Abstract
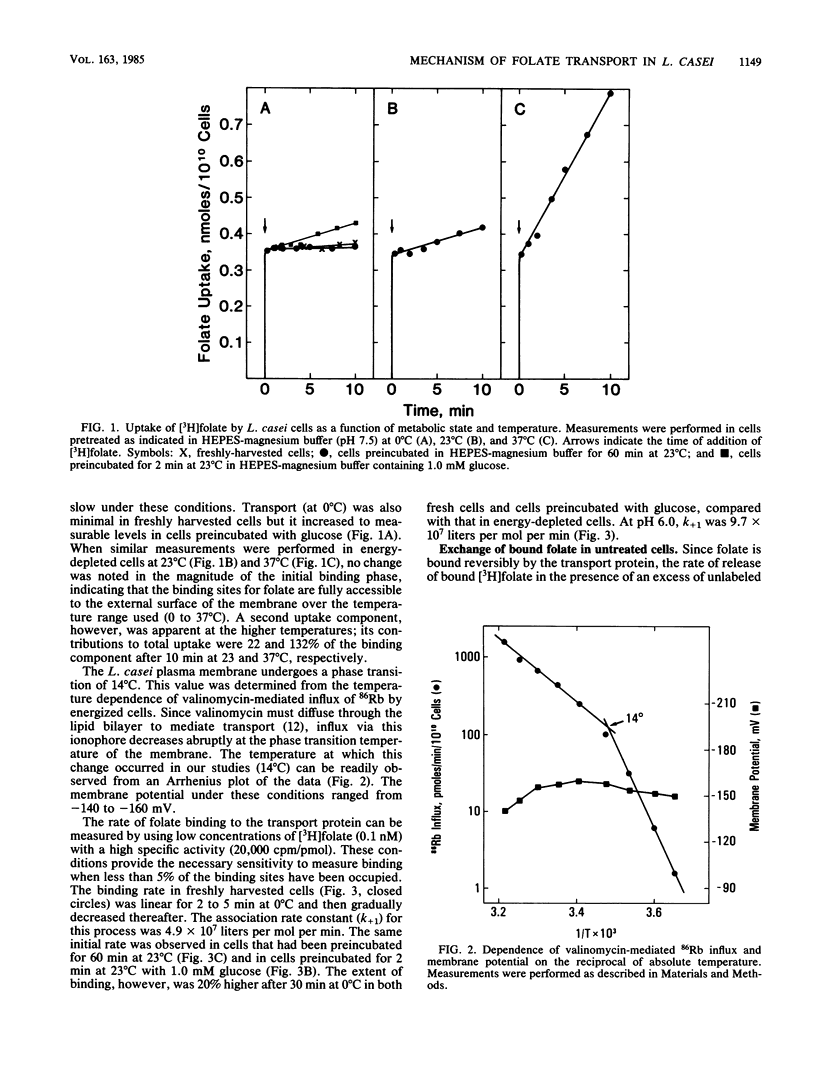
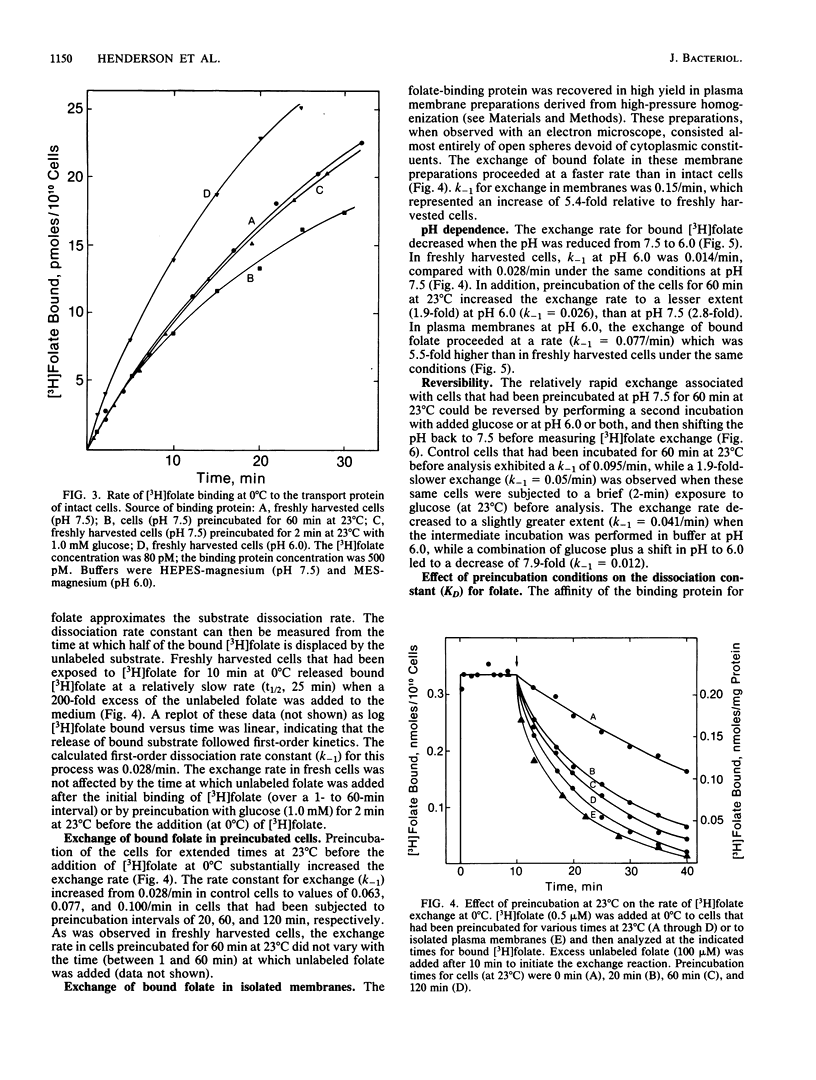
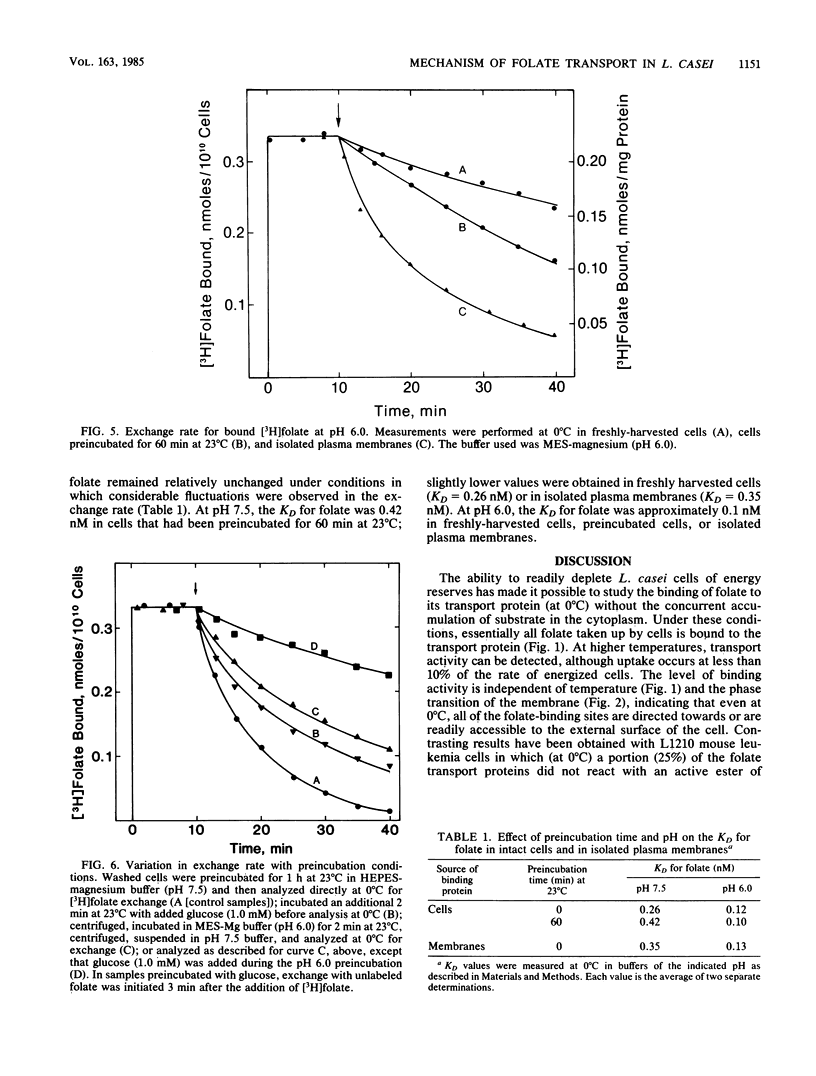
Lactobacillus casei cells contain a folate transport protein which exhibits a high affinity for folate. The dissociation constant (KD) for folate derived from binding parameters at the steady state (at 0 degree C) is 0.4 nM at pH 7.5 and 0.1 nM at pH 6.0. In the present study, folate binding to this protein at pH 7.5 (and 0 degree C) was shown to follow second-order kinetics and to proceed with an association constant (k+1) of 4.9 X 10(7) liter/mol per min. K+1 was not affected by preincubation conditions which alter the energetic state of the cell. Measurements on the extent of binding showed further that (at 0 degree C) essentially all unoccupied folate-binding sites reside at or are readily accessible to the outer surface of the membrane. In contrast, after saturating the binding site with [3H]folate, the first-order rate constant (k-1) for dissociation of the bound substrate (at 0 degree C) was found to vary substantially with the conditions employed. k-1 was 0.028/min in freshly harvested cells, but it increased by 2.8-fold in cells preincubated at 23 degrees C for 60 min and by 5.4-fold in isolated membranes. In addition, the faster rate observed in preincubated cells (k-1 = 0.077/min) returned to a slower rate after brief exposure of the cells to pH 6.0 (k-1 = 0.041/min), glucose (k-1 = 0.050/min), or both (k-1 = 0.012/min). k-1 was twofold lower at pH 6.0 than at pH 7.5 and was less dependent on the preincubation conditions, although it also increased substantially (5.5-fold) when the cells were converted to plasma membranes. The proposed explanation for these results is that folate transport protein of L. casei exists in two forms which can be distinguished by the accessibility of the binding site to the external medium and whose amounts are dependent upon the presence of bound folate, the pH, and the energetic state of the cell. It is suggested that these forms are transport proteins with binding sites oriented towards the inner and outer surfaces of the membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Henderson G. B., Huennekens F. M. Transport of folate compounds into Lactobacillus Casei. Arch Biochem Biophys. 1974 Oct;164(2):722–728. doi: 10.1016/0003-9861(74)90085-x. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Kojima J. M., Kumar H. P. Differential interaction of cations with the thiamine and biotin transport proteins of Lactobacillus casei. Biochim Biophys Acta. 1985 Mar 14;813(2):201–206. doi: 10.1016/0005-2736(85)90234-2. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Montague-Wilkie B. Irreversible inhibitors of methotrexate transport in L1210 cells. Characteristics of inhibition by an N-hydroxysuccinimide ester of methotrexate. Biochim Biophys Acta. 1983 Oct 26;735(1):123–130. doi: 10.1016/0005-2736(83)90267-5. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Potuznik S. Cation-dependent binding of substrate to the folate transport protein of Lactobacillus casei. J Bacteriol. 1982 Jun;150(3):1098–1102. doi: 10.1128/jb.150.3.1098-1102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Coupling of energy to folate transport in Lactobacillus casei. J Bacteriol. 1979 Aug;139(2):552–559. doi: 10.1128/jb.139.2.552-559.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Folate transport in Lactobacillus casei: solubilization and general properties of the binding protein. Biochem Biophys Res Commun. 1976 Feb 9;68(3):712–717. doi: 10.1016/0006-291x(76)91203-1. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Mechanism of folate transport in Lactobacillus casei: evidence for a component shared with the thiamine and biotin transport systems. J Bacteriol. 1979 Mar;137(3):1308–1314. doi: 10.1128/jb.137.3.1308-1314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Purification and properties of a membrane-associated, folate-binding protein from Lactobacillus casei. J Biol Chem. 1977 Jun 10;252(11):3760–3765. [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Kadner R. J., Huennekens F. M. The folate and thiamine transport proteins of Lactobacillus casei. J Supramol Struct. 1977;6(2):239–247. doi: 10.1002/jss.400060209. [DOI] [PubMed] [Google Scholar]
- Huennekens F. M., Vitols K. S., Henderson G. B. Transport of folate compounds in bacterial and mammalian cells. Adv Enzymol Relat Areas Mol Biol. 1978;47:313–346. doi: 10.1002/9780470122921.ch5. [DOI] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
- Linder E., Helin H., Chang C. M., Edgington T. S. Complement-mediated binding of monocytes to intermediate filaments in vitro. Am J Pathol. 1983 Sep;112(3):267–277. [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and metabolism of folates by bacteria. J Biol Chem. 1975 Mar 25;250(6):2243–2253. [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and utilization of methyltetrahydrofolates by Lactobacillus casei. J Biol Chem. 1976 Jun 10;251(11):3405–3410. [PubMed] [Google Scholar]


