Abstract
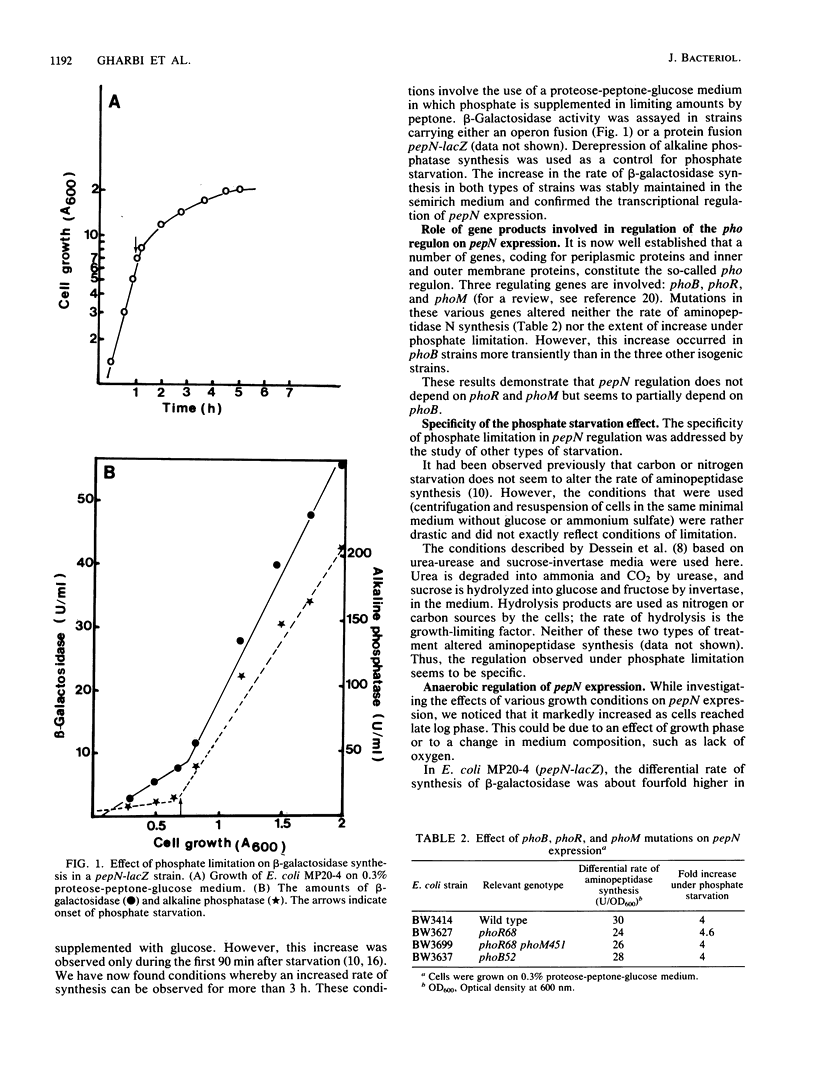
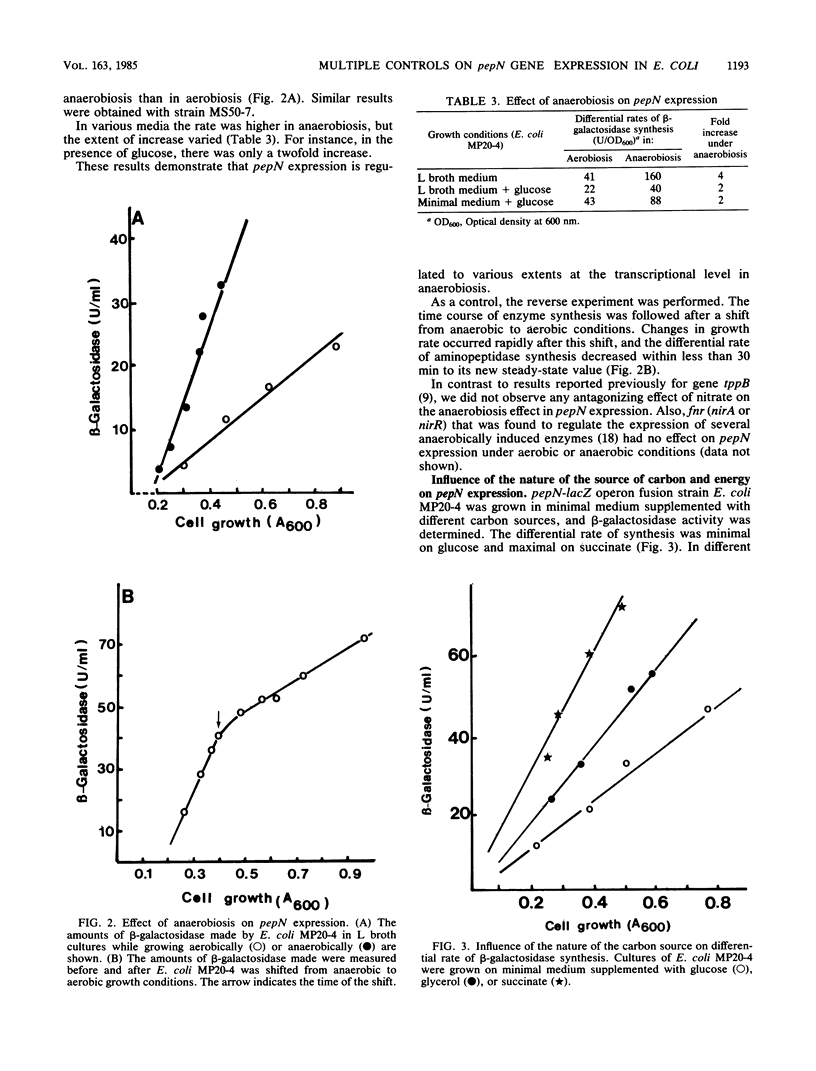
Three physiological conditions were shown to promote transcriptional regulation of pepN expression: phosphate limitation, the nature of the source of carbon and energy, and anaerobiosis. The transcriptional level of regulation can be deduced from the observation of these effects in strains carrying operon fusion pepN-lacZ. Mutations in the various genes phoB, phoM, phoR, crp, and fnr (oxrA) did not affect pepN expression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bally M., Murgier M., Lazdunski A. Cloning and orientation of the gene encoding aminopeptidase N in Escherichia coli. Mol Gen Genet. 1984;195(3):507–510. doi: 10.1007/BF00341454. [DOI] [PubMed] [Google Scholar]
- Bally M., Murgier M., Tommassen J., Lazdunski A. Physical mapping of the gene for aminopeptidase N in Escherichia coli K12. Mol Gen Genet. 1984;193(1):190–191. doi: 10.1007/BF00327436. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Bassford P. J., Jr Regulation of adenylate cyclase synthesis in Escherichia coli: studies with cya-lac operon and protein fusion strains. J Bacteriol. 1982 Sep;151(3):1346–1357. doi: 10.1128/jb.151.3.1346-1357.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chappelet-Tordo D., Lazdunski C., Murgier M., Lazdunski A. Aminopeptidase N from Escherichia coli: ionizable active-center groups and substrate specificity. Eur J Biochem. 1977 Dec 1;81(2):293–305. doi: 10.1111/j.1432-1033.1977.tb11952.x. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessein A., Schwartz M., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP. Mol Gen Genet. 1978 Jun 1;162(1):83–87. doi: 10.1007/BF00333853. [DOI] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski A., Murgier M., Lazdunski C. Evidence for an aminoendopeptidase localized near the cell surface of Escherichia coli. Regulation of synthesis by inorganic phosphate. Eur J Biochem. 1975 Dec 15;60(2):349–355. doi: 10.1111/j.1432-1033.1975.tb21009.x. [DOI] [PubMed] [Google Scholar]
- Lazdunski A., Pellissier C., Lazdunski C. Regulation of Escherichia coli K10 aminoendopeptidase synthesis. Effects of mutations involved in the regulation of alkaline phosphatase. Eur J Biochem. 1975 Dec 15;60(2):357–362. doi: 10.1111/j.1432-1033.1975.tb21010.x. [DOI] [PubMed] [Google Scholar]
- McCaman M. T., Villarejo M. R. Structured and catalytic properties of peptidase N from Escherichia coli K-12. Arch Biochem Biophys. 1982 Feb;213(2):384–394. doi: 10.1016/0003-9861(82)90564-1. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Schwartz G. Peptidase-deficient mutants of Escherichia coli. J Bacteriol. 1978 Aug;135(2):603–611. doi: 10.1128/jb.135.2.603-611.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgier M., Gharbi S. Fusion of the lac genes to the promoter for the aminopeptidase N gene of Escherichia coli. Mol Gen Genet. 1982;187(2):316–319. doi: 10.1007/BF00331136. [DOI] [PubMed] [Google Scholar]
- Murgier M., Pelissier C., Lazdunski A., Lazdunski C. Existence, localization and regulation of the biosynthesis of aminoendopeptidase in gram-negative bacteria. Eur J Biochem. 1976 Jun 1;65(2):517–520. doi: 10.1111/j.1432-1033.1976.tb10368.x. [DOI] [PubMed] [Google Scholar]
- Newman B. M., Cole J. A. The chromosomal location and pleiotropic effects of mutations of the nirA+ gene of Escherichia coli K12: the essential role of nirA+ in nitrite reduction and in other anaerobic redox reactions. J Gen Microbiol. 1978 May;106(1):1–12. doi: 10.1099/00221287-106-1-1. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. PHO-regulon of Escherichia coli K12: a minireview. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):243–249. [PubMed] [Google Scholar]
- Wanner B. L., McSharry R. Phosphate-controlled gene expression in Escherichia coli K12 using Mudl-directed lacZ fusions. J Mol Biol. 1982 Jul 5;158(3):347–363. doi: 10.1016/0022-2836(82)90202-9. [DOI] [PubMed] [Google Scholar]
- Wanner B. L. Overlapping and separate controls on the phosphate regulon in Escherichia coli K12. J Mol Biol. 1983 May 25;166(3):283–308. doi: 10.1016/s0022-2836(83)80086-2. [DOI] [PubMed] [Google Scholar]
- Yen C., Green L., Miller C. G. Degradation of intracellular protein in Salmonella typhimurium peptidase mutants. J Mol Biol. 1980 Oct 15;143(1):21–33. doi: 10.1016/0022-2836(80)90122-9. [DOI] [PubMed] [Google Scholar]



