Abstract
c-src deletion in mice leads to osteopetrosis as a result of reduced bone resorption due to an alteration of the osteoclast. We report that deletion/reduction of Src expression enhances osteoblast differentiation and bone formation, contributing to the increase in bone mass. Bone histomorphometry showed that bone formation was increased in Src null compared with wild-type mice. In vitro, alkaline phosphatase (ALP) activity and nodule mineralization were increased in primary calvarial cells and in SV40-immortalized osteoblasts from Src−/− relative to Src+/+ mice. Src-antisense oligodeoxynucleotides (AS-src) reduced Src levels by ∼60% and caused a similar increase in ALP activity and nodule mineralization in primary osteoblasts in vitro. Reduction in cell proliferation was observed in primary and immortalized Src−/− osteoblasts and in normal osteoblasts incubated with the AS-src. Semiquantitative reverse transcriptase-PCR revealed upregulation of ALP, Osf2/Cbfa1 transcription factor, PTH/PTHrP receptor, osteocalcin, and pro-alpha 2(I) collagen in Src-deficient osteoblasts. The expression of the bone matrix protein osteopontin remained unchanged. Based on these results, we conclude that the reduction of Src expression not only inhibits bone resorption, but also stimulates osteoblast differentiation and bone formation, suggesting that the osteogenic cells may contribute to the development of the osteopetrotic phenotype in Src-deficient mice.
Keywords: osteopetrosis, Src, osteoblast, differentiation, bone formation
Introduction
Targeted disruption of the c-src gene in mice has been demonstrated to cause osteopetrosis (Soriano et al. 1991a). Although the sarcoma (Src) gene product is ubiquitous and highly expressed in several cell types, including platelets (Golden et al. 1986) and neurons (Pyper and Bolen 1990), these mice develop only a bone phenotype (Soriano et al. 1991a). Therefore, much interest has been focused on the role of this gene in bone cells.
The Src family of nonreceptor tyrosine kinases consists of 10 proteins that contribute to many regulated events in cells (Thomas and Brugge 1997). These proteins share a high homology and consist of a unique NH2-terminal domain containing a short SH4 motif for myristoylation and palmitoylation, a SH3 and a SH2 domain involved in protein–protein interactions, and a COOH-terminal SH1 tyrosine kinase domain (Resh 1994; Wenqing et al. 1997). Several substrates have been demonstrated to be tyrosine phorphorylated by Src, including transmembrane tyrosine kinase receptors (platelet-derived growth factor [PDGF], epidermal growth factor [EGF], and macrophage colony stimulating factor [CSF]-1), focal adhesion associated kinases (focal adhesion kinase, proline-rich tyrosine kinase2, Cdk-activating kinase), adhesion molecules (vinculin), and soluble enzymes (enolase, phosphoglyceromutase, and lactate dehydrogenase) (Hunter and Cooper 1985; Parson and Parson 1997).
The recognized defect in Src−/− mice consists in the alteration of the bone resorbing cell function. Increased numbers of osteoclasts at the bone surface, which are inactive and lack a ruffled border, are remarkable features of this bone phenotype (Boyce et al. 1992; Horne et al. 1992; Lowe et al. 1993). Impairment of osteoclastic bone resorption leads to decreased bone remodeling, which results in small size, failure in incisor eruption, thickened growth plate, poorly developed cortex, persistence of endochondral primary spongiosa with widening and extension of trabecular bone in the distal methaphysis and diaphysis, and reduced bone marrow tissue that fills the very little remaining space of the bone cavity (Soriano et al. 1991a).
Osteoclast differentiation and bone resorption are also dependent on cells of the osteoblast lineage (Rodan and Martin 1981; Suda et al. 1997). However, Lowe et al. 1993 demonstrated that osteoblasts derived from c-src knockout mice successfully contributed to normal osteoclast differentiation and showed unremarkable morphological features relative to wild-type mice. This lead to the conclusion that the inherited defect is exclusively with mature osteoclasts and is autonomous from the bone marrow microenvironment. However, a detailed molecular analysis of osteoblast function has not been performed. A recent examination of the skeletal phenotype in older Src−/− mice has indicated that bone mass continues to increase with age, suggesting a continued imbalance between bone resorption and formation (Amling et al. 2000). Therefore, this present study was aimed at determining whether inhibition of Src expression in vivo or in vitro lead to alterations in osteoblast functions and bone formation, a feature which could contribute to the osteopetrotic phenotype, together with the decreased bone resorption.
Materials and Methods
Materials
DME, FCS, penicillin, streptomycin, and trypsin were from GIBCO BRL. Sterile plasticware was from Becton Dickinson. Anti-Src and anti-actin pAbs and HRP-conjugated secondary antibodies were from Santa Cruz Biotechnology, Inc. Antibody L-123 to osteopontin (Fisher et al. 1995) was donated by Dr. Larry Fisher (Craniofacial and Skeletal Diseases Branch, National Institute of Dental Research, National Institutes of Health, Bethesda, MD). Synthetic totally phosphorothioated oligodeoxynucleotide (ODN) sequences (Tanaka et al. 1996) were as follows: antisense (AS-src), 5′-GGGCTTGCTCTTGTTGCTGCCCAT-3′ and sense (S-src), 5′-ATGGGCAGCAACAAGAGCAAGCCC-3′. ODNs were purchased from M-Medical. Enhanced chemiluminescence kit was from Amersham Pharmacia Biotech. Primers and reagents for reverse transcriptase (RT)-PCR were from Promega. Benzoyl peroxide was from Polyscience Inc. All other reagents were of the purest grade from Sigma-Aldrich.
Histology and Histomorphometry
3- and 10-wk-old Src+/+ and Src−/− mice were injected with calcein (20 mg/kg body weight) followed by the same dose of demeclocycline at a 5-d interval. Animals were killed 2 d after the second injection and tibiae were dissected out, fixed in 3.7% formaldehyde in PBS, and embedded in methylmethacrylate by a procedure modified from Baron et al. 1983. Specifically, bones were dehydrated in graded acetone for 1 h in each of 70%, 90%, and twice in 100% acetone then infiltrated and embedded in 80% activated methylmethacrylate (Jowsey et al. 1965), 20% dibutylphthalate, and 4% benzoyl peroxide. 5-μm sections were stained with toluidine blue or unstained, with a coverslip on top, for analysis of fluorochrome labels. Histomorphometric analysis of the secondary spongiosa was carried out according to standard procedures (Parfitt et al. 1987) using the Osteomeasure system (OsteoMetrics, Inc.) in a region 200 μm below the chondro–osseous junction to the diaphysis.
Osteoblast Cultures
Primary cultures of calvarial osteoblasts were prepared by a modification of the sequential collagenase/trypsin digestion method (Robey and Termine 1985). In brief, calvaria were removed from 7–9-d-old CD1 mice or B6, 129tm/Sor Src+/+ and Src−/− mice, cleaned free from soft tissue, and digested with 1 mg/ml Clostridium histolyticum type IV collagenase and 0.025% trypsin for 20 min at 37°C in HBSS with gentle agitation. The procedure was repeated three times and cells from the second and third digestions were plated in petri dishes and grown to confluence in DME supplemented with antibiotics and 10% FCS. At confluence, cells were trypsinized by the standard procedure and plated in wells for experiments. The cells obtained with this method were positive for alkaline phosphatase (ALP) activity and expression of the osteoblast markers, parathyroid hormone/parathyroid hormone–related peptide (PTH/PTHrP) receptor and Osf2/Cbfa1 transcription factor, and of bone matrix proteins, osteopontin and bone sialoprotein II.
Immortalized Osteoblasts
Permanent osteoblast cell lines were prepared from Src+/+ and Src−/− mice (B6, 129tm/Sor) and immortalized by infection with the SV40 virus, according to Soriano et al. 1991b. Cells were grown in DME supplemented with 10% FCS and antibiotics in standard culture conditions. Cells were fed twice a week, trypsinized at confluence, and split 1:5.
ODNs
AS-src and S-src ODNs were diluted in culture medium in 100-μM stock solutions and stored in aliquots at −20°C until used. They were then diluted to the final concentration in medium and administered to the cells for 24 h–3 wk, according to the experimental design. The most effective concentration was 1 μM. Lower concentrations of AS-src had little effect, whereas concentrations >1 μM often resulted in nonspecific changes, which were also observed in S-src–treated cultures. Experiments in which osteoblasts were incubated with a mixture of ODNs and 5 μg/ml of the uptake enhancer lipofectamine resulted in effects similar to cultures incubated with the ODNs alone. Therefore, all the experiments illustrated below were performed at 1 μM of both AS-src and S-src ODNs without uptake enhancer.
Immunoblotting
Cells were lysed in ice-cold 0.1% SDS containing protease inhibitors and 1 mM sodium orthovanadate, and stored at −80°C until used. Protein content was measured using the Bradford method, and then 25–60 μg of cell protein in reducing sample buffer was subjected to 10% SDS-PAGE. Proteins were then transferred to nitrocellulose filter papers and probed with the primary antibody overnight at 4°C, followed by the secondary antibody for 1 h at room temperature. Bands were revealed by enhanced chemiluminescence detection.
RT-PCR
RNA was prepared from osteoblasts using the acid phenol technique. For RT-PCR, 1 μg/ml of total RNA was reverse-transcribed using Moloney-murine leukemia virus RT and the equivalent of 0.1 μg was added to PCR reactions. These were carried out in a final volume of 20 μl buffer containing 200 μM of deoxynucleoside triphosphates, 1.5 mM MgCl2, 10 pM of each primer, and 1 U of Thermus aquaticus DNA polymerase. PCR conditions and primer pairs used are listed in Table . For quantitative analysis, primers for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used along with the primers for the gene being analyzed. 10–30 μl of the PCR-amplified products were analyzed on 1–1.5% agarose gels containing ethidium bromide.
Table 1.
Primers and Conditions Used for RT-PCR
| Gene | Primers | PCR conditions | bp | References |
|---|---|---|---|---|
| ALP | Fw: 5′-GCCCTCTCCAAGACATATA-3′ Rv: 5′-CCATGATCACGTCGATATCC-3′ | 30 cycles: 95°C 1 min, 55°C 2 min, 72°C 1 min | 372 | Qu et al. 1998 |
| PTH/PTHrP receptor | Fw: 5′-CGCGGCCCTAGGCGGT-3′ Rv: 5′-TAGTTGGCCCACGTCC-3′ | 30 cycles: 95°C 1 min, 61°C 2 min, 72°C 1 min | 520 | Gene Bank accession number X78936 |
| Osf2/Cbfa1 | Fw: 5′-CCGCACGACAACCGCACCAT-3′ Rv: 5′-CGCTCCGGCCCACAAATCTC-3′ | 30 cycles: 94°C 30 s, 60°C 30 s, 72°C 30 s | 289 | Komori et al. 1997 |
| Osteocalcin | Fw: 5′-AAGCAGGAGGGCAATAAGGT-3′ Rv: 5′-AGCTGCTGTGACATCCATAC-3′ | 35 cycle: 95°C 1 min, 60°C 2 min, 72°C 1 min | 292 | Gene Bank accession number L24429 |
| COL1A2 | Fw: 5′- GCAATCGGGATCAGTACGAA-3′ Rv: 5′- CTTTCACGCCTTTGAAGCCA-3′ | 30 cycles: 95°C 1 min, 57.3°C 2 min, 72°C 1 min | 484 | Gene Bank accession number X58251 |
| Osteopontin | Fw: 5′-TCACCATTCGGATGAGTCTG-3′ Rv: 5′-ACTTGTGGCTCTGATGTTCC-3′ | 30 cycles: 95°C 1 min, 55°C 2 min, 72°C 1 min | 437 | Chackalaparampil et al. 1996 |
| GAPDH | Fw: 5′-CACCATGGAGAAGGCCGGGG-3′ Rv: 5′-GACGGACACATTGGGGGTAG-3′ | 25 cycle:, 95° 1 min, 55°C 2 min, 72°C 1 min | 418 | Qu et al. 1998 |
bp, Base pairs; Fw, forward; Rv, reverse.
DNA Staining
To stain the DNA and morphologically evaluate apoptosis, cells were fixed in Carnoy's fixative (methanol/glacial acetic acid, 3:1), incubated for 30 min in 0.5 μg/ml bis-benzimide, which specifically binds the adenine-thymine regions of the nucleic acid, rinsed twice in distilled water, mounted in glycerol/PBS, 1:1, and observed by conventional epifluorescence microscopy. The apoptotic nuclei were then enumerated and expressed as mean percentage of the total number of nuclei.
Cell Proliferation
Osteoblasts were plated in 24-well multiplates and grown to 70% confluence. Cells were then treated with the test compounds for 48 h and incubated for the final 4 h with 1 μCi/ml [3H]thymidine (specific activity 14.3 Ci/mmol). At the end of incubation cells were solubilized in 1 ml of 1% SDS to which 10 μl of 10 mg/ml BSA was added as a carrier protein, and precipitated by addition of 100 μl of 100% TCA. After incubation for 30 min at 4°C, the TCA-precipitable material was pelleted by centrifugation at 955 g, redissolved in 500 μl of 1% SDS, and counted in a scintillation spectrophotometer after addition of 500 μl of water and 9 ml of scintillation fluid.
ALP Activity
ALP activity was evaluated histochemically and biochemically, using the Sigma kits n.85 and 104-LS, respectively, according to the manufacturer's instruction (Sigma-Aldrich).
Nodule Mineralization
Osteoblasts were plated in 24-well multiplates and grown until confluent. Media were then replaced with mineralizing media (DME supplemented with 10% FCS, 10 mM β-glycerophosphate, 50 μg/ml ascorbic acid, with or without 10−7 M dexamethasone) containing, where indicated, 1 μM AS-Src or S-Src, and cells were cultured for an additional 3 wk. Media were replaced with fresh media containing the test compounds every 3 d. At the end of incubation, mineralization was detected with von Kossa staining. Quantitative analysis was performed by scanning densitometry on computer-assisted acquired images as described below.
Densitometric Analysis
Scanning densitometry of the areas of interest was performed using the Molecular Analyst software for the model 670 scanning densitometer (Bio-Rad Laboratories) to obtain the arbitrary density units. Normalization for immunoblotting and RT-PCR was performed using as internal controls the reference genes β-actin and GAPDH, respectively. The “gene of interest/reference gene” density ratio was then computed and represented in graphs.
Statistics
Data are expressed as mean percentage ± SEM of at least three independent experiments. Statistical significance was computed by the unpaired Student's t test. A P value < 0.05 was conventionally considered statistically significant.
Results
In Vivo Histomorphometry
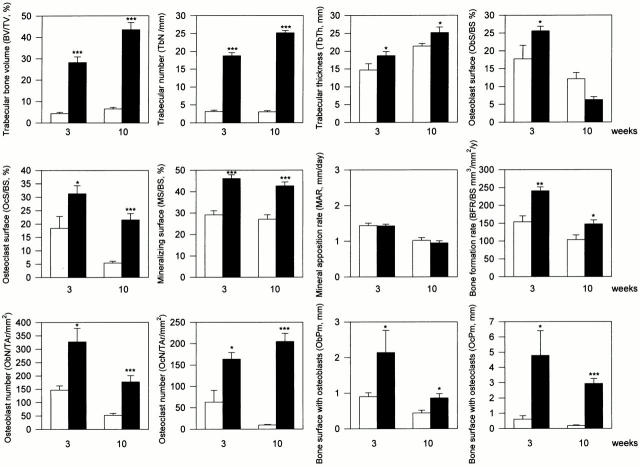
Histomorphometric analysis of 3- and 10-wk-old Src−/− and Src+/+ mice was carried out to determine whether bone formation was increased in the absence of Src in vivo (Fig. 1). As observed previously, both trabecular bone volume (BV/TV) and osteoclast surface (OcS/BS) were significantly increased in Src−/− mice. Bone formation was assessed by examining fluorochrome labels incorporated during mineralization in the same mice and an ∼50% increase in bone formation rate (BFR/BS) was observed at both 3 and 10 wk, which, for the most part, was due to an increase in mineralizing surface (MS/BS) rather than a change in mineral appositional rate (MAR). In contrast, the increase in osteoblast surface, expressed as a ratio of bone surface, in Src−/− mice was only significantly elevated at 3 wk.
Figure 1.
Histomorphometric analysis of tibiae from 3- and 10-wk-old Src+/+ and Src−/− mice. Samples from 3 and 10-wk-old Src+/+ and Src−/− mice were fixed, embedded in methylmethacrylate, and evaluated for histomorphometric analysis as described in the Materials and Methods. All values are mean ± SEM from at least three animals for each category. Src+/+; Src−/−; *P < 0.05, **P < 0.01 and ***P < 0.001 versus Src+/+ mice.
Since BV/TV and trabecular number (TbN) are both dramatically increased in osteopetrotic Src−/− mice, and since ObS/BS is expressed as a ratio of osteoblast surface to total bone surface, any effect of Src deficiency on the number of osteoblasts may be masked by the large extent of bone surface in Src−/− mice (trabecular bone surface was ∼3.4 times that of wild-type mice). To take this change into account, the total number of osteoblasts was also assessed by measuring osteoblast number relative to the area of secondary spongiosa (ObN/TAr), as well as the total bone surface lined with osteoblasts (ObPm). Indeed, when the increased trabecular bone surface is taken into account, we found that the total number of osteoblasts, indicated by both ObN/TAr and ObPm was significantly increased in Src−/− mice, about twofold at both 3 and 10 wk. As expected, osteoclast number and total osteoclast surface were both dramatically increased in Src−/− mice. Thus, Src−/− mice exhibit not only a cell autonomous defect in bone resorption (Soriano et al. 1991a; Lowe et al. 1993), but also a dramatic increase in osteoblast numbers and bone formation, changes that would contribute significantly to the osteopetrotic phenotype and explain the continued increase in bone mass in the knockout mice (Amling et al. 2000).
Cell Culture Study
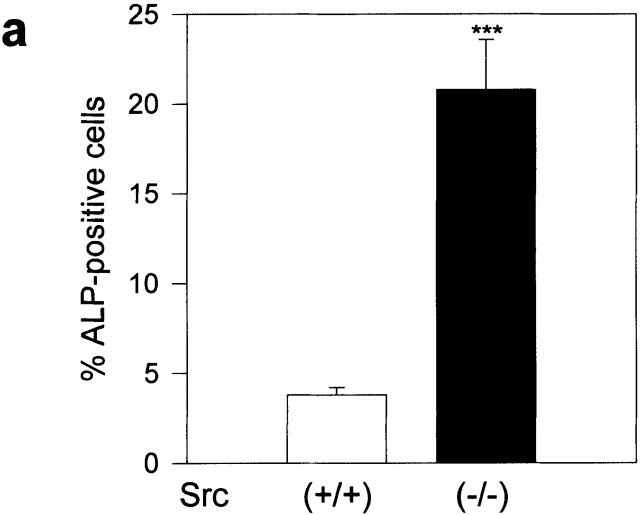
To determine whether the altered osteoblast phenotype was cell autonomous, primary calvarial cells harvested from Src null mice were cultured and analyzed for ALP expression and nodule mineralization (Fig. 2). We found that the number of ALP-positive cells was approximately fourfold higher (Fig. 2 a) and the rate of mineralized nodule formation was significantly increased (approximately twofold, Fig. 2 b) in Src null primary osteoblasts relative to wild-type cells. No morphological changes were observed in Src−/− osteoblasts, and DNA staining by bis-benzimide revealed the same numbers of apoptotic cells as the wild-type cells (Src+/+, 8.4 ± 0.7%; Src−/−, 7.7 ± 0.8% of the total number of nuclei; n = 3; P = 0.6). Therefore, these results suggest that the increased osteoblastic function observed in vivo in Src−/− mice is cell autonomous.
Figure 2.
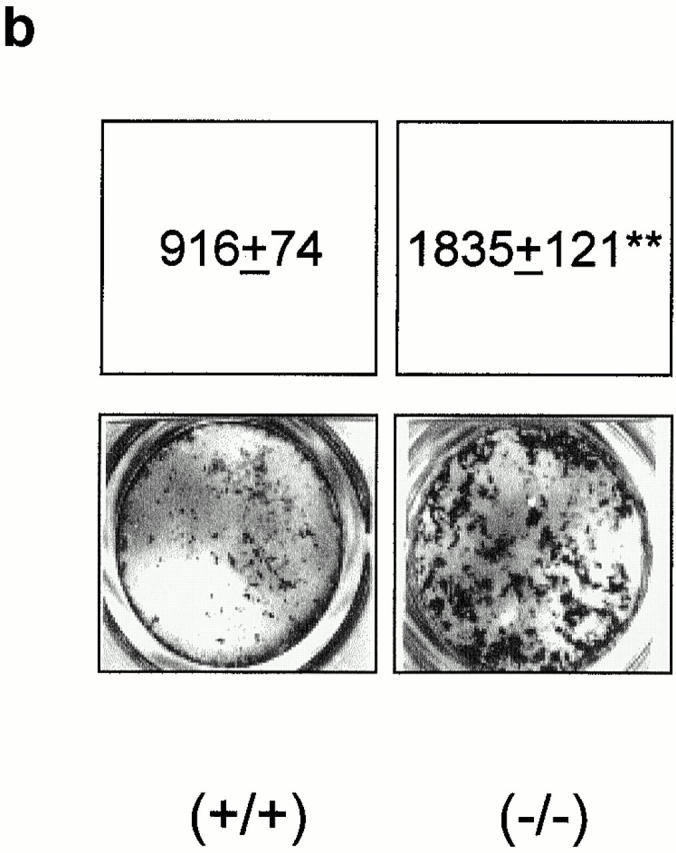
Src-deficient osteoblast phenotype. Primary calvarial osteoblasts were harvested from B6, 129tm/sor mice, as described in Materials and Methods, and cultured for detection of ALP activity and nodule mineralization. (a) 90% confluent osteoblasts were cultured for 1 wk, fixed, and stained histochemically to detect ALP activity using the Sigma kit n. 85. ALP-positive cells were counted and converted into a percentage versus the total number of cells. Data are the mean ± SEM of three independent experiments. ***P < 0.001 versus Src+/+. (b) 90% confluent osteoblasts were cultured for 3 wk in the presence of ascorbic acid and β-glycerophosphate, as described in Materials and Methods. Cultures were then fixed and mineralized (dark) nodules were detected by von Kossa staining. Quantitative analysis was performed by scanning densitometry, as described in Materials and Methods, and data from three independent experiments were expressed as arbitrary densitometric units (mean ± SEM). **P < 0.01 versus Src+/+.
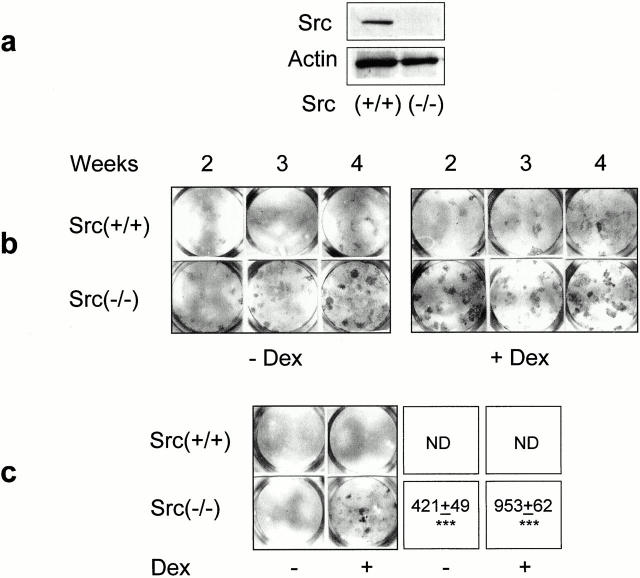
We then used SV40-immortalized osteoblasts from Src−/− or Src+/+ animals to further investigate this possibility. As expected, expression of the SV40 large T antigen did not modify the pattern of Src expression, with no protein in Src−/− cells, whereas normal protein levels were observed in Src+/+ osteoblasts (Fig. 3 a). These immortalized osteoblasts showed low ALP activity in standard culture conditions. However, ALP activity spontaneously increased in long-term cultures, with greater activity observed in Src−/− versus Src+/+ cells at any time point (Fig. 3 b). In addition, ALP activity was stimulated by treatment with the differentiating agent dexamethasone, with, once again, a higher level of ALP in Src−/− relative to Src+/+ cells (Fig. 3 b).
Figure 3.
Osteoblast phenotype in Src+/+ and Src−/− immortalized cells. (a) Src protein expression in immortalized osteoblasts. Confluent monolayers were lysed and 60 μg of total cell protein was electrophoresed in a 10% SDS-PAGE, blotted to nitrocellulose filter paper, and probed with Src pAb (diluted 1:250) at 4°C overnight, followed by the HRP-conjugated secondary antibody (diluted 1:5,000) at 37°C for 1 h. The filter was stripped and reprobed with anti-actin pAb (diluted 1:250). Bands were detected by ECL. This evaluation was repeated three times with similar results. (b) 90% confluent Src+/+ and Src−/− immortalized osteoblasts were cultured for the indicated times and subjected to determination of ALP, as described in the legend to Fig. 2. Cultures were treated with or without 10−7 M dexamethasone (Dex) for the entire time frame. Positive nodules are evident as dark spots. This experiment was repeated three times with similar results. (c) 90% confluent Src+/+ and Src−/− immortalized osteoblasts were cultured for 3 wk in the presence of ascorbic acid and β-glycerophosphate, as described in the Materials and Methods and with or without 10−7 M Dex for the entire time frame. Cultures were then fixed and subjected to von Kossa staining for the detection of nodule mineralization (dark areas). Quantitative analysis was performed by scanning densitometry, as described in the Materials and Methods, and data from three independent experiments were expressed as arbitrary densitometric units (mean ± SEM). ***P < 0.001 versus Src+/+; ND, non detectable.
In contrast to primary cultures, immortalized Src+/+ cells failed to form mineralized nodules in vitro, independent of time in culture and treatment with dexamethasone (Fig. 3 c). In contrast, Src−/− cells formed a few small mineralized nodules even in the absence of dexamethasone, whereas in the presence of dexamethasone these cultures underwent macroscopic nodule mineralization clearly visible by week 3 of culture (Fig. 3 c).
Taken together these findings suggest that Src deletion favors osteoblast differentiation and function in vitro and in vivo and in a cell autonomous manner. Therefore, Src may play a negative role in osteoblast differentiation and/or function. To test this hypothesis and elucidate whether the increased osteoblast function was directly related to Src deletion, we reduced Src expression in normal calvarial osteoblasts by the use of AS-src shown previously to inhibit Src synthesis in osteoclasts and affecting Src-dependent functions (Tanaka et al. 1996). In osteoblasts treated for 24 h with 1 μM AS-src or with the control sense (S-src) sequence, we found that AS-src reduced Src protein level by ∼60%, whereas the control oligonucleotide S-src was inactive (Fig. 4).
Figure 4.
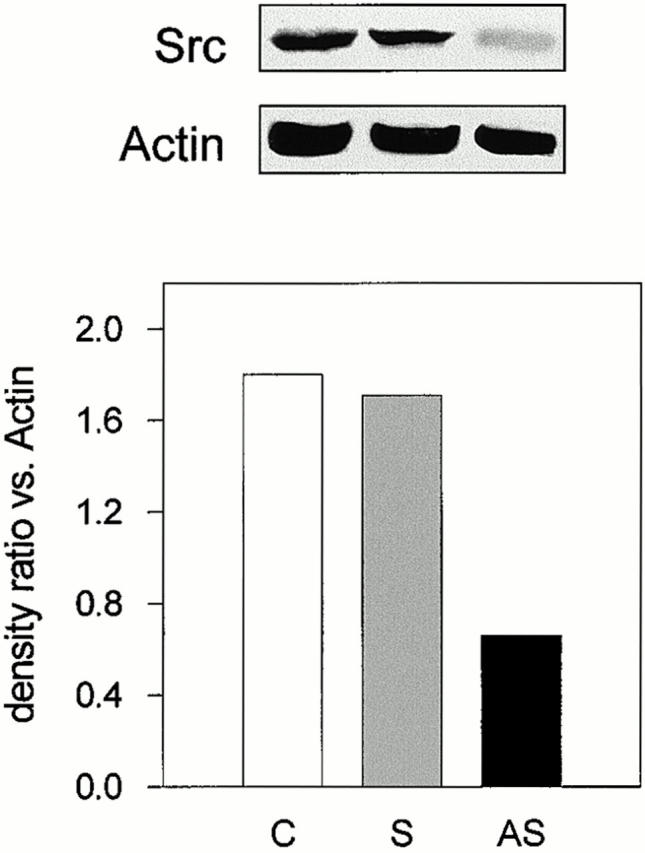
Immunoblotting analysis of Src expression in calvarial osteoblasts. Confluent calvarial osteoblasts from CD1 mice were treated with 1 μM AS-src or S-src for 24 h. Cells were lysed, subjected to SDS-PAGE, and immunoblotted for Src and actin, as described in the legend to Fig. 3. Bands were detected by ECL. Band densitometric analysis was performed as described in the Materials and Methods. The Src/actin ratio for each treatment was computed. Similar results were observed in three independent experiments performed with three different osteoblast preparations. Statistical significance between AS-src and S-src was <0.05. C, control; S, S-src ODN; AS, AS-src ODN.
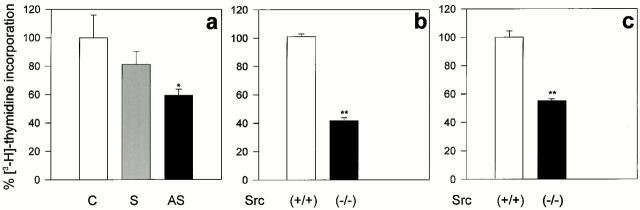
Using this assay, we determined whether treatment with the AS-src would result in alterations of the osteoblast proliferation/differentiation pattern. The incorporation of [3H]thymidine into the TCA-precipitable material derived from osteoblasts exposed to the ODNs for 48 h was significantly decreased by AS-src, but not by S-src, producing a significant 40% inhibition of cell proliferation (Fig. 5 a). A similar reduction of [3H]thymidine incorporation was observed in primary (Fig. 5 b) as well as in SV40-immortalized (Fig. 5 c) Src−/− versus Src+/+ osteoblasts.
Figure 5.
Osteoblast proliferation. (a) To assess osteoblast proliferation, 70% confluent cultures were treated for 48 h with 1 μM AS-src (AS), 1 μM S-src (S), or left untreated (C). 1 μCi/ml of [3H]thymidine was added for the last 4 h, then the cells were lysed, TCA-precipitated, and the radioactivity was measured by a β-spectrophotometer. Counts per minutes were then converted to a percentage of control and expressed as average ± SEM of three independent experiments. Statistical significance was <0.05 (*) versus S-src. (b) Primary osteoblasts and (c) immortalized Src+/+ and Src−/− osteoblasts were cultured to 70% confluence, 1 μCi/ml of [3H]thymidine was added for 4 h, and cells were processed as described in the legend to a. Data are the mean ± SEM of three independent experiment. Statistical significance was <0.01 (**) versus Src+/+.
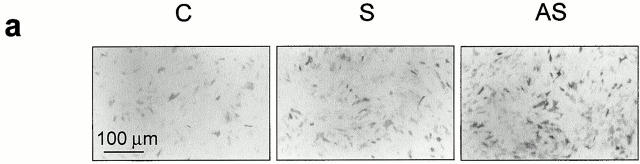
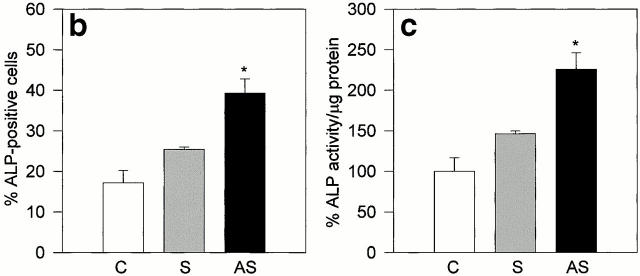
The effect of a reduction in Src expression could be to promote differentiation, which would only secondarily decrease cell proliferation. To determine whether inhibition of Src expression could favor osteoblast differentiation, we analyzed specific markers of the osteoblast phenotype. Treatment of osteoblasts with AS-src increased the number of ALP-positive osteogenic cells, whereas S-src was again inactive relative to control (Fig. 6 a). Further confirmation of enhancement of ALP activity was demonstrated by the quantitative analyses, using histochemical (Fig. 6 b) and biochemical (Fig. 6 c) assays, which showed a greater than twofold increase of the enzyme activity in AS-src- versus S-src–treated and control cultures.
Figure 6.
ALP activity. (a) 90% confluent calvarial osteoblasts from CD1 mice were treated for 3 d with 1 μM AS-src, 1 μM S-src, or left untreated. Cells were fixed, stained histochemically for ALP activity using the Sigma kit n. 85, and observed by conventional light microscopy. (b) The number of ALP-positive cells was counted and expressed as mean percentage ± SEM of control. Data are from three independent experiments. *P < 0.05 versus S-src. (c) Osteoblasts were lysed and ALP activity was evaluated biochemically using the Sigma kit n. 104-LS and p-nitrophenyl phosphate were used as substrate. Activity, normalized versus protein content, was then converted as a percentage of control. Data are mean ± SEM of three independent experiments, *P < 0.05 versus S-src. C, control; S, S-src ODN; AS, AS-src ODN.
We then measured nodule mineralization in the presence of the ODNs, which was added repetitively to the medium during the entire length of the culture period (see Materials and Methods). Fig. 7 shows that AS-src, but not S-src, was able to stimulate nodule mineralization over a period of 3 wk, whether or not cells were cultured in the presence of dexamethasone (Fig. 7).
Figure 7.
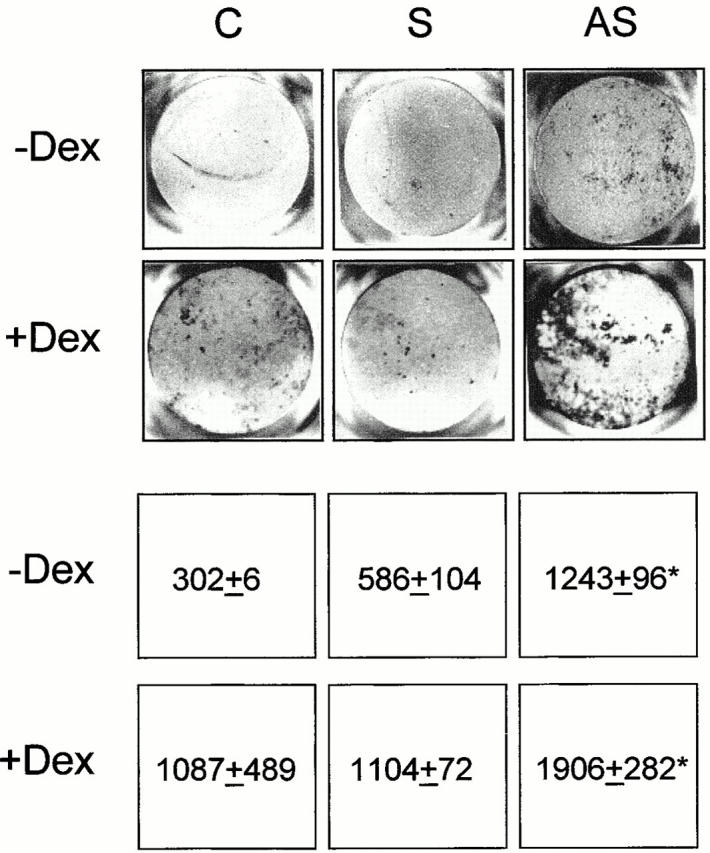
Nodule mineralization. Osteoblasts were plated in 24-well multiplates, grown to 90% confluence, and incubated for 3 wk with ascorbic acid and β-glycerophosphate, as described in the Materials and Methods, with the addition of 1 μM AS-src or S-src, with or without 10−7 M dexamethasone (Dex). Cultures were fixed and mineralization (dark areas) was evidenced by the von Kossa reaction. Quantitative analysis was performed by scanning densitometry, as described in the Materials and Methods, and data from three independent experiments are expressed as arbitrary densitometric units (mean ± SEM). *P < 0.05 versus S-src. C, control; S, S-src ODN; AS, AS-src ODN.
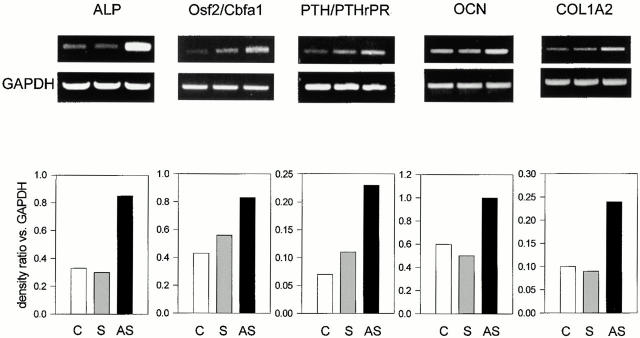
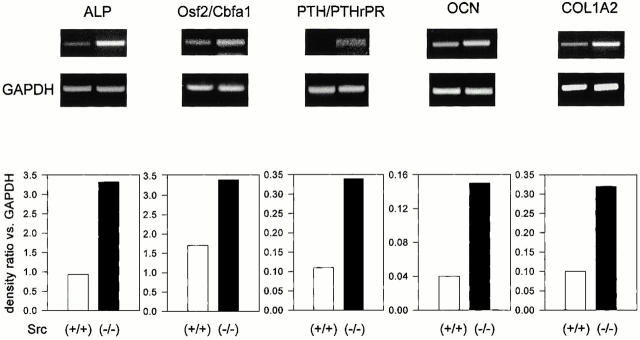
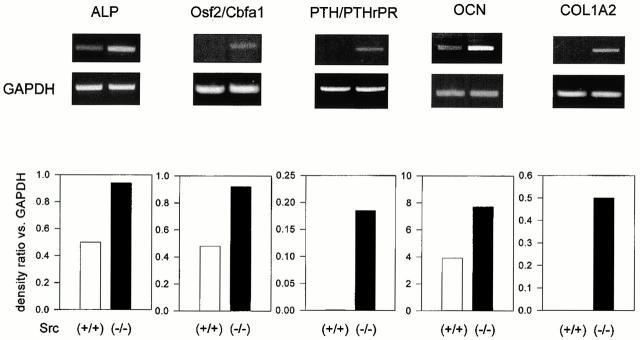
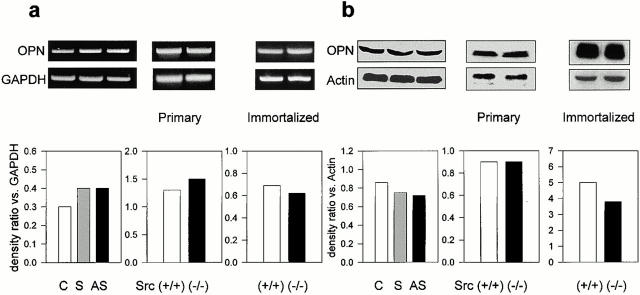
We then examined whether Src deficiency affected the expression of marker genes of the osteoblast phenotype. For this purpose we performed semiquantitative RT-PCR using primers for the housekeeping gene GAPDH along with primers for the gene being analyzed and measured the relative ratios by densitometry. Fig. 8 shows that the transcription of ALP was increased by AS-src, but not by S-src. In addition, two other osteoblast markers, the Osf2/Cbfa1 transcription factor and the PTH/PTHrP receptor, were similarly transcriptionally upregulated by the AS-src versus controls and S-src–treated cultures (Fig. 8). Finally, the matrix proteins osteocalcin (OCN) and pro-alpha 2(I) collagen (COL1A2) were significantly increased by the AS-src. Interestingly, similar increases were observed in primary (Fig. 9) as well as in immortalized (Fig. 10) Src−/− osteoblasts as compared with wild-type cells. In contrast, the pattern of expression of osteopontin, a bone matrix sialoprotein that is not osteoblast specific, was not modified by treatment with AS-src, or in primary, as well as in immortalized Src−/− osteoblasts relative to control cell (Fig. 11).
Figure 8.
Transcriptional regulation of osteoblast genes. 90% confluent osteoblasts were incubated for 3 d with 1 μM AS-src, 1 μM S-src, or left untreated, and RNA was extracted. 1 μg RNA was reverse-transcribed and the equivalent of 0.1 μg was subjected to PCR using primer pairs and conditions as described in the legend to Table . Densitometric analysis was performed as described in Materials and Methods, and then the density ratio between the gene being analyzed and the constitutive gene GAPDH was computed. Similar results were observed in three independent experiments, with P < 0.05 for AS-src treated versus S-src. C, control; S, S-src ODN; AS, AS-src ODN.
Figure 9.
Transcriptional regulation of osteoblast genes in primary Src+/+ and Src−/− calvarial cells. Src+/+ and Src−/− primary osteoblasts were grown to confluence, then RNA was extracted. 1 μg RNA was reverse-transcribed and the equivalent of 0.1 μg was subjected to PCR using primer pairs and conditions as described in the legend to Table . Densitometric analysis was performed as described in the Materials and Methods, then the density ratio between the gene being analyzed and the constitutive gene GAPDH was computed. Similar results were observed in three independent experiments, with P < 0.05 for Src−/− versus Src+/+.
Figure 10.
Transcriptional regulation of osteoblast genes in immortalized cells. Src+/+ and Src−/− immortalized osteoblasts were grown to confluence, then RNA was extracted. 1 μg RNA was reverse-transcribed and the equivalent of 0.1 μg was subjected to PCR using primer pairs and conditions as described in the legend to Table . Densitometric analysis was performed as described in the Materials and Methods, and then the density ratio between the gene being analyzed and the constitutive gene GAPDH was computed. Similar results were observed in three independent experiments, with P < 0.05 for Src−/− versus Src+/+.
Figure 11.
Expression of osteopontin. RNA or proteins were extracted from 90% confluent osteoblasts treated for 3 d with 1 μM AS-src, 1 μM S-src, or left untreated, or from Src+/+ and Src−/− primary and immortalized osteoblasts. (a) RT-PCR was performed using the osteopontin (OPN) primer pairs and conditions as described in the legend to Table . Densitometric analysis was performed as described in the Materials and Methods, then the density ratio between the gene being analyzed and the constitutive gene GAPDH was computed. Similar results were observed in three independent experiments. Differences are not statistically significant. (b) 25 μg protein for each sample was electrophoresed in a 10% SDS-PAGE, blotted to nitrocellulose filter paper, and probed with L-123 anti-OPN pAb (diluted 1:250) at 4°C overnight, followed by the HRP-conjugated secondary antibody (diluted 1:5,000) at 37°C for 1 h. Filters were stripped and reprobed with anti-actin pAb (diluted 1:250). Bands were detected by ECL and densitometric analysis was performed as described in the Materials and Methods, and then the OPN/actin density ratio was computed. Similar results were observed in three independent experiments. Differences are not statistically significant. C, control; S, S-src ODN; AS, AS-src ODN.
Discussion
Analysis of osteoblast characteristics in the absence of Src shows that Src deletion or decreased expression leads to a marked increase in osteoblast differentiation and bone formation, both in vivo and in vitro. Our in vivo observations clearly show that besides a continuous increase in bone mass (Amling et al. 2000), Src−/− mice exhibit a profound increase in the number of osteoblasts and in the rate of bone formation. Primary osteoblasts derived from the calvaria of Src−/− mice show an increased expression of various osteoblast markers, decreased proliferation, and an accelerated rate of mineralized nodule formation. Similar results were obtained with Src−/− immortalized osteoblast cell lines and with primary osteoblasts treated with Src-antisense oligonucleotides (AS-Src). Thus, Src deletion leads to a cell autonomous increase in osteoblast differentiation and bone formation. These results suggest that under wild-type conditions Src plays a negative role in these processes.
Despite its heterogeneity, osteopetrosis is defined as an osteoclast disease. Defects in bone resorption, due to either defective osteoclast differentiation or to defective function of the cell, are the cause of all forms of osteopetrosis (Whyte 1999). This is in contrast with osteosclerosis, another pathological situation in which bone mass is abnormally high, where it is assumed that the defect is increased bone formation rather than decreased bone resorption (Whyte 1999). However, these two abnormalities in bone cell differentiation and/or function are not mutually exclusive. So far, little has been done to determine whether, notwithstanding the osteoclast defects, osteoblasts could also contribute to the phenotype in some forms of osteopetrosis (Marks et al. 1989; Shalhoub et al. 1994).
We have recently performed histomorphometric analyses in aging osteopetrotic Src−/− mice and found that bone mass continues to increase with time in the absence of Src. In adult animals, this continued bone formation in the face of impaired resorption eventually leads to the development of extreme osteopetrosis and extramedullary hematopoiesis (Amling et al. 2000). However, this study did not allow us to determine whether bone formation was at a normal level, albeit higher than the defective bone resorption, or whether it was increased above the normal level of bone formation. Although both situations would lead to a progressive increase in bone mass, the mechanisms involved are quite distinct. Only in the case where bone formation is higher than normal would the osteoblast potentially be defective. Our dynamic histomorphometric data clearly show that this is the case in the Src knockout mice. We observed a marked increase in most parameters of bone formation in these mutant mice; the bone formation rate increased to 150% of control values and the osteoblast number and surface more than doubled. The increased bone formation in vivo appeared to relate mainly to increased osteoblast number rather than increased matrix production, which may indicate increased proliferation, accelerated differentiation, or extended lifespan of the osteoblast. Thus, not only is bone formation maintained, despite the decrease in bone resorption known to be present in these animals (Soriano et al. 1991a; Boyce et al. 1992; Lowe et al. 1993), but it is actually increased above the normal level, explaining the progressive increase in bone mass with time observed in this study and a previous one (Amling et al. 2000). Most interestingly, our in vitro data indicate that these changes are cell autonomous, since Src−/− cultured primary osteoblasts or immortalized cells as well as normal osteoblasts treated with AS-Src show a decrease in proliferation, an increase in the expression of osteoblast markers, and an increase in the ability to form mineralized nodules in culture. Since osteoblast apoptosis is not decreased in Src−/− primary osteoblasts, it is unlikely that the osteoblast lifespan is markedly extended in the absence of Src. Therefore, the increase in the number of osteoblasts in vivo is mostly due to increased differentiation. Whether or not the precursor pool is also induced to proliferate cannot be determined in vivo. However, in vitro Src deletions lead to decreased cell proliferation. One possibility is that the early precursor pool is not present in the cultures such that increased differentiation depletes the pool of preosteoblasts, whereas in vivo the precursor pool could be continuously replenished at the same rate as cells move along their differentiation pathway. Therefore, we propose that the increase in bone mass and bone formation observed in vivo is likely to be the result of a cell-autonomous enhancement of osteoblast differentiation when Src is deleted or when its expression is markedly depressed, as in our antisense experiments. This suggests that under normal circumstances, c-Src is a negative regulator of osteoblast differentiation and bone formation.
On the basis of the in vivo observations, we attempted to investigate whether Src could affect the osteoblasts in vitro. Primary calvarial cells harvested from Src-deficient mice indeed showed a greater differentiated phenotype relative to their wild-type counterpart. Our observation that ALP activity and nodule mineralization in culture was enhanced in Src−/− versus Src+/+ strongly supports our hypothesis. Moreover, we further addressed this issue and analyzed several functional and molecular aspects of osteoblast differentiation in two additional osteoblast models, which were more readily available in culture. These were (a) calvarial cells harvested previously from Src deficient or wild-type mice and subsequently immortalized in culture with the SV40 large T antigen, and (b) normal calvarial osteoblasts where Src deficiency was achieved by introduction into the cell of a Src-antisense oligonucleotide (AS-src). As described below, the fact that the same findings were confirmed in all the osteoblast models used in this study reasonably supports our hypothesis.
Immortalized calvarial cells showed only a weak osteoblast phenotype under basal conditions. However, spontaneous increase in ALP activity could be seen in long-term cultures. This is the time when the cells achieve postconfluency, a condition which in our hands represents a stimulus towards osteoblast differentiation (Migliaccio et al. 1998). In addition, these cells showed normal responsiveness to glucocorticoids, which significantly stimulated ALP activity. A remarkable feature of immortalized Src-deficient cells was a greater ALP activity, at any time of culture and treatment with dexamethasone, relative to normal Src expressing osteoblasts. Higher levels of ALP activity confirmed the presence of a more differentiated phenotype, as indicated by the increased matrix mineralizing ability that could be observed in Src-deficient cells but not in wild-type immortalized osteoblasts.
Experiments performed with calvarial cells harvested from normal mice where Src expression was downregulated by the phosphorothioated Src antisense ODN further confirmed direct involvement of Src in inhibiting osteoblast differentiation. AS-src is a complementary nucleic acid sequence that hybridizes to target the AUG translational start site of the Src mRNA and form an DNA–RNA duplex, resulting in the block of translation of the mRNA into the protein, and activation of RNase H, which destroys the duplex (Alama et al. 1997). This ODN has been successfully used previously to inhibit Src expression in mouse osteoclasts (Tanaka et al. 1996). In the same study, the sense ODN was found to be an appropriate control, which was also confirmed here. The antisense-specific reduction of Src levels demonstrated that the changes observed were indeed due to the antisense sequence-specific properties of AS-src and not to sequence-independent effects (Wagner 1995; Stein 1997; Bennett 1998). In this model, Src expression was not completely inhibited (∼60%). However, enhanced ALP activity and nodule mineralization were observed relative to the control cultures. Therefore, we believe that increased ALP activity and nodule mineralization ability reflect conditions directly related to the deficiency in Src expression in osteoblasts.
Increased ALP activity and mineral deposition were not the only features indicating a negative role of Src in osteoblast differentiation. Osteogenic cells are known to express various specific markers that distinguish them from other matrix-forming cells of mesenchyme origin. Among these, we have selected a few genes that characterize the osteoblast phenotype and have investigated their Src-dependent transcriptional regulation. We observed in Src-deficient osteoblasts, as well as in cells treated with AS-src, a significant upregulation of ALP, Osf2/Cbfa1 transcription factor, PTH/PTHrP receptor, and matrix proteins OCN and COL1A2 gene transcripts relative to normal Src-expressing cells. These markers are considered highly predictive of the differentiated osteoblast phenotype. The transcriptional regulation of these genes is not well characterized. The ALP gene promoter is known to possess a consensus sequence (GGGCGG) for SP1 binding (Weiss et al. 1988), whereas at least three promoters have been described in the PTH/PTHrP receptor gene, one of which, the P2, is also found in mouse (Manen et al. 1998). In contrast, the promoter of the recently identified Osf2/Cbfa1 transcription factor (Komori et al. 1997; Ducy et al. 1997) is still uncharacterized, and only regulation by bone morphogenetic proteins 4 and 7, vitamin D3, and Osf2/Cbfa1 itself has been identified so far (Ducy et al. 1997, Ducy et al. 1999; Tsuji et al. 1998). The COL1A2 promoter is known to possess SP1 and SP3 binding sites (Ihn and Trojanowska 1997), whereas the OCN promoter is regulated by AP-1–related proteins and, interestingly, by the Osf2/Cbfa1 transcription factor (Frendo et al. 1998; Stein 1997). Therefore, it is possible that the concomitant upregulation of expression of these genes in a condition of Src deficiency does not necessarily imply a direct relationship with Src, but rather an indirect regulation by Src-dependent intracellular signaling events.
Our results indicate that the modifications induced by Src deficiency in osteoblasts lead to the deposition of a matrix capable of greater and/or faster mineralization. Bone matrix is a mixture of extracellular proteins arranged to promote mineral deposition. Particular interest has been recently focused on osteopontin, whose expression has been found to be increased in ia (incisor absent), op (osteopetrotic), and tl (toothless) osteopetrotic rats (Shalhoub et al. 1994; Marks et al. 1989), but reduced in mouse Src−/− fibroblasts (Chackalaparampil et al. 1996). Osteopontin is a sialoprotein of bone necessary for osteoclast attachment and bone resorption (Duong and Rodan 1998), which signals the cell through the vitronectin receptors inducing changes in cytosolic free-calcium concentration and phosphorylation patterns (Duong and Rodan 1998). The osteopontin gene promoter has a CCAAT box, located at −53 to −122 from the transcription start site, functioning as a v-Src response element; v-Src stimulates osteopontin expression in NIH 3T3 fibroblasts (Tezuka et al. 1996). However, Chackalaparampil et al. 1996 showed that in in vivo assays normal levels of osteopontin accumulated in the bone matrix of Src−/− mice. Despite the increased deposition and mineralization of matrix in Src−/− cultures, we could not detect changes in osteopontin expression in osteoblasts treated with the AS-src or in primary and immortalized osteoblasts derived from the Src-deficient mice.
In conclusion, our study provides evidence that Src-deficient osteoblasts exhibit a cell autonomous alteration that lead to increased osteoblast numbers and bone formation in vivo, as well as accelerated differentiation, and increased matrix production, and/or mineralization in vitro. These alterations contribute to the development of the osteopetrotic phenotype observed in Src-deleted mice. Thus, Src exerts a negative regulatory influence on osteoblasts and bone formation in normal cells.
Acknowledgments
This work was supported by Telethon grants Nos. E.456 and E.831 to A. Teti. A. Teti was also supported by the Hoechst Marion Roussel grant No. R96006 and by the “Ministero dell'Università e della Ricerca Scientifica e Tecnologica-Università dell'Aquila-Cofinanziamento 1997.” R. Baron was supported by the National Institutes of Health grant AR 42927. Miss Susanne Voit and Dr. Anna Taranta were recipients of Fellowships from Comitato Telethon Fondazione Onlus, grant No. E.456. Silvia Migliaccio was supported by an Eli Lilly grant.
Footnotes
M. Marzia and N.A. Sims share first authorship on this work. R. Baron and A. Teti contributed equally to this work.
B.F. Boyce's present address is Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, NY 14610.
Abbreviations used in this paper: ALP, alkaline phosphatase; AS-src, Src-antisense oligodeoxynucleotide; COL1A2, pro-alpha 2(I) collagen; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OCN, osteocalcin; ODN, oligodeoxynucleotide; PTH/PTHrP, parathyroid hormone/parathyroid hormone related peptide; RT, reverse transcriptase; S-src, Src-sense oligodeoxynucleotide; Src, sarcoma.
References
- Alama A., Barbieri F., Cagnoli M., Schettini G. Antisense oligonucleotides as therapeutic agents. Pharmacol. Res. 1997;36:171–178. doi: 10.1006/phrs.1997.0227. [DOI] [PubMed] [Google Scholar]
- Amling M., Neff L., Priemel M., Priemel A.F., Schilling A.F., Rueger J.M., Baron R. Progressive increase in bone mass and development of odontomas in aging osteopetrotic c-Src–deficient mice. Bone. 2000;In press doi: 10.1016/s8756-3282(00)00373-2. [DOI] [PubMed] [Google Scholar]
- Baron R., Vignery A., Neff L., Silverglate A., Maria A.S. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker R.R., editor. Bone HistomorphometryTechnique and Interpretation. CRC Press; Boca Raton, FL: 1983. pp. 13–35. [Google Scholar]
- Bennett C.F. Antisense oligonucleotidesis the glass half full or half empty? Biochem. Pharmacol. 1998;55:9–19. doi: 10.1016/s0006-2952(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Boyce B.F., Yoneda T., Lowe C., Soriano P., Mundy G.R. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackalaparampil I., Peri A., Nemir M., Mckee M.D., Lin P.H., Mukherjee B.B., Mukherjee A.B. Cells in vivo and in vitro from osteopetrotic mice homozygous for c-src disruption show suppression of synthesis of osteopontin, a multifunctional extracellular matrix protein. Oncogene. 1996;12:1457–1467. [PubMed] [Google Scholar]
- Ducy P., Starbuck M., Priemel M., Shen J., Pinero G., Geoffroy V., Amling M., Karsenty G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P., Zhang R., Geoffrey V., Ridall A.L., Karsenty G. Osf2/Cbfa1a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Duong L.T., Rodan G.A. Integrin-mediated signaling in the regulation of osteoclast adhesion and activation. Front. Biosci. 1998;3:D757–D768. doi: 10.2741/A319. [DOI] [PubMed] [Google Scholar]
- Fisher L.W., Stubbs J.T., III, Young M.F. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop. Scand. 1995;66:61–65. [PubMed] [Google Scholar]
- Frendo J.L., Xiao G., Fuchs S., Franceschi R.T., Karsenty G., Ducy P. Functional hierarchy between two OSE2 elements in the control of osteocalcin gene expression in vivo. J. Biol. Chem. 1998;273:30509–30516. doi: 10.1074/jbc.273.46.30509. [DOI] [PubMed] [Google Scholar]
- Golden A., Nemeth S.P., Brugge J. Blood platelets express high levels of pp60c-src-specific tyrosine kinase activity. Proc. Natl. Acad. Sci. USA. 1986;83:852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne W.C., Neff L., Chatterjee D., Lomri A., Levy J.B., Baron R. Osteoclasts express high levels of pp60c-src in association with intracellular membranes. J. Cell Biol. 1992;119:1003–1013. doi: 10.1083/jcb.119.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J.A. Protein-tyrosine kinases. Ann. Rev. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Ihn H., Trojanowska M. Sp3 is a transcriptional activator of the human alpha2(I) collagen gene. Nucleic Acid Res. 1997;25:3712–3717. doi: 10.1093/nar/25.18.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowsey J, Kelly P.J., Riggs B.L., Bianco A.J., Scholz D.A., Gershon-Cohen J. Quantitative microradiographic studies of normal and osteoporotic bone. J. Bone Joint Surg. 1965;47A:785–806. [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R.T., Gao Y.-H., Inada M., Sato M., Okamoto R., Kitamura Y., Yoshiki S., Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Lowe C., Yoneda T., Boyce B.F., Chen H., Mundy G.R., Soriano P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc. Natl. Acad. Sci. USA. 1993;90:4485–4489. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manen P.D., Palmer G., Bonjour J.P., Rizzoli R. Sequence and activity of parathyroid hormone/parathyroid hormone-related receptor promoter region in human osteoblast-like cells. Gene. 1998;18:49–56. doi: 10.1016/s0378-1119(98)00386-2. [DOI] [PubMed] [Google Scholar]
- Marks S.C., Jr., Mackowiak S., Shaloub V., Lian J.B., Stein G.S. Proliferation and differentiation of osteoblasts in osteopetrotic ratsmodification in expression of genes encoding cell growth and extracellular matrix proteins. Connect. Tissue Res. 1989;21:107–113. doi: 10.3109/03008208909050001. [DOI] [PubMed] [Google Scholar]
- Migliaccio S., Bernardini S., Wetsel W.C., Korach K.S., Faraggiana T., Teti A. Protein kinase C modulates estrogen receptors in differentiated osteoblastic cells in vitro. Steroids. 1998;63:352–354. doi: 10.1016/s0039-128x(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R. Bone histomorphometrystandardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J. Bone Miner. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Parson T.J., Parson S.J. Src family tyrosine kinasescooperating with growth factor and adhesion signaling pathways. Curr. Opin. Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- Pyper J.M., Bolen J. Identification of a novel neuronal c-src exon expressed in human brain. Mol. Cell Biol. 1990;10:2035–2040. doi: 10.1128/mcb.10.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q., Perälä-Heape M., Kapanen A., Dahllund J., Salo J., Väänänen H.K., Härkönen P. Estrogen enhances differentiation of osteoblasts in mouse bone marrow culture. Bone. 1998;22:201–209. doi: 10.1016/s8756-3282(97)00276-7. [DOI] [PubMed] [Google Scholar]
- Resh M.D. Myristylation and palmitylation of Src family membersthe facts of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Robey P.G., Termine J.D. Human bone cells in vitro. Calcif. Tissue Int. 1985;37:453–460. [PubMed] [Google Scholar]
- Rodan G.A., Martin T.J. Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif. Tissue Int. 1981;33:349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Shalhoub V., Bettencourt B., Jackson M.E., MacKay C.A., Glimcher M.J., Marks S.C., Jr., Stein G.S., Lian J.B. Abnormalities of phosphoprotein gene expression in three osteopetrotic rat mutantselevated mRNA transcripts, protein synthesis, and accumulation in bone of mutant animals. J. Cell Physiol. 1994;158:110–120. doi: 10.1002/jcp.1041580114. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice Cell. 64 1991. 693 702a [DOI] [PubMed] [Google Scholar]
- Soriano P., Friedrick G., Lawinger P. Promoter interaction in retrovirus introduced into fibroblasts and embryonic stem cells J. Virol 65 1991. 2314 2319b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C.A. Controversies in the cellular pharmacology of oligodeoxynucleotides. Ciba Found. Symp. 1997;209:79–89. [PubMed] [Google Scholar]
- Suda T., Nakamura I., Jimi E., Takahashi N. Regulation of osteoclast function. J. Bone Min. Res. 1997;12:869–879. doi: 10.1359/jbmr.1997.12.6.869. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Amling M., Neff L., Peyman A., Uhlmann E., Levy J.B., Baron R. c-cbl is downstream of c-src in a signaling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- Tezuka K., Denhart D.T., Rodan G.A., Harada S. Stimulation of mouse osteopontin promoter by v-Src is mediated by a CCAAT box-binding factor. J. Biol. Chem. 1996;271:22713–22717. doi: 10.1074/jbc.271.37.22713. [DOI] [PubMed] [Google Scholar]
- Thomas S.M., Brugge J.S. Cellular functions regulated by Src family kinases. Ann. Rev. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Ito Y., Noda M. Expression of the PEB2aA/AML3/CBFA1 gene is regulated by BMP4/7 heterodimer and its overexpression suppresses type I collagen and OCN gene expression in osteoblastic mesenchymal cells. Bone. 1998;22:87–92. doi: 10.1016/s8756-3282(97)00267-6. [DOI] [PubMed] [Google Scholar]
- Wagner R.W. The state of the art in antisense research. Nat. Med. 1995;1:1116–1118. doi: 10.1038/nm1195-1116. [DOI] [PubMed] [Google Scholar]
- Weiss M.J., Ray K., Henthorn P.S., Lamb B., Kadesch T., Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J. Biol. Chem. 1988;263:12002–12010. [PubMed] [Google Scholar]
- Wenqing X., Harrison S.C., Eck M.J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- Whyte M.P. Sclerosis bone disorders. In: Favus M.J., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Fourth edition. Lippincott-Raven Publishers; Philadelphia: 1999. pp. 367–383. [Google Scholar]