burdens where therapies are more likely to be effective. Although this trial was not designed to assess efficacy, evidence of an antitumor effect was suggested in patients with minimal tumor burden as manifested only by rising PSAs (24). All patients showed evidence of rising PSAs prior to treatment and throughout the first 26 weeks of the trial. However, as patients continued to be observed for the 6–9 months after treatment, favorable changes in the PSA slopes occurred in some patients. Observations indicated that a treatment effect could occur within 3 months after completion of the trial. Of five evaluatable patients without evidence of bone metastases, an attenuation of log PSA slopes was observed in two patients. Though the confidence intervals between pre- and posttreatment PSA slopes show no overlap, suggesting a significant difference in slope after treatment, the difference in slope was relatively small.
Overall, this analysis demonstrates the optimal dose, relevant immunogenicity, and safety of a synthetic globo H-KLH plus QS-21 vaccine. It also suggests approaches for augmenting the efficacy of antibody-inducing vaccines against prostate cancer. Evidence of disease stability and decreased PSA slope were seen in patients vaccinated while free of grossly detectable disease. This result was consistent with our previous findings that treatment of solid tumors with vaccine-induced or passively administered antibodies should be restricted to the adjuvant setting, where micrometastasis and circulating tumor cells are the targets (ref. 1, reviewed in ref. 12). Consequently, future enrollment will be restricted to patients with rising PSA but no other evidence of disease. The vaccine-induced antibodies reacted with the cell surface and were capable of inducing complement-mediated tumor cell lysis in only 50% of patients. However, even in these minimal-disease patients, decreases in PSA slope were relatively modest. Consequently, increased vaccine potency will be required. This potency may be provided by the use of additional immunological adjuvants to further augment the immunogenicity of KLH conjugate plus QS-21 vaccines. In addition, in light of the antigenic heterogeneity of tumors and the heterogeneity of human immune responses against any given antigen, polyvalent vaccines will be required. If the effect of immunization with several antigens on PSA slope were additive, a falling PSA might well be expected.
We also note that the concept of using PSA slope profiles for assessing early treatment effects of such vaccines must await further validation in phase II and phase III clinical trials.
Acknowledgments
We extend our appreciation to Dr. Alan Houghton for his many suggestions, which have contributed toward the development of the vaccine therapy program in prostate cancer. This work was supported by the National Institutes of Health (Grants AI-16943, CA-28824, CA-71506, and CA-08748), Cancer Research Institute, CaP CURE, Swim Across America, and the PepsiCo Foundation.
ABBREVIATIONS
- KLH
keyhole limpet hemocyanin
- PSA
prostate-specific antigen
- MMCCH
4-(4-N-maleimidomethyl)cyclohexane-1-carbonylhydrazide
References
- 1.Livingston P O, Ragupathi G. Cancer Immunol Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ragupathi G. Cancer Immunol Immunother. 1996;43:152–157. doi: 10.1007/s002620050316. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Zhang S L, Cheung N V, Ragupathi G, Livingston P O. Cancer Res. 1998;54:2844–2849. [PubMed] [Google Scholar]
- 4.Ragupathi G, Meyers M, Adluri S, Ritter G, Livingston P O. Proc Am Assoc Cancer Res. 1997;38:398. (abstr.). [Google Scholar]
- 5.Dickler M, Gilewski T, Ragupathi G, Adluri R, Koganti R R, Longenecker B M, Houghton A N, Norton L, Livingston P O. Proc Am Soc Clin Oncol. 1998;16:439a. (abstr.). [Google Scholar]
- 6.Kudryashov V, Kim H M, Ragupathi G, Danishefsky S J, Livingston P O, Lloyd K O. Cancer Immunol Immunother. 1998;45:281–286. doi: 10.1007/s002620050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones P C, Sze L L, Liu P Y, Morton D L, Irie R. J Natl Cancer Inst. 1981;66:249–254. [PubMed] [Google Scholar]
- 8.Livingston P O, Wong G Y C, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves M J, Helling F, Ritter G, Oettgen H F, Old L J. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 9.Bremer E G, Levery S B, Sonnino S, Ghidoni R, Canevari S, Kannagi R, Hakomori S. J Biol Chem. 1984;259:14773–14777. [PubMed] [Google Scholar]
- 10.Adobati E, Panza L, Russo G, Colnaghi M, Canevari S. Glycobiology. 1997;7:173–178. doi: 10.1093/glycob/7.2.173. [DOI] [PubMed] [Google Scholar]
- 11.Menard S, Tagliabue E, Canevari S, Fossati G, Colnaghi M I. Cancer Res. 1983;43:1295–1300. [PubMed] [Google Scholar]
- 12.Livingston P O. Cancer Biol. 1995;6:357–366. doi: 10.1016/1044-579x(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Cordon-Cardo C, Zhang H S, Reuter V E, Adluri S, Hamilton W B, Lloyd K O, Livingston P O. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Zhang H S, Reuter V E, Slovin S F, Scher H I, Livingston P. Clin Cancer Res. 1998;4:295–302. [PubMed] [Google Scholar]
- 15.Bilodeau M T, Park T K, Hu S S, Randolph J T, Danishefsky S J. J Am Chem Soc. 1995;117:7840–7841. [Google Scholar]
- 16.Ragupathi G, Park T K, Zhang S, Kim I J, Graber L, Adluri R, Lloyd K O, Danishefsky S J, Livingston P O. Angew Chem Int Ed Engl. 1997;36:125–128. [Google Scholar]
- 17.Helling F, Zhang S, Shang A, Adluri S, Calves M, Koganty R, Longenecker B M, Yao T-J, Oettgen H F, Livingston P O. Cancer Res. 1995;55:2783–2788. [PubMed] [Google Scholar]
- 18.Ragupathi R, Slovin S F, Adluri D, Sames D, Kim I J, Kim H M, Spassova M, Bornmann W G, Lloyd K O, Scher H I, Livingston P O, Danishefsky S J. Angew Chem Int Ed Engl. 1999;38:563–566. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Danishefsky S J, Bilodeau M T. Angew Chem Int Ed Engl. 1996;35:1381–1419. [Google Scholar]
- 20.Ragupathi G, Koganty R R, Qui D X, Lloyd K O, Livingston P O. Glycoconjugate J. 1998;15:217–221. doi: 10.1023/a:1006936826730. [DOI] [PubMed] [Google Scholar]
- 21.Livingston P O, Ritter G, Srivastava P, Calves M J, Oettgen H F, Old L J. Cancer Res. 1989;49:7045–7050. [PubMed] [Google Scholar]
- 22.Kudryashov V, Ragupathi G, Kim I, Danishefsky S J, Livingston P O, Lloyd K O. Glycoconjugate J. 1998;15:243–249. doi: 10.1023/a:1006992911709. [DOI] [PubMed] [Google Scholar]
- 23.Efron B, Tibshirani R J. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. pp. 170–172. [Google Scholar]
- 24.Scher H I, Slovin S F, Kelly W K, Livingston P O, Danishefsky S J, Fazzari M, Terry K, Heller G. Proc Am Soc Clin Oncol. 1998;17:324a. (abstr. 1247). [Google Scholar]