Abstract
The basement membrane is a highly intricate and organized portion of the extracellular matrix that interfaces with a variety of cell types including epithelial, endothelial, muscle, nerve, and fat cells. The laminin family of glycoproteins is a major constituent of the basement membrane. The sixteen known laminin isoforms are formed from combinations of α, β, and γ chains, with each chain containing specific domains capable of interacting with cellular receptors such as integrins and other extracellular ligands. In addition to its role in the assembly and architectural integrity of the basement membrane, laminins interact with cells to influence proliferation, differentiation, adhesion, and migration, processes activated in normal and pathologic states. In vitro these functions are regulated by the posttranslational modifications of the individual laminin chains. In vivo laminin knock-out mouse studies have been particularly instructive in defining the function of specific laminins in mammalian development and have also highlighted its role as a key component of the basement membrane. In this review, we will define how laminin structure complements function and explore its role in both normal and pathologic processes.
Keywords: Laminin, Basement Membrane, Extracellular Matrix, Integrin, Development
I. INTRODUCTION
The basement membrane (BM) is a complex and highly organized yet dynamic matrix of extracellular material that interfaces with epithelial, endothelial, nerve, fat and muscle cells to provide mechanical support and stability, structural compartmentalization, regulation of cellular activities, and a physical reservoir for cellular growth factors. Basement membranes are specialized for different tissue types but have a basic cross sectional structure divided into the lamina lucida, lamina densa, and the sublamina densa. The lamina densa which defines the electron dense region of the basement membrane, is comprised predominantly of polymeric networks of collagen and laminin integrated by crosslinkers such as nidogen and perlecan (Ghohestani et al. 2001). The laminin family of glycoproteins, first discovered as a product of mouse Engelbreth-Holm-Swarm sarcoma (EHS) cells almost three decades ago (Timpl et al. 1979), play a significant role in basement membrane assembly, architecture, and regulation of cellular differentiation, adhesion, and migration. This review will serve to explore the relationship between laminin structure and function, including its role in pathologic processes, from the molecular to the organismal level.
II. LAMININ EVOLUTION
Laminins exhibit cross-species similarities in domain sequence. Similar to other basement membrane constituents, laminins are evolutionarily ancient and conserved gene products found in both vertebrates and invertebrates. It is thought that the present day family of laminins arose a single laminin gene, similar to the one found in Hydra vulgaris, through a series of gene duplications and modifications. Laminin α and β chains from Hydra have been cloned and show much sequence similarities to both vertebrate and invertebrate laminin α and β chains(Sarras et al. 1994; Zhang et al. 2002). Invertebrate have been found to express 4 different subunits of laminins (β chain, γ chain; αA and αB in C. elegans and α1,2 and α3,5 in D. melanogaster), which are capable of assembling into 2 different laminin trimers throughout development. From similarities in sequence, it seems that the mammalian α1 and α2 chains branched with the αA/α1,2 lineage, whereas the mammalian α5 chains branched with the αB/α3,5 lineage(Miner et al. 2004b). Interestingly and consistent with its evolutionary history, α1/laminin 1 and α5/laminin 10 are the only laminins which show widespread expression during embryogenesis and are crucial during mammalian embryonic development, as will be discussed in subsequent sections.
III. FAMILY OF LAMININ PROTEINS
Laminins are extracellular heterotrimeric glycoproteins composed of various combinations of α, β, and γ chains. These large molecules are 400–900 kDa in weight and exhibit a cross shape. To date, five α, four β, and three γ chains (Miner and Yurchenco 2004b), as well as chain splice variants, have been identified to create sixteen known laminins (laminins 1-15) in mammals (Aumailley et al. 2005) (table 1). The number of combinations that can be created from the three chains exceeds the number of laminins identified, as not all combinations of the three chains have yet been found to occur.
Table 1.
Laminin Isoforms and Chain Composition
| Isoform | α | β | γ | Sites of expression | Proposed function | Phenotypes of knockout mouse | Associated Human Disease |
|---|---|---|---|---|---|---|---|
| Laminin 1 | 1 | 1 | 1 | Epithelia of early embryogenesis, adult reproductive organs, kidney, liver (Ekblom et al. 2003) | Early embryogenesis | Alpha 1 deletion: Perimplantation lethality E7 (Yurchenco and Wadsworth 2004) | N/A |
| Laminin 2 | 2 | 1 | 1 | Muscle cells (extrasynaptic) (Brandenberger et al. 1996) | Muscle cell structural integrity | Alpha 2 deletion: Adult lethality, muscular dystrophy (Miyagoe et al. 1997) | Congenital muscular dystrophy A1 (CMDA1) (Helbling-Leclerc et al. 1995) |
| Laminin 3 | 1 | 2 | 1 | Placenta(Champliaud et al. 2000) | N/A | Alpha 1 deletion: Perimplantation lethality E7 (Yurchenco and Wadsworth 2004) | N/A |
| Laminin 4 | 2 | 2 | 1 | Muscle cell (neuromuscular junction) (Brandenberger et al. 1996) | Muscle cell structural integrity | Alpha 2 deletion: Adult lethality, muscular dystrophy (Miyagoe et al. 1997) | Congenital muscular dystrophy A1 (CMDA1) (Helbling-Leclerc et al. 1995) |
| Laminin 5 | 3 A | 3 | 2 | Skin(Rousselle et al. 1991), placenta, mammary glands(Doliana et al. 1997) | Hemidesmosome formation, cellular migration | Alpha 3 deletion: Neonatal lethality from blistering condition (Ryan et al. 1999) | Junctional epidermolysis bullosa |
| Laminin 5B | 3 B | 3 | 2 | Skin, uterus, lung (Doliana et al. 1997) | Not known | N/A | N/A |
| Laminin 6 | 3 A | 1 | 1 | Skin, amnion (Marinkovich et al. 1992b) | Association with laminin 5 for ECM assembly | Alpha 3 deletion: Neonatal lethality from blistering condition (Ryan et al. 1999) | N/A |
| Laminin 7 | 3 A | 2 | 1 | Skin, Amnion (Champliaud et al. 1996) | Association with laminin 5 for ECM assembly | Alpha 3 deletion: Neonatal lethality from blistering condition (Ryan et al. 1999) | N/A |
| Laminin 8 | 4 | 1 | 1 | Vascular endothelial cells, peripheral nerves, muscle fibers, developing kidney, developing skeletal muscle (Thyboll et al. 2002), platelet, white blood cells (Wondimu et al. 2004) | Neutrophil migration/extrava sation(Wondimu et al. 2004), endothelial development | Alpha 4 deletion: Embryonic and neonatal hemorrhaging, locomotion defects (Thyboll et al. 2002) | N/A |
| Laminin 8 | 4 | 1 | 1 | Vascular endothelial cells, peripheral nerves, muscle fibers, developing kidney, developing skeletal muscle (Thyboll et al. 2002), platelet, white blood cells(Wondimu et al. 2004) | Neutrophil migration/extrava sation(Wondimu et al. 2004), endothelial development | Alpha 4 deletion: Embryonic and neonatal hemorrhaging, locomotion defects (Thyboll et al. 2002) | N/A |
| Laminin 9 | 4 | 2 | 1 | Vascular endothelial cells, peripheral nerves, muscle fibers, developing kidney, developing skeletal muscle (Thyboll et al. 2002) | N/A | N/A | N/A |
| Laminin 10 | 5 | 1 | 1 | Vascular endothelial cells, skin, placenta (Champliaud et al. 2000), developing embryo | Embryogenesis, BMZ structural scaffold, hair development | Alpha 5 deletion: Late embryonic lethality E16.5 (exencephaly, syndactyly, placentopathy) (Miner et al. 1998) | N/A |
| Laminin 11 | 5 | 2 | 1 | Placenta (Champliaud et al. 2000), neuromuscular junction, renal glomeruli (Miner et al. 1999) | N/A | N/A | N/A |
| Laminin 12 | 2 | 1 | 3 | Kidney capillaries/arterioles, Leydig cells of testis (Iivanainen et al. 1999) | ? | N/A | N/A |
| Laminin 13 | 3 | 2 | 3 | Hippocampus (Egles et al. 2007) | CNS synaptic organization (Egles et al. 2007) | N/A | N/A |
| Laminin 14 | 4 | 2 | 3 | Retina/CNS(Libby et al. 2000), hippocampus (Egles et al. 2007) | CNS synaptic organization (Egles et al. 2007) | N/A | N/A |
| Laminin 15 | 5 | 2 | 3 | Retina/CNS(Libby et al. 2000) | CNS synaptic organization (Libby et al. 2000) | N/A | N/A |
Each chain in the laminin molecule consists of rodlike, globular, and coiled coil regions (see Figure II) (Colognato et al. 2000). The separate chains are held together at the coiled coil regions by disulfide bonds (Ekblom et al. 2003; Miner and Yurchenco 2004b). The largest chain is the α chain, which contains the long arm on the C terminal end and a short arm on the N terminal end. The C terminal end of the long arm consists of the LG 1-5 domains, which are involved in interactions with cellular receptors such as integrins and dystroglycans. The N terminal end of the short arm is also capable of binding to integrin receptors, although it is more associated with polymerization of the molecule (Patarroyo et al. 2002). The short arm of α chains displays great diversity in length. Laminins1-4 and 12 have full length α chains short arms, laminins 5-9 have truncated α chains short arms, and laminin 10-11 have elongated full length α chains short arms (Cheng et al. 1997; Colognato and Yurchenco 2000). The β and γ chains are involved in interactions with molecules in the extracellular matrix (ECM). For example, the γ3 chain of laminin 15 (Gersdorff et al. 2005) and the γ1 chain of laminin1 binds nidogen(Gerl et al. 1991), while the β3 chain of laminin 5 binds to collagen VII (Chen et al. 1999). Laminin 5 is the only laminin with truncated β and γ short arms (Cheng et al. 1997)
Figure 2.
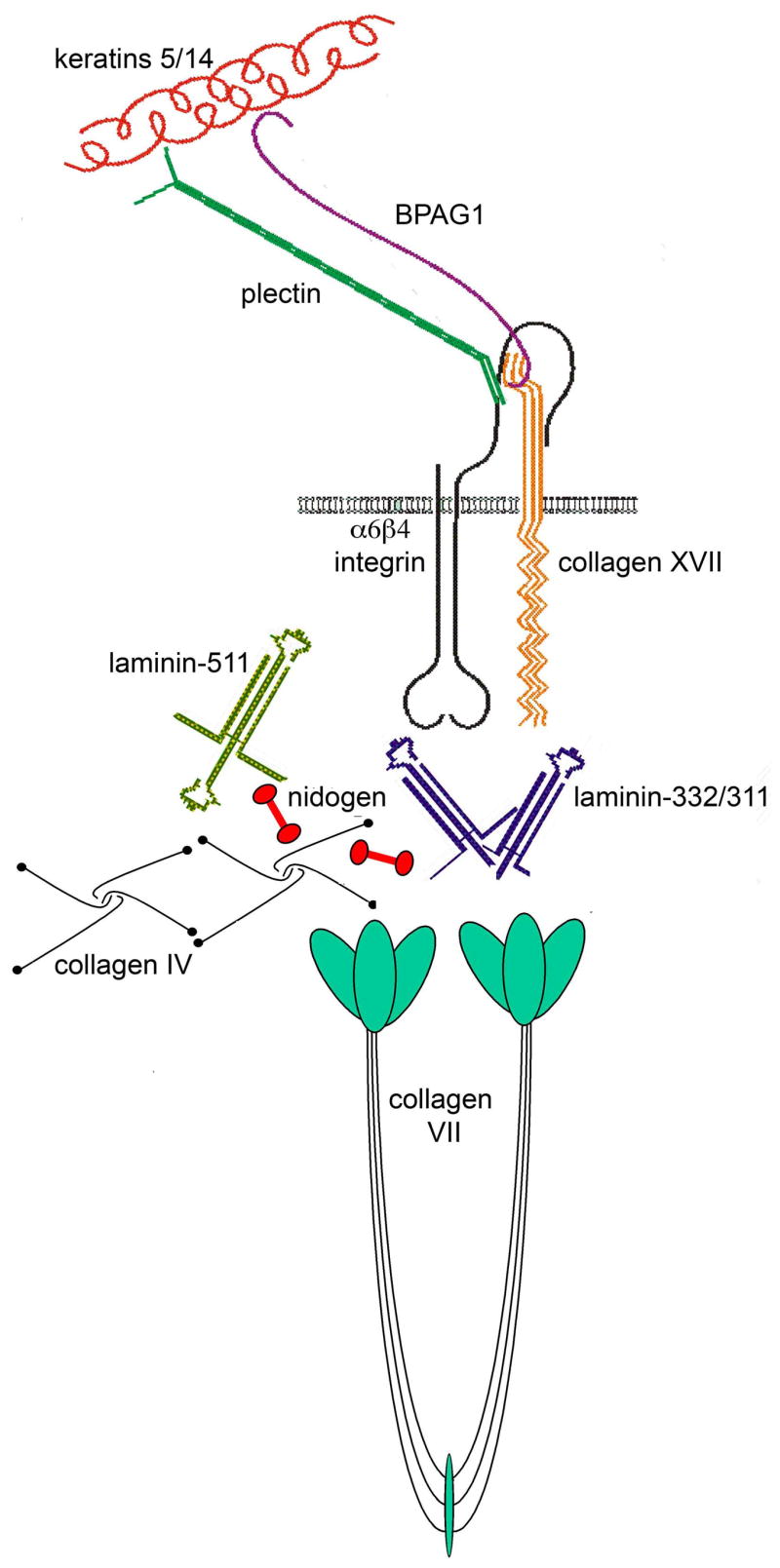
Structure and composition of dermal-epidermal basement membrane zone. Laminin 511 and collagen IV constitute the major polymeric networks of the lamina densa, connected by nidogens as well as interactions with heparan sulfate proteoglycans such as perlecan. Laminin 332 is a component of the hemidesmosome-anchoring filament complex, bridging the cell surface with the dermis. It is linked to the rest of the ECM through its interactions with type VII collagen and laminin 311.
Within the rough endoplasmic reticulum, individual laminins subunits are co-translationally glycosylated with high mannose oligosaccharide side chains at the N terminus. Glycosylation stabilizes the subunits and protects these products from degradation (Morita et al. 1985). Different laminins are glycosylated in varying amounts, depending on the number of glycosylation concensus sites (Champliaud et al. 2000). Laminin trimer assembly begins first by the stable association of the β and γ chain (Figure 3). The α chain then combines with this dimeric complex in order for the trimeric molecule to be secreted. This is the rate limiting step (Yurchenco et al. 1997; Schneider et al. 2006). The laminin trimer then undergoes terminal glycosylation within the Golgi organelle (Morita et al. 1985) and is then secreted out of the cell, deposited, and undergoes proteolytic processing of its chains. The processing that occurs, as will be described in subsequent sections, affects important cellular processes such as movement. Both epithelial and mesenchymal cells contribute to the deposition of laminin into the basement membrane (Patarroyo et al. 2002).
Figure 3.
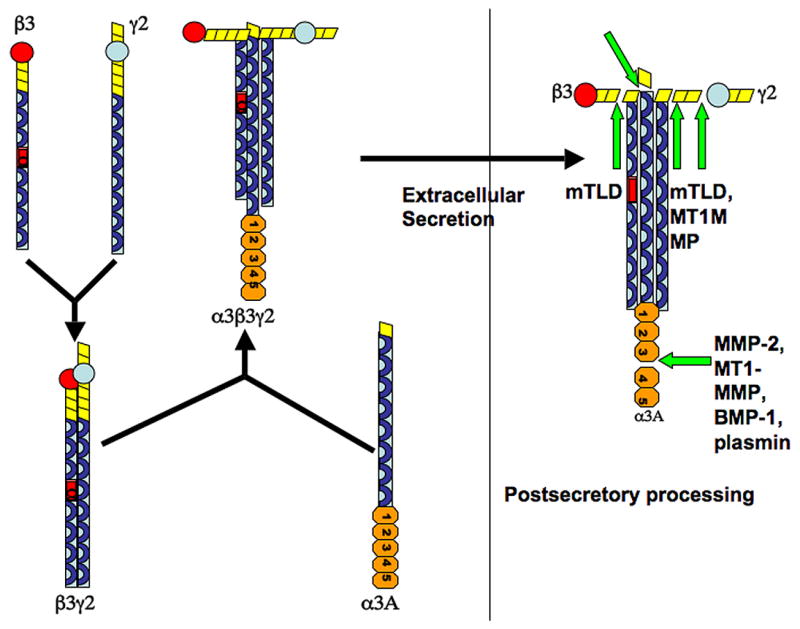
Assembly of laminin heterotrimers. Shown here is the assembly of the laminin 332 heterotrimer. Individual chains have all been glycosylated prior to dimmer/trimer assembly. Assembly begins first by the stable association of the β and γ chains. The α chain then combines with this dimer in order for the trimeric molecule to be secreted out of the cell. Addition of the α chain is the rate limiting step (Schneider et al. 2006). Proteolytic processing occurs after the trimer is secreted extracellularly.
The nomenclature for laminins has been recently revised in 2005 (Aumailley et al. 2005). Traditionally, laminins were named by numbering them in the order in which they were discovered. The new simplified nomenclature assigns a three digit number to each laminin, with each digit representing the number of the individual chain type found within the trimer. For example laminin 5, which consists of α3β3γ2 chains, is now termed laminin 332. Although this section has utilized the traditional system to introduce the laminin family in a logical fashion, the subsequent sections in this paper will employ the new nomenclature system when appropriate.
IV. LAMININS IN PROMOTING BASEMENT MEMBRANE ASSEMBLY AND TISSUE INTEGRITY
Laminins play a prominent role in providing structure to the ECM and anchorage for cells to the basement membrane. Those laminins containing full-length chains in all short arms are capable of polymerization. According to the three-arm interaction model, the three N terminals short arms are believed to interact with the N terminal short arms of other laminins to produce a lattice-type supramolecular network (Cheng et al. 1997). N terminal bonding is calcium dependent (Miner and Yurchenco 2004b). In addition to polymerization, laminin molecules are incorporated into the ECM by interactions with other ECM molecules such as collagen IV, nidogen, and fibulin, or other laminins. For example laminin 332, which has truncations in all short arms and hence cannot self-polymerize, can bind to laminin 311, laminin 321, and collagen VII via its β3 chain (Chen et al. 1999). Its γ2 chain can associate with collagen VII, nidogen, and fibulin (Sasaki et al. 2001; Schneider et al. 2006). By associating with nidogen, it is connected into the extensive collagen IV network; by association with other laminins, it is capable of forming heteropolymers within the ECM (Schneider et al. 2006). Laminin 332 is unique amongst laminins in that it also serves to anchor cells to the basement membrane via hemidesmosome complexes. The C terminal G domains of the α3 chain binds to the surface integrins (α6β4, α3β1), which connect to cytokeratin filaments within the cell, while the N terminal short arms connect to collagen VII, which provides anchorage to the dermis (figure 2).
The importance of laminins in maintaining tissue integrity is demonstrated in the group of diseases known as junctional epidermolysis bullosa (JEB), a blistering skin condition in humans whereby laminin 332 is functionally compromised by genetic mutations in any of its three chains or antibodies against any of these chains. The skin splits at the level of the hemidesmosome. The Herlitz variant of JEB is particularly severe, as laminin 332 is completely absent in this condition (Varki et al. 2006). Deletion of laminin 332’s hemidesmosomal binding partner α6 or β4 integrin chains in mice also creates a similar blistering phenotype (Georges-Labouesse et al. 1996; van der Neut et al. 1996). The role of laminins in supporting tissue integrity is also demonstrated in diseases of muscular dystrophy. Laminin 211 is found predominantly in skeletal muscle tissues and serves as an anchor between the dystrophin glycoprotein complex (DGC) that lies at the cytoplasmic surface of muscle cells and the surrounding extracellular matrix. LAMA2 gene mutations in humans result in congenital muscular dystrophy 1A (CMD1A) (Helbling-Leclerc et al. 1995). A similar phenotype is found in mice with deletions of the laminin α2 chain (Miyagoe et al. 1997). A discussion of laminin receptors and muscular dystrophies is provided in the following section. The pathologies of these diseases are exactly what one would predict based on our understanding of the structure and function of laminins in the basement membrane.
V. INTERACTIONS OF LAMININS WITH CELLS
The major cell surface receptors for laminins include integrins and nonintegrin molecules. At least eight integrins are known to interact with laminins (Givant-Horwitz et al. 2005) including α1β1, α2β1, α5ν1, α3β1, α6β1, α6β4, αvβ3, αvβ5, and α7β1 (Burkin et al. 1999; Tzu 2005). Each integrin recognize particular sequences within the laminin α chain and thus binds only to specific set of laminins. The recognition/binding site on the integrin receptor is formed by a combination of its α and β chains, although the α chain may contribute more to ligand specificity (Tarone et al. 2000). Integrins recognize and bind to laminins mostly at the C terminal LG tandem of the α chain, although this could also take place at the N terminal domain (full length chains) and laminin 4b domain (α3 chain). In addition, interactions of integrin with the laminin β and γ chain have also been reported (Patarroyo et al. 2002). Recently, the third glutamic acid residue from the C terminal of the gamma chain has been found to play an important role in laminin-integrins binding (Ido et al. 2007). Once bound to its ligand, the intracellular cytoplasmic portion of the integrin can activate focal adhesion kinases (FAK), small rho GTPases, and MAPK pathways to effect cellular activities (Givant-Horwitz et al. 2005). Laminins also interact with nonintegrin cellular receptors such as a dystroglycan, 32/67 kDa laminin receptor, 67 kDa elastin-laminin receptor, galactoside-binding lectin, galactosyltransferase, heparin sulfate proteoglycans, and Ig-related basal cell adhesion molecule (Patarroyo et al. 2002). Again, most of these interactions occur at the C terminal LG1-5 of the α chain, although interactions with other chains may also occur. The importance of laminin-receptor interactions can be underscored by human conditions and mouse models in which laminin receptors are functionally absent. Junctional epidermolysis bullosa with pyloric atresia (JEB-PA) is an example of a skin blistering condition that involves a defective α6β4 integrin, a component of hemidesmomes (Ashton et al. 2001). Congenital muscular dystrophies have been observed in humans with missing α7β1 integrins, the main integrins found in myocytes, and mutations within the dystrophin glycoprotein complex, a complex of cytoplasmic bound to dystrophin, that links the actin cytoskeleton of the muscle cell to its extracellular matrix. Duchenne’s muscular dystrophy results when the absence of dystrophin disrupts the integrity of the DCG and hence alters the mechanical stability of the muscle cell, such that the cell becomes susceptible to injury with the stresses of repetitive contractile forces (Burkin and Kaufman 1999; Kanagawa et al. 2006) Normally, the alpha chain of the dimeric dystroglycan receptor binds to the C terminal LG4-5 domains of the laminin molecule (Sasaki et al. 2004). Similar muscular dystrophy phenotypes are replicated in knockout mouse models with the respective receptor deletions (Dowling et al. 1996; Mayer et al. 1997; Rooney et al. 2006). Interestingly, in humans, there have been no genetic conditions associated with primary mutations of the dystroglycan gene. Deletions of the dystroglycan gene in mouse models have resulted in embryonic lethality as a result of failure to form Reichert’s membrane, accompanied by an absence of laminin and collagen networks. It is thought that dystroglycan facilitates incorporation of laminins into the ECM, which in turn allows collagen IV network assembly. Such data suggests that dystroglycan plays an essential role in early embryogenesis. The curious lack of data on human muscular dystrophies associated with dystroglycan defects may be a result of the embryonic lethal nature of a mutation in such an important gene (Williamson et al. 1997).
VI. LAMININ PROCESSING
As mentioned previously, laminin molecules can undergo multiple posttranslational modifications before reaching its final form. How these hetermotrimeric molecules are processed affects the dynamics of cellular movement. Processing of each of the three chains by specific enzymes has been reported (Figure II), with specific effects on the interacting cell. These studies have mostly focused on laminin 332 processing.
The laminin-332 α3 chain can be processed at both C and N terminals. C terminal processing occurs in both splice variants α3A, a shorter 190kDa form, and α3B, a longer 325kDa form. Cleavage of these two splice variants occurs between the LG3 and LG4 domains and produces 165 kDa and 280 kDa products, respectively (Aumailley et al. 2003). Plasmin, MMP2, MT1MMP, and BMP1 are all enzymes reported to participate in C terminal α3 processing (Veitch et al. 2003). Since LG4-G5 is involved in binding to cellular receptors such as syndecan and dystroglycans, and integrins, processing of the C terminal α3 chain is thought to regulate cellular movement. This complex topic will be further explored in the next section. In addition, α3A N terminal processing can also occur. Cleavage at the LEc tandem shortens the chain from 165 kDa to 145kDa. The proteolytic enzyme involved at this site has not been identified, however it is thought that BMP-1 is not a likely participant (Amano et al. 2000). In human skin basement membranes, it was observed that all laminin-332 have processed α3 C terminals (Aumailley et al. 2003) whereas only half are found with processed α3 N terminals (Marinkovich et al. 1992a). This suggests that processing has a physiologic role in humans.
Although most studies have focused on the laminin-332 α3 chain, processing of α chains found in other laminins has also been described. Laminin α4 and α2 chain C-terminal processing occurring at the LG3-G4 region, as in α3 chains, have been described in mice (Talts et al. 1998; Talts et al. 2000). The significance of such processing in in vitro and in vivo systems has been less well described. Laminin α5 chain also undergoes cleave at multiple sites by MT1-MMP to generate 45 kDa, 310 kDa, 190 kDa, and 160 kDa fragment products. Processing of this chain in laminin 511 is associated with decreased adhesiveness of human prostate cancer cells to the underlying laminin 511 substratum and hence increased transmigratory potential (Bair et al. 2005).
There have been few studies focusing on human laminin 332 β3 chain processing. Laminin-332 β3 chain cleavage occurs at the N terminus and reduces the chain from 140 kDa to 80–90 fragments in one study (Udayakumar et al. 2003), and to both 80 and 110 kDa fragments in another study (Nakashima et al. 2005). β3 proteolysis carried out by MT1MMP has been observed in human prostate cancer cells (Udayakumar et al. 2003) and MMP7 in human colon cancer cells (Remy et al. 2006). These studies concluded that β3 processing facilitated tumor cell migration. Interestingly, β3 chain proteolysis can also occur in the presence of MMP inhibitors, suggesting that other proteinases produced endogenously from cells such as BMP-1/mTLD may be the physiologic mediators (Nakashima et al. 2005). β3 chain processing seems to be species specific, as rat β3 chains do not undergo processing (Udayakumar et al. 2003).
Numerous studies have focused on laminin-332 γ2 processing. The γ2 chain is a 155 kDa chain that undergoes processing at the N terminal. Earlier studies by Koshikawa suggested that MMP2 and MT1MMP participate in cleaving the rat γ2 chain to 80 kDa and 100 and 80 kDa fragments respectively (Koshikawa et al. 2000). However, a subsequent study by Veitch revealed that unlike rat γ2, the human γ2 chain does not undergo processing by MMP2 or MT1MMP. Instead, BMP-1 isoforms were found to process the 165 kDa γ2 chain to 105 kDa. In particular, mTLD may be the physiologic enzyme, as it is the main BMP isoform found in human skin. γ2 processing occurred even in the presence of MMP inhibitors. The difference between enzymatic action was attributed to the likely difference in cleavage site sequences between rats and humans. To underscore the physiologic importance of BMP’s role in laminin-332 processing in an in vivo system, Veitch demonstrated that skin in BMP null mice exhibited abnormal laminin-332 processing as well as abnormal hemidesmosomes in the lamina densa (Veitch et al. 2003). Koshikawa then contradicted the findings by demonstrating that MT1MMP was actually able to process human monomeric and hetermotrimer-bound γ2 into 105kDa and 85kDa fragments. In addition, similar to rat γ2 processing, an internal DIII fragment (25 or 27 kDa) is also generated in the process. The released DIII fragment which binds to EFGR was demonstrated to activate migration of cells. When a comparison of the cleavage site sequences was performed between rat and human γ2, it was found that the first cleavage site was conserved while the second site(s) was not (Koshikawa et al. 2005). The reason for the discrepancy in data between the two groups is unclear. However, an analogy can be drawn with the discrepancies found in the different studies that examined β3 chain processing.
The importance of in vivo laminin-332 processing using BMP-1 knock out mice was described previously. In human skin, only processed γ2 is found, suggesting that γ2 processing could have an important physiologic role (Marinkovich et al. 1992a). Defective γ2 processing in humans results in cylindromatosis, a rare genetic disorder describing multiple benign epithelial tumors. Laminin-332 α and γ chains are found mostly in the unprocessed 165kDa and 155 kDa forms, the basement membrane thickens and develops abnormally in structure, and the different integrin receptors are found in improper ratios. In addition, laminin-332’s interaction with other ECM components is altered, as binding to partner molecules such as collagen VII is dependent on its processed state (Tunggal et al. 2002).
VII. LAMININS AND CELL MIGRATION
The ECM provides critical signals to interfacing cells to direct the dynamics of cellular motion. For some time, there appeared to be contradictory data on effects of laminin-332 on cellular motility(Goldfinger et al. 1998). Certain cell lines were known to produce a laminin-332 matrix which promoted migration(Zhang et al. 1996) whereas other cell lines produced laminin-332 which promoted hemidesmosome formation (Baker et al. 1996; O’Toole et al. 1997). Goldfinger noted that that the laminin-332 α3 chains secreted by cell lines which favored migration were intact, while laminin-332 α3 secreted by cell lines which promoted hemidesmosome formation were processed (Goldfinger et al. 1998). It is now thought that processing of laminin chains, especially the α and γ chains of laminin-332, play a role in regulation of cell migration via haptotaxis and chemotaxis (Veitch et al. 2003; Koshikawa et al. 2004; Koshikawa et al. 2005).
Processing of laminin α chains is thought to alter its binding ability to various integrins, by differential exposure of binding sites/sequences to integrin receptors. The intact α3 chain is capable of binding, via its LG2-LG3 domains, to α3β1 and α6β1 integrins, both of which signal the cell to migrate. Experiments involving antibody mediated activation of α3β1 and α6β1 integrins initiate the MAPkinase ERK1/2 pathway that ultimately results in cellular movement. Processing of the α3 chain at the LG3-LG4 domains may expose an internal binding site that allows binding to α6β4 the integrin constituting hemidesmosomes, thereby theoretically increasing hemidesmosomal formation and inhibiting migration (Hintermann et al. 2004). Experiments involving antibody mediated activation of α6β4 results in erbB2 phosphorylation and initiation of the PI-3K pathway, which inhibits α3β1. This suppression of integrin activity by another integrin is known as trans-dominant inhibition and the exact mechanism in unclear (Hintermann et al. 2001). Experiments show that α6β4 signaling ultimately leads to increased cadherin related cell-cell adhesion that could inhibit cellular movement (Hintermann et al. 2005). Although there is a general concensus that α6β4 supports adhesion, controversy exists regarding its role in cell migration. Some recent studies show that α6β4 supports migration (Nikolopoulos et al. 2005; Sehgal et al. 2006). One study suggested that α6β4, via rac signaling, helps organize laminin-332 organization within the ECM and hence affects the track of cellular movement (Sehgal et al. 2006). It should be noted that this model of laminin-integrin mediated movement is specific to laminin-332, as other integrin-laminin interactions produce different results (Hintermann and Quaranta 2004).
Processing of the laminin γ chain is also implicated in cell migration. The DIII fragment that is released during γ2 processing binds to erbB1, part of the EGFR family, initiating the ERK 1/2 pathway. ErbB1 activation phosophorylates the beta chain of α2β4 and causes hemidesmosomal disassembly, necessary for cellular movement. ErbB1 in turn, also activates expression of MMP2, which contributes to further processing of laminin 5 γ chain for cell migration (Schenk et al. 2003).
VIII. WOUND HEALING AND TUMOR INVASION
Given the role of laminin 332 in cell migration, it is not surprising that laminin-332 is implicated in processes such as wound healing and tumor invasion. Interestingly, keratinocytes activated at the leading edges of wounds are found to express high levels of unprocessed laminin-332 α3 chains, while other quiescent keratinocytes are found to express processed laminin α3 chains. In vitro studies demonstrate that these leading keratinocytes deposit the unprocessed laminin-332 at the rear of the cell, forming a trail of laminin-332 deposits (see Figure 4), underneath which the collagen substratum is removed (Frank et al. 2004). α3β1 and α6β4 integrins are also both found at leading wound edges and seem to play a role in wound healing, as antibodies against either integrin partially impede wound closure in cell culture models, while antibodies against both integrins impede wound closure more so. The α6β4 integrins are proposed to form “immature hemidesmosomes” and is therefore consistent with its role in wound healing (Goldfinger et al. 1999). Laminin 332-α6β4 integrin interaction at wound edges activates the PI3K signal pathway, allowing for α3β1 integrin mediated cellular spreading over laminin-332. Deposition of laminin-332 over the exposed collagen during wounding thus causes leading keratinocytes to switch from rho GTPase signaling associated with collagen-α2β1 integrin to PI3K signaling associated with laminin 332-integrins for spreading during wound repair (Nguyen et al. 2000). However, even before keratinocyte are activated from quiescent to leading, α3β1 interactions with endogenous laminin-332 are still required to increase the rho GTPase levels necessary for collagen mediated spreading and subsequent laminin-332 deposition. Nguyen et al posit that even though the endogenous, processed laminin-332 has been traditionally considered inhibitory to migration, the activation state of the cell contributes to its motility as much as the state of laminin processing. Hence, local inflammatory mediators can activate quiescent cells at the wound site to spread in response to processed laminin-332 –α3β1 integrin interaction (Nguyen et al. 2001).
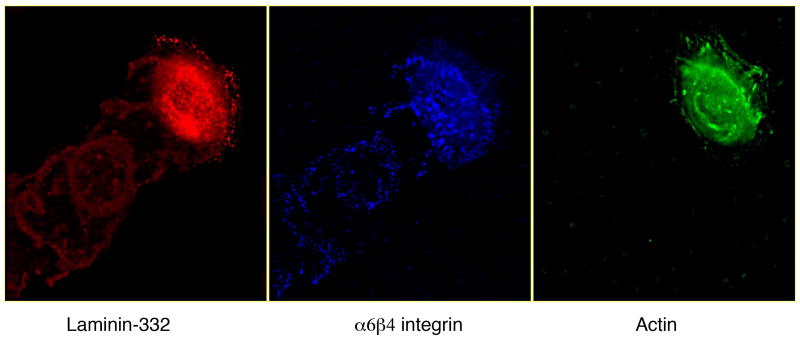
Figure 4.
Laminin 332 is a major component of the extracellular matrix of many epithelial cells. Shown here by triple label indirect immunofluorescence microscopy, laminin 332 localizes to trails behind migrating human keratinocytes along with its ligand α6β4 integrin. Actin staining is also included to indicate position of the cell.
Many epithelial human cancers, including lung, colon, and squamous cell have been found to express high amounts of the laminin γ2 chain especially at its leading edges (Pyke et al. 1995; Kainulainen et al. 1997; Maatta et al. 1999; Ono et al. 1999; Yamamoto et al. 2001). The high expression levels of γ2 have been found to correlate with cancer invasiveness and its detection has been proposed to serve both clinical diagnostic and prognostic purposes (Noel et al. 2005). Most of the γ2 chains detected in these tumor cells were located in the cytoplasm and in monomeric forms (Katayama et al. 2004), which interestingly is more sensitive to processing compared to the heterotrimeric forms (Koshikawa et al. 2005). Laminin γ2 chain processing, as demonstrated in a number of studies, plays a role in tumor cell invasion. Human breast cancer cells are stimulated to migrate when the γ2 chain is processed with the addition of exogenous MMP2 (Giannelli et al. 1997). In addition, migration is also induced when the γ2 chain is processed by endogenous MMP2 activated by cellular MT1-MMP or MT1MMP alone, providing more of a physiologic model for spatial control of migration (Koshikawa et al. 2000). A clinical study of pancreatic cancer patients found that patients with metastatic pancreatic cancer to the liver had a significantly higher level of processed γ2 N terminal fragments within the serum compared to lower stage pancreatic cancers and normal individuals (Katayama et al. 2005). This finding is not surprising, since the soluble N terminal γ2 fragments are released into the extracellular fluids upon proteolysis and the amount of proteolysis correlates the invasiveness of the tumor (Katayama and Sekiguchi 2004).
IX. TISSUE DISTRIBUTION OF LAMININ ISOFORMS
Expression patterns of laminin isoforms are regulated both temporally and spatially during development. This results in a specific distribution of laminin isoforms within an organism at any given time. Regulation of α chain expression is an important determinant of the laminin isoform found in a tissue. The α1 chain, found in laminin 1 (111) and 3 (121), is heavily expressed in epithelial cells during early embryogenesis. Its expression becomes more restricted as the organism develops and is found in adult reproductive organs, kidney, and liver (Ekblom et al. 2003). The α1 chain was once thought to be more ubiquitously expressed, as the 4C7 antibody used to detect its expression actually detected α5 chain expression instead (Falk et al. 1999). Despite its minor appearance in adult organisms, laminin 111 has been one of the most well studied laminins and is orthologous to laminins found in invertebrates, suggesting its evolutionarily ancient history (Colognato and Yurchenco 2000; Yurchenco et al. 2004). The α2 chain, found in laminins 2 (211), 4 (221), and 12 (213) is mostly localized to the neuromuscular system such as basement membranes of the myofiber sarcolemma and the neuromuscular junction. The α3 chain, found in laminins 5 (332), 6 (311), and 7 (321), is found in skin and other epithelia. Laminin 5 (332), 6 (311), and 7 (321) are often found bound to each other in human skin and amnion (Hirosaki et al. 2002). The α4 chain, found in laminins 8 (411), 9 (421), and 14 (423), is localized to cells of mesenchymal origin. Laminin 8 along with laminin 10 is the major laminins of the vascular endothelial cells. Laminin 8 is also found on secreted by cells derived from the bone marrow (Wondimu et al. 2004). The α5 chain, found in laminins 10 (511), 11 (521), and 15 (523), is widely expressed throughout the body in adult epithelial, neuromuscular, and vascular tissues. It is also found in significant quantities during embryogenesis, along with the α1 chain (Colognato and Yurchenco 2000).
X. LAMININS AND DEVELOPMENT
Early Embryogenesis
Knock out mouse models have imparted valuable information on the critical role of laminins in development. Through such studies, laminins-111 and 511 have been found to assume essential roles during embryonic development. Laminin-111 is highly expressed during early embryogenesis and deletion of any of its subunits results in peri-implantation lethality. γ1 subunit and β1 subunit deletion resulted in the failure of formation of laminins and consequently the blastocyst’s visceral and parietal (Reichert’s) basement membranes. These embryos died after implantation at E5.5. However, both γ1 and β1 are chains common to laminin-511, which is also widely expressed in the embryo. By specifically deleting the α1 chain, embryos survived to E7, possibly due to partial compensation by the α5 chain. A visceral basement membrane was detectable in these embryos, however Reichert’s membrane failed to develop (Yurchenco and Wadsworth 2004). Moreover, overexpression of α5 in the homozygous α1 null embryos allowed gastrulation to proceed (Miner et al. 2004a). These experiments suggest that laminin-111 plays an important role in basement membrane assembly, is partially compensated by laminin-511, and is critical for early embryogenesis to proceed. It can be argued that lethality was prevented from occurring at an earlier stage due to maternal contribution of laminins to the homozygous embryos (Miner and Yurchenco 2004b).
Further insight into the importance of laminins to basement membrane initiation and assembly was obtained from a number of studies that examined the effects of deleting other major components known to contribute to the structural integrity of the basement membrane. For some time, it was not known whether basement membrane assembly was dependent on formation of the collagen network, with subsequent integration of laminins via linkers such as nidogen, or whether assembly was simply dependent on the formation of the laminin network. As demonstrated in studies examining collagen IV knockout mice, early embryogenesis proceeded with normal deposition and initiation of basement membrane assembly. However, these mice died at E 10.5–11.5 with abnormal basement membranes and a structurally inadequate Reichert’s membrane. This suggested that despite collagen IV’s prominent role in stabilizing the basement membrane architecture via formation of a polymerizing network, it is not necessary in the initiation of basement membrane assembly (Poschl et al. 2004). Thus laminins appear essential for basement membrane development in the early stages of embryogenesis, while collagen IV is needed as the basement membrane continues to develop. Other studies involving nidogen knock out mice found that absence of nidogen 1 or 2 did not affect embryogenesis or basement membrane architecture. Such mice develop normally and are fertile (Murshed et al. 2000; Schymeinsky et al. 2002). Because the nidogen isoforms could functionally compensate for each other, nidogen 1 and 2 double knockouts were created. In these mice, embryogenesis and organogenesis proceed unimpeded. However, these mice cannot survive beyond birth as a result of abnormal basement membrane architecture affecting late heart and lung development (Bader et al. 2005). Taken together, these experiments demonstrate that laminin alone, especially laminin-111, can provide sufficient architectural support for basement membrane assembly in early embryogenesis, but additional molecular linkers and networks are required later in development for complete integrity of the basement membrane and hence proper organ formation and embryonic viability.
Embryonic Organogenesis
Laminin-511, along with laminin-111, is also widely expressed in all basement membranes during embryogenesis, with an expression pattern that becomes more restricted as development proceeds. Its absence disrupts the development of multiple organs, consistent with its ubiquitous pattern of expression through much of embryogenesis. Embryonic lethality occurs later than homozygous laminin-111 null mice, at E16.5, presumably because its presence is critical at later stages of embryogenesis. Miner et al performed several studies with α5 knockout mice and noted a variety of developmental anomalies within the neural, limb, and placental tissues of homozygous null mutants, consistent with areas of the body where α5 is normally expressed within the basement membrane. Distal extremities of mutant embryos failed to septate into individual digits. When sections of the limbs were examined under the microscope, it was found that the ectodermal basement membrane was discontinuous and the mesenchyme had extruded through the defects to cap the ectoderm. This abnormal reorganization of the forelimb caused by mesenchymal displacement was suggested to result in the observed syndactyly. Most mutant embryos also developed exencephaly. The lack of proper anterior neural tube closure was likely secondary to structurally abnormal basement membranes near the neural folds, which have been thought to be responsible for tube closure. Examination of the placental labyrinth in mutants revealed large vessels with reduced branching and trophoblasts that do not properly adhere to the basement membrane. This last developmental abnormality is likely the largest contributor to embryonic lethality (Miner et al. 1998). .
In addition to the three main developmental anomalies noted, Miner also noted defects in the lung and kidneys of the homozygous α5 mutant embryos. In subsequent studies, Miner found that the α5 chain is the main α chain type involved in the development of the pleural basement membrane and is necessary for proper septation of the lung. However, lung morphogenesis and vascular development were not impaired, and it is thought that either expression of other alpha chains is required, or that compensation by other alpha chains may be sufficient to compensate for α5 deficiency (Nguyen et al. 2002). The α5 chain also contributes to later stages of lung development, as demonstrated by experiments using inducible lung epithelial cell-specific α5 knockouts. It was found that mutant fetuses exhibited enlarged alveoli, impaired or absent differentiation of type I and II alveolar cells, and decreased capillary density. These developmental defects occurred despite structurally normal alveolar basement membranes and preservation of α5 within the vascular endothelial cells, suggesting that laminin-511 plays important signaling functions that direct lung development (Nguyen et al. 2005). Kidneys of α5 homozygous mutants also showed abnormal development. A small percentage of mutants had unilateral or bilateral renal agenesis, thought to be caused by defects in the ureteral outgrowth towards mesenchymal tissue, with which it interacts to generate reciprocal signals for induction of kidney formation. The basement membrane of the Wolffian duct, from which the ureteral bud arises, normally expresses the α5 chain. Glomeruli in these mice also lacked a basement membrane and hence could not support proper endothelial and podocyte attachment to form structurally intact glomeruli (Miner et al. 2000).
The α5 chain has also been implicated in cutaneous development, particularly hair follicle formation. Laminin-511 expression is increased around the basement membrane of hair follicles and has been shown to be upregulated throughout the anagen phase (Li et al. 2003; Sugawara et al. 2007). In knockout mouse studies, the dermal-epidermal basement membrane of α5 homozygous mutant mouse embryos was noted to be discontinous. Mutant skin revealed reduced hair follicles in the skin compared to wild type controls. Grafting of the mutant embryonic skin onto nude mice revealed that laminin-511 deficient skin continued to display absent hair development compared to wild type control grafts. Interestingly, hair development could be restored with addition of human exogenous laminin-511 (Li et al. 2003).
As the α3β1 and α6β1 integrins are known to be the main cellular receptors for laminin 511, it is not surprising that similar developmental abnormalities can be replicated in mice with α3 or α6 integrin deletions. Although mice with individual mutations in α3 or α6 integrins exhibit some similarities with homozygous laminin α5 mutants, the similarities become striking when both α3 and α6 are mutated. Such mice exhibit syndactyly, exencephaly, abnormalities in lung septation/alveolarization, and urogenital abnormalities. These similarities can be explained by the necessity for both integrin receptors to interact with laminin-511 during development of these specific organs. On the other hand, some of the developmental abnormalities in these mice not observed with laminin-511 mutants can be explained by the need for additional integrin-ligand interactions independent of laminin-511 (De Arcangelis et al. 1999). Conditional ablation of the β1 integrin in the skin of mice produced a similar phenotype to the skin findings of laminin α5 knockout mice. The dermal-epidermal basement membranes of these mutants were ultrastructurally abnormal and failed to support hair follicle development. Additionally, these mice exhibited severe blistering, possibly related to an indirect need for β1 integrins in hemidesmosomal assembly (Brakebusch et al. 2000; Raghavan et al. 2000).
XI. CONCLUSIONS
Much advancement has been made in our understanding of laminin biology in recent years. Laminins are now recognized to be one of the most important basement membrane components, with diverse structural and active regulatory functions that arise from the interactions of its various domains to cellular receptors and other ligands. In vitro and in vivo knock out mouse studies have elucidated key roles of laminins on cellular behavior and embryonic development. Additional studies are still needed to further characterize the molecular and signal transduction determinants of cellular motility and its relevance to pathologic processes that arise from abnormal cellular motility or adhesion. The construction of different laminin conditional knock out mouse models in the future can also help further clarify the developmental role laminins within the postnatal organism. Information derived from such studies may allow us to develop effective clinical tools to diagnose and treat congenital conditions, malignancies, and disease states that arise from perturbation of normal laminin function.
Figure 1.
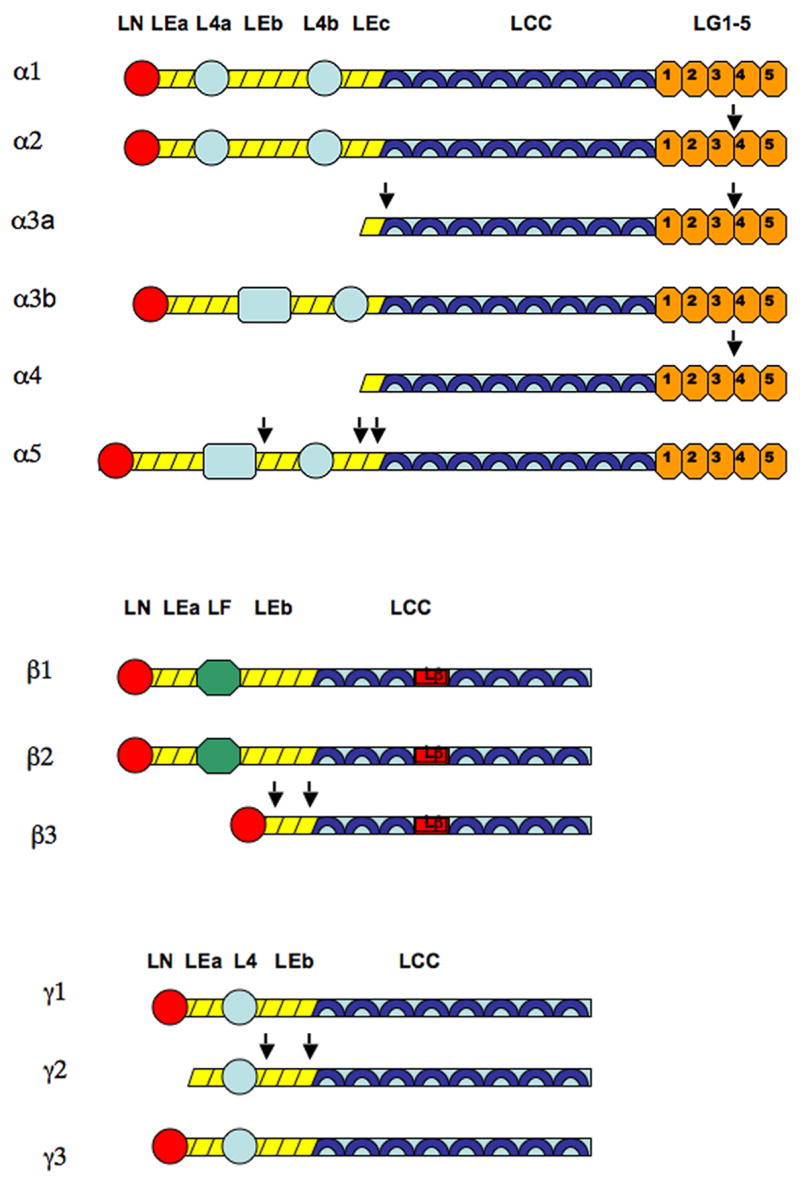
Domains of laminin alpha, beta, and gamma chains. Short arms of each chain are comprised of rodlike EGF-like tandems (LEa, LEb, LEc) and globular domains (LN, L4a, L4b, L4, LF). Long arms of each chain are comprised of alpha helical coils (LCC). Beta chains have a characteristic interruption of the coiled-coiled structure, known as the laminin β-knob domain (Lβ). Alpha chains also have a distinctive globular domain, consisting of five repeats (LG1-5), at the C terminal of the long arm (Patarroyo et al. 2002).
Laminin 332 processing occurs in alpha, beta, and gamma chains. Note that processing occurs at both N and the LG3-G4 site of the C terminals in the alpha 3A chain (Aumailley et al. 2003). The α2 and α4 chains also undergo processing at the LG3-G4 site (Talts et al. 1998; Talts et al. 2000). The α5 chain undergoes N terminal processing at multiple sites within the EGF-like domains (Bair et al. 2005). The β3 chain undergoes processing at two sites along its short arm (Nakashima et al. 2005). Processing of the γ chain occurs at two sites as well, producing an internal DIII fragment that is implicated in cell motility (Koshikawa et al. 2005; Veitch et al. 2003). Cleavage sites are indicated by arrows.
Abbreviations
- BM
basement membrane
- ECM
extracellular matrix
- JEB
junctional epidermolysis bullosa
- JEB-PA
junctional epidermolysis bullosa with pyloric atresia
- MMP
metalloproteinase
- EGFR
epidermal growth factor receptor
- MAPK
mitogen activated protein kinase
- FAK
focal adhesion kinase
- JNK
c-Jun amino-terminal kinase
- ERK
extracellular signal-regulated kinase
- PI-3K
phosphatidylinositol-3 kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, Keene DR, Hudson DL, Nishiyama T, Lee S, Greenspan DS, Burgeson RE. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem. 2000;275:22728–22735. doi: 10.1074/jbc.M002345200. [DOI] [PubMed] [Google Scholar]
- Ashton GH, Sorelli P, Mellerio JE, Keane FM, Eady RA, McGrath JA. Alpha 6 beta 4 integrin abnormalities in junctional epidermolysis bullosa with pyloric atresia. Br J Dermatol. 2001;144:408–414. doi: 10.1046/j.1365-2133.2001.04038.x. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Aumailley M, El Khal A, Knoss N, Tunggal L. Laminin 5 processing and its integration into the ECM. Matrix Biol. 2003;22:49–54. doi: 10.1016/s0945-053x(03)00013-1. [DOI] [PubMed] [Google Scholar]
- Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25:6846–6856. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SE, Hopkinson SB, Fitchmun M, Andreason GL, Frasier F, Plopper G, Quaranta V, Jones JC. Laminin-5 and hemidesmosomes: role of the alpha 3 chain subunit in hemidesmosome stability and assembly. J Cell Sci. 1996;109 ( Pt 10):2509–2520. doi: 10.1242/jcs.109.10.2509. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. Embo J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger R, Kammerer RA, Engel J, Chiquet M. Native chick laminin-4 containing the beta 2 chain (s-laminin) promotes motor axon growth. J Cell Biol. 1996;135:1583–1592. doi: 10.1083/jcb.135.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. doi: 10.1007/s004410051279. [DOI] [PubMed] [Google Scholar]
- Champliaud MF, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J Cell Biol. 1996;132:1189–1198. doi: 10.1083/jcb.132.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champliaud MF, Virtanen I, Tiger CF, Korhonen M, Burgeson R, Gullberg D. Posttranslational modifications and beta/gamma chain associations of human laminin alpha1 and laminin alpha5 chains: purification of laminin-3 from placenta. Exp Cell Res. 2000;259:326–335. doi: 10.1006/excr.2000.4980. [DOI] [PubMed] [Google Scholar]
- Chen M, Marinkovich MP, Jones JC, O’Toole EA, Li YY, Woodley DT. NC1 domain of type VII collagen binds to the beta3 chain of laminin 5 via a unique subdomain within the fibronectin-like repeats. J Invest Dermatol. 1999;112:177–183. doi: 10.1046/j.1523-1747.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-assembly of laminin isoforms. J Biol Chem. 1997;272:31525–31532. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–3968. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- Doliana R, Bellina I, Bucciotti F, Mongiat M, Perris R, Colombatti A. The human alpha3b is a ‘full-sized’ laminin chain variant with a more widespread tissue expression than the truncated alpha3a. FEBS Lett. 1997;417:65–70. doi: 10.1016/s0014-5793(97)01251-9. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egles C, Claudepierre T, Manglapus MK, Champliaud MF, Brunken WJ, Hunter DD. Laminins containing the beta2 chain modulate the precise organization of CNS synapses. Mol Cell Neurosci. 2007;34:288–298. doi: 10.1016/j.mcn.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Ekblom P, Lonai P, Talts JF. Expression and biological role of laminin-1. Matrix Biol. 2003;22:35–47. doi: 10.1016/s0945-053x(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Falk M, Ferletta M, Forsberg E, Ekblom P. Restricted distribution of laminin alpha1 chain in normal adult mouse tissues. Matrix Biol. 1999;18:557–568. doi: 10.1016/s0945-053x(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Frank DE, Carter WG. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci. 2004;117:1351–1363. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Gerl M, Mann K, Aumailley M, Timpl R. Localization of a major nidogen-binding site to domain III of laminin B2 chain. Eur J Biochem. 1991;202:167–174. doi: 10.1111/j.1432-1033.1991.tb16358.x. [DOI] [PubMed] [Google Scholar]
- Gersdorff N, Kohfeldt E, Sasaki T, Timpl R, Miosge N. Laminin gamma3 chain binds to nidogen and is located in murine basement membranes. J Biol Chem. 2005;280:22146–22153. doi: 10.1074/jbc.M501875200. [DOI] [PubMed] [Google Scholar]
- Ghohestani RF, Li K, Rousselle P, Uitto J. Molecular organization of the cutaneous basement membrane zone. Clin Dermatol. 2001;19:551–562. doi: 10.1016/s0738-081x(00)00175-9. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Givant-Horwitz V, Davidson B, Reich R. Laminin-induced signaling in tumor cells. Cancer Lett. 2005;223:1–10. doi: 10.1016/j.canlet.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JC. The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999;112 ( Pt 16):2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JC. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tome FM, Schwartz K, Fardeau M, Tryggvason K, et al. Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995;11:216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Hintermann E, Bilban M, Sharabi A, Quaranta V. Inhibitory role of alpha 6 beta 4-associated erbB-2 and phosphoinositide 3-kinase in keratinocyte haptotactic migration dependent on alpha 3 beta 1 integrin. J Cell Biol. 2001;153:465–478. doi: 10.1083/jcb.153.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann E, Quaranta V. Epithelial cell motility on laminin-5: regulation by matrix assembly, proteolysis, integrins and erbB receptors. Matrix Biol. 2004;23:75–85. doi: 10.1016/j.matbio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Hintermann E, Yang N, O’Sullivan D, Higgins JM, Quaranta V. Integrin alpha6beta4-erbB2 complex inhibits haptotaxis by up-regulating E-cadherin cell-cell junctions in keratinocytes. J Biol Chem. 2005;280:8004–8015. doi: 10.1074/jbc.M406301200. [DOI] [PubMed] [Google Scholar]
- Hirosaki T, Tsubota Y, Kariya Y, Moriyama K, Mizushima H, Miyazaki K. Laminin-6 is activated by proteolytic processing and regulates cellular adhesion and migration differently from laminin-5. J Biol Chem. 2002;277:49287–49295. doi: 10.1074/jbc.M111096200. [DOI] [PubMed] [Google Scholar]
- Ido H, Nakamura A, Kobayashi R, Ito S, Li S, Futaki S, Sekiguchi K. The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin gamma chains in integrin binding by laminins. J Biol Chem. 2007;282:11144–11154. doi: 10.1074/jbc.M609402200. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Morita T, Tryggvason K. Molecular cloning and tissue-specific expression of a novel murine laminin gamma3 chain. J Biol Chem. 1999;274:14107–14111. doi: 10.1074/jbc.274.20.14107. [DOI] [PubMed] [Google Scholar]
- Kainulainen T, Autio-Harmainen H, Oikarinen A, Salo S, Tryggvason K, Salo T. Altered distribution and synthesis of laminin-5 (kalinin) in oral lichen planus, epithelial dysplasias and squamous cell carcinomas. Br J Dermatol. 1997;136:331–336. [PubMed] [Google Scholar]
- Kanagawa M, Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J Hum Genet. 2006;51:915–926. doi: 10.1007/s10038-006-0056-7. [DOI] [PubMed] [Google Scholar]
- Katayama M, Funakoshi A, Sumii T, Sanzen N, Sekiguchi K. Laminin gamma2-chain fragment circulating level increases in patients with metastatic pancreatic ductal cell adenocarcinomas. Cancer Lett. 2005;225:167–176. doi: 10.1016/j.canlet.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Katayama M, Sekiguchi K. Laminin-5 in epithelial tumour invasion. J Mol Histol. 2004;35:277–286. doi: 10.1023/b:hijo.0000032359.35698.fe. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. J Biol Chem. 2005;280:88–93. doi: 10.1074/jbc.M411824200. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Schenk S, Moeckel G, Sharabi A, Miyazaki K, Gardner H, Zent R, Quaranta V. Proteolytic processing of laminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. Faseb J. 2004;18:364–366. doi: 10.1096/fj.03-0584fje. [DOI] [PubMed] [Google Scholar]
- Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, Bradley M, Keene DR, Oro AE, Miner JH, Marinkovich MP. Laminin-10 is crucial for hair morphogenesis. Embo J. 2003;22:2400–2410. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatta M, Soini Y, Paakko P, Salo S, Tryggvason K, Autio-Harmainen H. Expression of the laminin gamma2 chain in different histological types of lung carcinoma. A study by immunohistochemistry and in situ hybridization. J Pathol. 1999;188:361–368. doi: 10.1002/(SICI)1096-9896(199908)188:4<361::AID-PATH363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992a;267:17900–17906. [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Keene DR, Burgeson RE. The dermal-epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992b;119:695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Li C. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–289. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004a;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Miner JH, Patton BL. Laminin-11. Int J Biochem Cell Biol. 1999;31:811–816. doi: 10.1016/s1357-2725(99)00030-8. [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004b;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- Morita A, Sugimoto E, Kitagawa Y. Post-translational assembly and glycosylation of laminin subunits in parietal endoderm-like F9 cells. Biochem J. 1985;229:259–264. doi: 10.1042/bj2290259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed M, Smyth N, Miosge N, Karolat J, Krieg T, Paulsson M, Nischt R. The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol. 2000;20:7007–7012. doi: 10.1128/mcb.20.18.7007-7012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y, Kariya Y, Yasuda C, Miyazaki K. Regulation of cell adhesion and type VII collagen binding by the beta3 chain short arm of laminin-5: effect of its proteolytic cleavage. J Biochem (Tokyo) 2005;138:539–552. doi: 10.1093/jb/mvi153. [DOI] [PubMed] [Google Scholar]
- Nguyen BP, Gil SG, Carter WG. Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J Biol Chem. 2000;275:31896–31907. doi: 10.1074/jbc.M006379200. [DOI] [PubMed] [Google Scholar]
- Nguyen BP, Ren XD, Schwartz MA, Carter WG. Ligation of integrin alpha 3beta 1 by laminin 5 at the wound edge activates Rho-dependent adhesion of leading keratinocytes on collagen. J Biol Chem. 2001;276:43860–43870. doi: 10.1074/jbc.M103404200. [DOI] [PubMed] [Google Scholar]
- Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, Miner JH. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol. 2005;282:111–125. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Nguyen NM, Miner JH, Pierce RA, Senior RM. Laminin alpha 5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev Biol. 2002;246:231–244. doi: 10.1006/dbio.2002.0658. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Puri C, Tacchetti C, Giancotti FG. Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol Cell Biol. 2005;25:6090–6102. doi: 10.1128/MCB.25.14.6090-6102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JC, Fernandez-Aguilar S, Fayt I, Buxant F, Ansion MH, Simon P, Anaf V. Laminin-5 gamma 2 chain expression in cervical intraepithelial neoplasia and invasive cervical carcinoma. Acta Obstet Gynecol Scand. 2005;84:1119–1123. doi: 10.1111/j.0001-6349.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Ono Y, Nakanishi Y, Ino Y, Niki T, Yamada T, Yoshimura K, Saikawa M, Nakajima T, Hirohashi S. Clinocopathologic significance of laminin-5 gamma2 chain expression in squamous cell carcinoma of the tongue: immunohistochemical analysis of 67 lesions. Cancer. 1999;85:2315–2321. [PubMed] [Google Scholar]
- O’Toole EA, Marinkovich MP, Hoeffler WK, Furthmayr H, Woodley DT. Laminin-5 inhibits human keratinocyte migration. Exp Cell Res. 1997;233:330–339. doi: 10.1006/excr.1997.3586. [DOI] [PubMed] [Google Scholar]
- Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy L, Trespeuch C, Bachy S, Scoazec JY, Rousselle P. Matrilysin 1 influences colon carcinoma cell migration by cleavage of the laminin-5 beta3 chain. Cancer Res. 2006;66:11228–11237. doi: 10.1158/0008-5472.CAN-06-1187. [DOI] [PubMed] [Google Scholar]
- Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119:2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145:1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarras MP, Jr, Yan L, Grens A, Zhang X, Agbas A, Huff JK, St John PL, Abrahamson DR. Cloning and biological function of laminin in Hydra vulgaris. Dev Biol. 1994;164:312–324. doi: 10.1006/dbio.1994.1201. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Fassler R, Hohenester E. Laminin: the crux of basement membrane assembly. J Cell Biol. 2004;164:959–963. doi: 10.1083/jcb.200401058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Gohring W, Mann K, Brakebusch C, Yamada Y, Fassler R, Timpl R. Short arm region of laminin-5 gamma2 chain: structure, mechanism of processing and binding to heparin and proteins. J Mol Biol. 2001;314:751–763. doi: 10.1006/jmbi.2001.5176. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Muhle C, Pacho F. Biological function of laminin-5 and pathogenic impact of its deficiency. Eur J Cell Biol. 2006 doi: 10.1016/j.ejcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Schymeinsky J, Nedbal S, Miosge N, Poschl E, Rao C, Beier DR, Skarnes WC, Timpl R, Bader BL. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol. 2002;22:6820–6830. doi: 10.1128/MCB.22.19.6820-6830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal BU, DeBiase PJ, Matzno S, Chew TL, Claiborne JN, Hopkinson SB, Russell A, Marinkovich MP, Jones JC. Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K, Tsuruta D, Kobayashi H, Ikeda K, Hopkinson SB, Jones JC, Ishii M. Spatial and temporal control of laminin-332 (5) and -511 (10) expression during induction of anagen hair growth. J Histochem Cytochem. 2007;55:43–55. doi: 10.1369/jhc.6A6920.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts JF, Mann K, Yamada Y, Timpl R. Structural analysis and proteolytic processing of recombinant G domain of mouse laminin alpha2 chain. FEBS Lett. 1998;426:71–76. doi: 10.1016/s0014-5793(98)00312-3. [DOI] [PubMed] [Google Scholar]
- Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, Timpl R. Structural and functional analysis of the recombinant G domain of the laminin alpha4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275:35192–35199. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- Tarone G, Hirsch E, Brancaccio M, De Acetis M, Barberis L, Balzac F, Retta SF, Botta C, Altruda F, Silengo L. Integrin function and regulation in development. Int J Dev Biol. 2000;44:725–731. [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22:1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Tunggal L, Ravaux J, Pesch M, Smola H, Krieg T, Gaill F, Sasaki T, Timpl R, Mauch C, Aumailley M. Defective laminin 5 processing in cylindroma cells. Am J Pathol. 2002;160:459–468. doi: 10.1016/S0002-9440(10)64865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzu J, Li J, Marinkovich MP. Basement membrane and extracellular matrix molecules in the skin. In: Miner JH, editor. Extracellular Matrix in Development and Disease. Vol 15: Advances in Developmental Biology. Elsevier; 2005. pp. 129–151. [Google Scholar]
- Udayakumar TS, Chen ML, Bair EL, Von Bredow DC, Cress AE, Nagle RB, Bowden GT. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003;63:2292–2299. [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Varki R, Sadowski S, Pfendner E, Uitto J. Epidermolysis bullosa. I. Molecular genetics of the junctional and hemidesmosomal variants. J Med Genet. 2006;43:641–652. doi: 10.1136/jmg.2005.039685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch DP, Nokelainen P, McGowan KA, Nguyen TT, Nguyen NE, Stephenson R, Pappano WN, Keene DR, Spong SM, Greenspan DS, Findell PR, Marinkovich MP. Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin-5 in keratinocytes and skin. J Biol Chem. 2003;278:15661–15668. doi: 10.1074/jbc.M210588200. [DOI] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- Wondimu Z, Geberhiwot T, Ingerpuu S, Juronen E, Xie X, Lindbom L, Doi M, Kortesmaa J, Thyboll J, Tryggvason K, Fadeel B, Patarroyo M. An endothelial laminin isoform, laminin 8 (alpha4beta1gamma1), is secreted by blood neutrophils, promotes neutrophil migration and extravasation, and protects neutrophils from apoptosis. Blood. 2004;104:1859–1866. doi: 10.1182/blood-2004-01-0396. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Itoh F, Iku S, Hosokawa M, Imai K. Expression of the gamma(2) chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Clin Cancer Res. 2001;7:896–900. [PubMed] [Google Scholar]
- Yurchenco PD, Quan Y, Colognato H, Mathus T, Harrison D, Yamada Y, O’Rear JJ. The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci U S A. 1997;94:10189–10194. doi: 10.1073/pnas.94.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kramer RH. Laminin 5 deposition promotes keratinocyte motility. Exp Cell Res. 1996;227:309–322. doi: 10.1006/excr.1996.0280. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fei K, Agbas A, Yan L, Zhang J, O’Reilly B, Deutzmann R, Sarras MP., Jr Structure and function of an early divergent form of laminin in hydra: a structurally conserved ECM component that is essential for epithelial morphogenesis. Dev Genes Evol. 2002;212:159–172. doi: 10.1007/s00427-002-0225-4. [DOI] [PubMed] [Google Scholar]