Abstract
Keratinocyte proliferation and migration are essential to cutaneous wound healing and are, in part, mediated in an autocrine fashion by epidermal growth factor receptor (EGFR)–ligand interactions. EGFR ligands are initially synthesized as membrane-anchored forms, but can be processed and shed as soluble forms. We provide evidence here that wound stimuli induce keratinocyte shedding of EGFR ligands in vitro, particularly the ligand heparin-binding EGF-like growth factor (HB-EGF). The resulting soluble ligands stimulated transient activation of EGFR. OSU8-1, an inhibitor of EGFR ligand shedding, abrogated the wound-induced activation of EGFR and caused suppression of keratinocyte migration in vitro. Soluble EGFR–immunoglobulin G-Fcγ fusion protein, which is able to neutralize all EGFR ligands, also suppressed keratinocyte migration in vitro. The application of OSU8-1 to wound sites in mice greatly retarded reepithelialization as the result of a failure in keratinocyte migration, but this effect could be overcome if recombinant soluble HB-EGF was added along with OSU8-1. These findings indicate that the shedding of EGFR ligands represents a critical event in keratinocyte migration, and suggest their possible use as an effective clinical treatment in the early phases of wound healing.
Keywords: TGF-α, HB-EGF, amphiregulin, tissue repair, matrix metalloprotease
Introduction
Successful wound healing involves a number of processes including inflammation, cell proliferation, cell migration, vascular permeability and angiogenesis, as well as matrix deposition and tissue remodeling (for review see Martin 1997). Particularly critical are cell proliferation and migration, which are driven by growth factors and cytokines released coordinately into the injury sites. In cutaneous wound healing, keratinocytes play a central role, not only as a key structural cell type in the repairing skin, but also as the source of a number of growth factors. Prominent among these keratinocyte-derived factors are ligands for the epidermal growth factor receptor (EGFR).
The EGFR ligand family is composed of six members including the epidermal growth factor (Cohen 1964), transforming growth factor-α (TGF-α; Derynck et al. 1984), amphiregulin (AR; Shoyab et al. 1989), heparin-binding EGF-like growth factor (HB-EGF; Higashiyama et al. 1991), betacellulin (Shing et al. 1993), and epiregulin (Toyoda et al. 1995). All members of this family are synthesized as membrane-anchored forms, which are then processed to give bioactive soluble factors. This processing can be stimulated by treating cells with various agents, including the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA; for review see Massague and Pandiella 1993).
Within the EGFR ligand family, TGF-α, AR, and HB-EGF have been extensively characterized and clearly shown to be autocrine growth factors for keratinocytes (Coffey et al. 1987; Cook et al. 1991; Hashimoto et al. 1994). The transmembrane forms of these factors are also capable of stimulating the growth of adjacent cells including keratinocytes via cell–cell contact, a process termed juxtacrine stimulation (Brachmann et al. 1989; Wong et al. 1989; Higashiyama et al. 1995; Goishi et al. 1995; Inui et al. 1997).
Marikovsky et al. 1993 reported that wound fluid in skin contains processed bioactive EGFR ligands, suggesting that regulation of EGFR ligand shedding might be a physiologically important event in wound healing. Therefore, we investigated the possible role of EGFR ligand shedding in response to wounding, focusing in particular on the early EGFR-related molecular events which are evoked by wound stimuli. We also applied a newly established inhibitor of EGFR ligand shedding to wound healing models, in both in vitro and in vivo experiments.
Materials and Methods
Materials
Human EGFR antibodies were purchased from Upstate Biotechnology. Phosphotyrosine antibody PY-20 was obtained from Transduction Laboratories. Neutralizing polyclonal antibody No. 197 was raised in a goat against a 75–amino acid form of recombinant human HB-EGF and was obtained from D. Damm (Scios Inc., Mountain View, CA; Hashimoto et al. 1994). Anti-EGFR neutralizing antibody was a generous gift from Dr. U. Rodeck (Wister Institute, Philadelphia, PA). AG1478 was obtained from Calbiochem. TPA was obtained from Wako Pure Chemical Industry. Sulfo-NHS-biotin was purchased from Pierce Chemical Co. An enhanced chemiluminescence (ECL) kit was obtained from Amersham Pharmacia Biotech.
Metalloprotease Inhibitors
More than five hundred hydroxamic acid–based inhibitors of metalloproteases, along with a series of nonhydroxamic derivatives, were synthesized by Kanebo Ltd. The hydroxamic acid–based inhibitors included the following: OSU8-1, [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsuccinyl]-l-3-(5,6,7,8-tetra-hydro-1-naphthyl)alanine-N-methylamide; OSU7-6, [4-(N-hydroxyamino)-2R-isobutylsuccinyl]-l-3-(1-naphthyl)-alanine-N-methylamide; and OSU9-6, [6,7-dihydroxy-3-(N-hydroxycarbamoyl)-2-(4-methoxybenzenesulfonyl)-1,2,3,4-tetrahydroisoquinoline]. OSU8-1, OSU7-6, and OSU9-6 work as competitive inhibitors.
Construction of Alkaline Phosphatase–EGF-Related Ligand Fusion Vectors
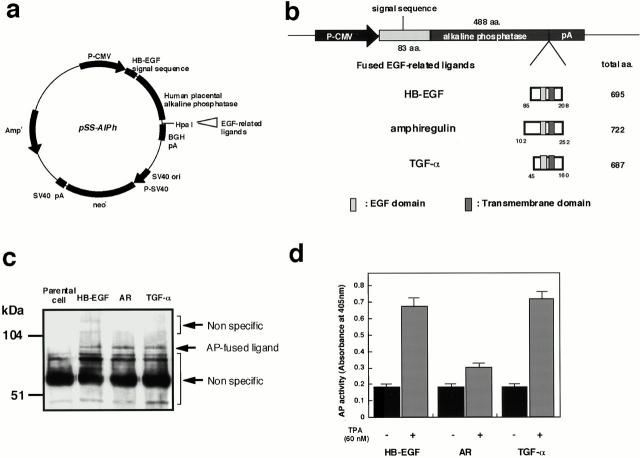
The coding region for human placental alkaline phosphatase (AP) was obtained from the APtag-1 expression vector (a gift from Dr. J. Flanagan, Harvard Medical School, Boston, MA) and inserted into the multiple cloning site of pRc/CMV (Invitrogen). A BstBI-MscI fragment of the human HB-EGF cDNA, which encodes the signal sequence and prosequence, was fused to the 5′ end of the AP sequence to create the plasmid pSS-AlPh (see Fig. 1 a). As pSS-AlPh has a unique HpaI site overlapping the termination codon of the AP coding region, one of the following DNA fragments encoding human EGF–related ligands were fused in-frame at this site: MscI-PstI fragment of the HB-EGF cDNA (0.45 kb); HinfI fragment of the AR cDNA (0.5 kb); or MseI-SauIII fragment of the TGF-α cDNA (0.38 kb). The plasmids resulting from these ligations were introduced into CHO cells using the calcium phosphate method (Chen and Okayama 1988), and stably transfected clones were isolated.
Figure 1.
Expression of AP-tagged EGFR ligands. (a) Structure of the AP-tag parental expression plasmid, pSS-AlPh. (b) A schematic diagram of the region within the expression plasmids derived from pss-AlPh that encode the fusions of AP-tag and EGFR ligands. The coding region for the AP-tag HB-EGF fusion was constructed such that, in the encoded fusion, the AP sequence (488 amino acids) was inserted at Ala84 of the HB-EGF precursor. In the AP-tag AR and AP-tag TGF-α fusion proteins, AR (residues 102–252) and TGF-α (residues 45–160), respectively, were substituted for HB-EGF (residues 85–208). (c) Expression of AP-tag HB-EGF, AP-tag AR, and AP-tag TGF-α on the surface of transfected CHO cells. Biotinylated AP-tag HB-EGF, AP-tag AR, and AP-tag TGF-α were immunoprecipitated with human placental AP antibody and analyzed by SDS-PAGE and Western blotting. Biotinylated proteins on the Western blot membranes were detected by HRP-conjugated avidin and ECL. (d) AP activity in the conditioned media of transfectants expressing AP-tag HB-EGF, AP-tag AR, and AP-tag TGF-α. Cells were treated with or without 60 nM TPA for 30 min at 37°C. AP activity was measured as described in Materials and Methods. Each bar is the average of triplicate values.
Cell-surface Biotinylation, Immunoprecipitation, and Western Blotting
CHO cell transfectants stably expressing either wild-type HB-EGF, AR, or TGF-α, or AP-tagged fusions of these ligands, were seeded in 6-cm dishes at a density of 2 × 105 cells/dish and cultured for 24 h with Ham's F12/10% FCS. Cells were washed three times with ice-cold Hanks buffer and biotinylated with 0.1 mg/ml of sulfo-NHS-biotin in 50 mM Hepes, pH 7.5, and 0.15 M NaCl for 15 min on ice. Excess reagent was quenched and removed by washing with ice-cold Ham's F12/10% FCS. Cells were lysed with a buffer containing 1% Triton X-100, 3 mM EDTA, 1 mM (p-amidinophenyl) methanesulfonyl fluoride HCl, 1 μg/ml aprotinin, 5 μM 3,4-dichloroisocoumarin, and 0.4 M NaCl in 20 mM Hepes, pH 7.2. After centrifugation of the lysates at 15,000 rpm for 15 min, supernatants were collected and incubated with 2 μg of human placental alkaline phosphatase antibody (Dako), HB-EGF antibody No. H6 (Higashiyama et al. 1995), AR antibody No. AAR1 (Hashimoto et al. 1994), or TGF-α antibody (a gift from Dr. R. Derynck, University of California, San Francisco, San Francisco, CA) for 2 h at 4°C, followed by incubation with 10 μl of protein A trisacryl (50% suspension; Pierce Chemical Co.) for 2 h at 4°C. After centrifugation of the mixes, the pellets were analyzed by SDS-PAGE and Western blotting as described previously (Goishi et al. 1995).
Preparation of EGFR Extracellular Domain–Immunoglobulin G-Fcγ Fusion Protein
A fusion protein consisting of the extracellular domain of EGFR and human immunoglobulin G-Fcγ (EGFR-Fc) was produced as follows. A BglII site was engineered into an EGFR expression plasmid (Gotoh et al. 1994) at the point encoding the extracellular domain/transmembrane domain junction of this receptor. A SacI-BglII fragment encoding the extracellular domain of EGFR was isolated from the resulting plasmid and fused to the 5′ end of the coding region for IgFc in pcDNA-Fcγ (a gift from Dr. H. Ishiguro, Fujita Health University, Aichi, Japan). The plasmid obtained as a result of this ligation was introduced into CHO cells using the calcium phosphate method (Chen and Okayama 1988). A stable transfectant was cloned, amplified, and incubated in the presence of serum-free medium. EGFR-Fc fusion protein was purified from the resulting conditioned medium using a protein A column (5 ml Pharmacia HiTrap) on an FPLC system.
Screening of EGFR Ligand Shedding Inhibitors
Stable transfectants expressing AP-tagged EGFR ligands were seeded in 96-well plates at a density of 2 × 104 cells/well and incubated for 24 h. The cells were washed with Mg+ and Ca2+-free PBS [PBS(−)] and incubated for 10 min with 1 μM of an inhibitor. TPA (60 nM) was added to stimulate inducible processing, and the plates were incubated for a further 30 min. 100-μl aliquots of the conditioned media were transferred to 96-well plates and heated for 10 min at 65°C to inactivate endogenous alkaline phosphatases. An equal volume of 2× AP buffer (2 M diethanolamine, pH 9.8, 1 mM MgCl2, 20 mM l-homoarginine, and 24 mM p-nitrophenylphosphate) was added to each well and gently mixed at room temperature until color development was apparent in positive control wells. AP activity was determined by the measurement of absorbance at 405 nm with a microplate reader.
Keratinocyte Culture and Wound Assay
Human keratinocytes were cultured in optimized nutrient medium MCDB153 (Nissui Co.) supplemented with 5 μg/ml insulin, 0.5 μM hydrocortisone, 0.1 mM ethanolamine, 0.1 mM phosphoethanolamine, and 150 μg/ml bovine hypothalmic extract (BHE) as previously described (Hashimoto et al. 1994). The stock solution of MCDB153 used in this case was supplemented with 0.1 mM CaCl2, and contained elevated concentrations of amino acids (0.75 mM isoleucine, 0.24 mM histidine, 0.09 mM phenylalanine, 0.045 mM tryptophan, and 0.075 mM tyrosine) and kanamycin (0.1 mg/ml).
For wounding experiments, cells were seeded on type I collagen–coated 10-cm dishes or 6-well plates. After reaching subconfluency, the cells were re-fed with a BHE-free medium and wounded in either of two ways. To obtain conditioned media from wounded keratinocytes, cell layers were wounded by linear, cross-stripe scrapes 5 mm apart using the tip of a micropipette (tip-scraping). For the growth and migration assay, cell layers were scraped with a wide silicone rubber cell scraper (rubber scraping). In either case, the cells were washed with fresh medium once to remove floating cells and re-fed with fresh medium without BHE in the absence or presence of EGFR neutralizing antibody No. 425 (10 μg/ml), AG1478 (30 nM), OSU8-1 (1 μM), or HB-EGF (20 ng/ml). The conditioned media from these cultures were collected for analysis after various periods of incubation at 37°C.
Growth Factor Activity Assays
To measure the mitogenic activities of EGFR ligands, an EP170.7 cell growth factor assay was carried out as described previously (Blotnick et al. 1994). Cells were plated in 96-well plates (2 × 104 cells/100 μl/well). 100-μl aliquots of samples were added to each well, and the plates were incubated for 36 h. [3H]Thymidine (1 μCi/well; 1 mCi = 37 MBq) was added to the wells, and the plates were incubated for an additional 6 h. The EP170.7 cells were harvested and analyzed for the incorporation of [3H]thymidine into DNA using a 1205 Betaplate system (Amersham Pharmacia Biotech). To neutralize HB-EGF activity, samples were preincubated with human HB-EGF neutralizing antibody No. 197 at a final concentration of 10 μg/ml before addition to the assay wells.
A juxtacrine growth factor assay was carried out as described previously (Higashiyama et al. 1995). Keratinocytes (105 cells/well) were plated in 24-well plates and incubated for 12 h. The cell layers were washed with fresh medium containing 2 M NaCl to remove soluble HB-EGF and AR trapped by cell-surface heparan sulfate proteoglycans, and were then fixed with 5% buffered formalin for 5 min. The fixed cells were washed twice with RPMI 1640/10% FCS, and EP170.7 cells (105 cells/500 μl/well) were added to the wells along with [3H]thymidine. After a 6-h incubation, the EP170.7 cells were harvested and analyzed for incorporation of [3H]thymidine into DNA.
Northern Blot Hybridization
Total RNA was isolated from primary cultured human keratinocytes as described previously (Chomczynski and Sacchi 1987). Total RNA was electrophoresed on a 1% agarose gel and transferred onto a Zeta-probe membrane (Bio-Rad Laboratories) by capillary action. The membrane filter was hybridized with 32P-labeled human HB-EGF, AR, or TGF-α cDNA probes that corresponded to the HindIII-XbaI, ApaI-XbaI, and HindIII-XbaI fragments, respectively. The filter was washed once in 2× SSC, 0.1% SDS, and then twice in 0.2× SSC, 0.1% SDS at 55°C, before exposure to a scientific imaging film (Eastman Kodak Co.).
Migration Assay
The migration of keratinocytes was assayed in a Boyden chamber as previously described (Boyden 1962; Higashiyama et al. 1993). Keratinocytes (5 × 105 cells/dish) were plated on type I collagen–coated 10-cm dishes and incubated for 72 h. The subconfluent keratinocytes were detached from the dishes by incubation with trypsin-EDTA (0.05% trypsin and 0.5 mM EDTA) at 37°C. To avoid excess exposure to trypsin, the incubation was carried out for 1 min or less. The cells were washed once with the supplemented MCDB153 medium described above, resuspended in the same medium, and diluted to a final cell concentration of 105 cells/ml. To set up the migration assay, samples were added to the bottom wells of a 48-well Boyden chamber (Neuro Probe Inc.) and an 8-μm pore size polyvinylpyrrolidone-free polycarbonate membrane (Neuro Probe Inc.) precoated with type I collagen (10 μg/ml in PBS; Nitta Gelatin Co.) was placed above these wells. 50 μl of the keratinocyte suspension were added to each upper well of the 48-well chamber, and the chamber was incubated for 4 h at 37°C in a humidified atmosphere of air/5% CO2. Cells adhering to the upper surface of the filter membrane were removed by scraping with a rubber blade. Cells that had migrated through the filter were fixed with buffered formalin overnight, and then stained with Gill's hematoxylin overnight. The filter was mounted between two glass slides with 90% glycerol, and the number of keratinocytes that had crossed the filter in each well was determined by counting the stained keratinocytes under a microscope.
Immunoprecipitations and Phosphotyrosine Western Blotting
Keratinocytes (5 × 105 cells/dish) were plated on type I collagen–coated 10-cm dishes and incubated for 72 h. The subconfluent keratinocytes were starved for 2 h in BHE-free medium with or without 1 μM of OSU8-1, 50 μM of cycloheximide, or 10 μg/ml HB-EGF neutralizing antibody No. 197, and then wounded by tip-scraping followed by incubation for the indicated times at 37°C. Lysates were prepared in 1 ml of PBS(−) containing 5 mM EDTA, 100 μM sodium orthovanadate, 100 μM sodium pyrophosphate, 1 mM sodium fluoride, 5 μM 3,4-dichloroisocumarin, 1 mg/ml aprotinin, and 1% Triton X-100. Cell lysates were centrifuged at 10,000 rpm for 10 min at 4°C and the supernatants were incubated with 1 μg of anti-EGFR antibodies for 12 h at 4°C with end-over-end rotation. 15 μl of protein A–Sepharose (50% suspension; Amersham Pharmacia Biotech) were added to each lysate/antibody mixture and incubated for 12 h at 4°C with end-over-end rotation. The mixtures were centrifuged and the protein A–Sepharose beads were washed three times with lysis buffer, resuspended in 20 μl of 2× SDS-PAGE sample buffer (0.5 M Tris-HCl, pH 7.2, 1% SDS, 100 mM β-mercaptoethanol, and 0.01% bromophenol blue), and boiled for 5 min. Aliquots of the boiled samples were fractionated on 6% SDS-PAGE gels and were transferred to PVDF membranes (Millipore Corporation). The PVDF membranes were blocked with 5% skimmed milk in PBS(−) overnight at 4°C. The membranes were incubated for 6 h with a second antibody as indicated in the text and figures. After three washes with 0.05% Tween 20 in PBS(−) at 10-min intervals, the membranes were treated with ABC reagents (Vector Laboratories) for 20 min at room temperature. The membranes were again washed three times with 0.05% Tween 20 in PBS(−), and were treated with ECL detection reagents for 1 min at room temperature before exposure to scientific imaging films (Eastman Kodak Co.).
In Vivo Wound Assay
An in vivo wound assay was performed as previously described (Tsuboi et al. 1992). Female BALB/c mice (n = 15) were housed individually and used at 8 wk of age. A vehicle solution, consisting of 1.5% carboxymethylcellulose in sterilized PBS solution, was used for the control. OSU8-1 alone (10 mM) and a cocktail of 10 mM OSU8-1 plus 5 μg/ml HB-EGF were prepared in the same solution. Mice were anesthetized with diethylether, and two full-thickness-round wounds were prepared on the back of each mouse with a punch biopsy instrument (6-mm diam). After the operation, 500 μl of the test solution was applied to each wound. One wounded side of each mouse was used for vehicle treatment, whereas the other side received either OSU8-1 treatment (n = 10) or a cocktail treatment of OSU8-1 and HB-EGF (n = 5). The wounds were left open, and the mice were treated daily with these reagents for 5 d. The mice were killed at day 6, and the wounds were excised and fixed in 10% buffered formalin. After fixation, the samples were sectioned parallel to the anterior-posterior axis and stained with antikeratin/cytokeratin antibody (Nichirei Ltd.).
Results
Screening and Characterization of Inhibitors for EGFR Ligand Shedding
Expression plasmids encoding fusions between AP and three different EGFR ligands (AP-tag HB-EGF, AP-tag AR, and AP-tag TGF-α) were constructed as shown in Fig. 1 (a and b), and were stably transfected into CHO cells. Fusion protein expression on the surface of the transfected cells was analyzed by cell-surface biotinylation followed by immunoprecipitation with an anti-AP antibody. Proteins of the expected size (∼88 kD) were expressed almost equally in the respective transfected cell lines (Fig. 1 c). To determine whether the fusions could be processed to release soluble versions of the AP-tagged ligands, TPA-inducible shedding of these ligands was tested. AP activity in the conditioned media of the three transfectants was measured after a 30-min incubation with or without 60 nM TPA. TPA induced the shedding of AP-tag HB-EGF and AP-tag TGF-α (Fig. 1 d), which is consistent with previous observations on TPA-stimulated shedding (Pandiella and Massague 1991; Goishi et al. 1995). However, TPA was less effective, at inducing the shedding of AP-tag AR (Fig. 1 d). The shed ligands were also able to activate the EGF receptor (data not shown).
Using the AP-tag EGFR ligand transfectants, >500 synthetic compounds were tested for the ability to inhibit shedding of the ligands. Since it has been reported that a matrix metalloprotease (MMP) is involved in the shedding of HB-EGF and TGF-α (Suzuki et al. 1997; Izumi et al. 1998; Peschon et al. 1998), these compounds were designed as potential MMP inhibitors. The most effective compound was OSU8-1. 1 μM OSU8-1 markedly blocked the shedding of AP-tag HB-EGF and AP-tag AR, and partially blocked the shedding of AP-tag TGF-α (Fig. 2 a). A 10-fold higher concentration of OSU8-1 (10 μM) significantly blocked the TPA-inducible shedding of all three AP-tagged EGFR ligands (Fig. 2 a). Cell-surface biotinylation and immunoprecipitation of wild-type HB-EGF, TGF-α, and AR also revealed the abrogation of their TPA-inducible shedding by 10 μM of OSU8-1 (Fig. 2 b). We examined the inhibitory spectrum of OSU8-1 with regard to the following three representative MMPs: MMP-1, MMP-3, and MMP-9. As shown in Table , OSU8-1 effectively inhibited the activities of all three MMPs with IC50s of 0.3–2.9 nM. We also selected two more compounds, OSU9-6 and OSU7-6 that showed similar inhibitory activities for the shedding of EGFR ligands, but which displayed distinguishable inhibitory activities for the three tested MMPs (Table ). These three inhibitors were used for characterization of EGFR ligand shedding in the wound experiments described below.
Figure 2.
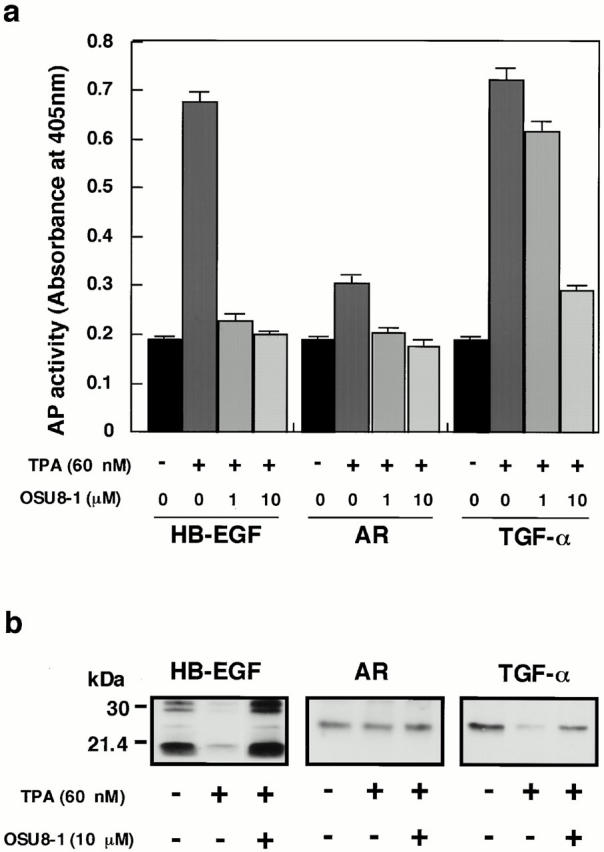
Effect of OSU8-1 on TPA-inducible shedding of EGFR ligands. (a) AP activities in the conditioned media of CHO transfectants expressing AP-tag HB-EGF, AP-tag AR, and AP-tag TGF-α treated with or without 60 nM of TPA in the presence or absence of OSU8-1. Cells were preincubated with the indicated concentration of OSU8-1 for 10 min at 37°C. The cells were incubated with fresh media containing the indicated concentrations of TPA and OSU8-1 for 30 min at 37°C. AP activity was measured as described in Materials and Methods. Each bar is the average of triplicate values. (b) CHO transfectants expressing wild-type (non–AP-tag fused) HB-EGF, AR, and TGF-α were biotinylated and preincubated with or without 10 μM OSU8-1 for 10 min at 37°C. Cells were incubated with fresh media containing the indicated concentrations of TPA and OSU8-1 for 30 min at 37°C, followed by cell lysis, immunoprecipitation with antibodies against the shed ligands, SDS-PAGE, Western blotting, and avidin-HRP/ECL detection. The wild-type HB-EGF shows different processing forms (Goishi et al. 1995).
Table 1.
Inhibition Spectra of EGFR-ligand Shedding Inhibitors
| Percent inhibition at 1 μM | IC50 | |||||
|---|---|---|---|---|---|---|
| Compounds | HB-EGF | AR | TGF-α | MMP-1 | MMP-3 | MMP-9 |
| nM | nM | nM | ||||
| OSUB8-1 | 95 | 70 | 51 | 0.30 | 2.9 | 0.30 |
| OSUB9-6 | 47 | 35 | 5 | 10,000 | 10,000 | >1,000 |
| OSUB7-6 | 44 | 30 | 5 | 0.35 | 2.0 | 0.40 |
Wounding of Cultured Human Keratinocytes Induces the Production of Active and Soluble EGFR Ligands
To investigate EGFR ligand shedding in response to wounding, subconfluent cultures of keratinocytes were scraped with the tip of a micropipette. The production of bioactive soluble EGFR ligands in conditioned medium collected at different times after the wounding was determined using a DNA synthesis assay. Fig. 3 a shows a typical time course for the release of EGFR ligands in the early phase after wounding (0–210 min). The production of soluble EGFR ligands showed two peaks during this time period, at 60 and 210 min after wounding. EGFR activation in the wounded keratinocyte cultures themselves was observed starting at 90 min after wounding. EGFR activation continued for at least 60 min (90–150 min after wounding) and was reactivated at 210 min (Fig. 3 b). Four independent experiments showed almost identical patterns of ligand production and EGFR activation. The addition of 1 μM OSU8-1 abrogated completely the release of EGFR ligands and EGFR activation (Fig. 3, a and b).
Figure 3.
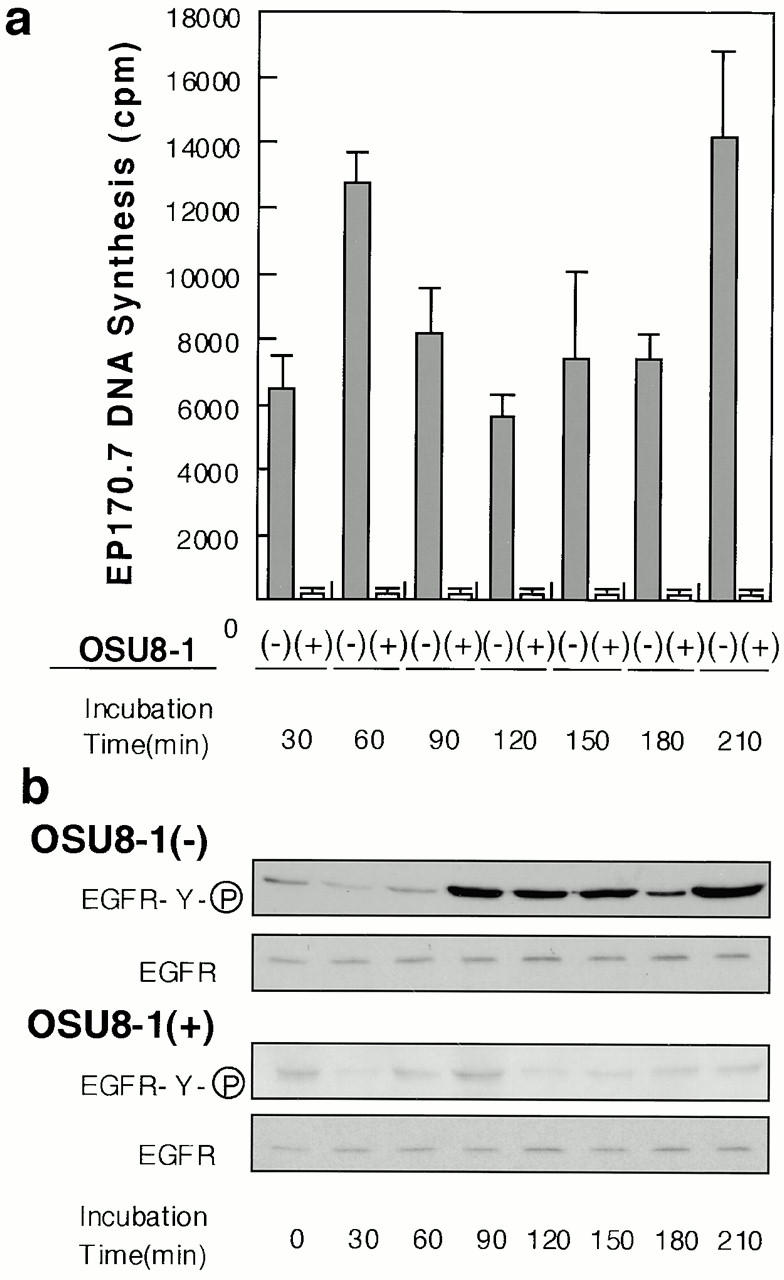
Production of soluble EGFR ligands and activation of EGFR in cultured keratinocytes after wounding. (a) Detection of shed EGFR ligands produced by wounded keratinocytes. The levels of released EGFR ligands in conditioned media collected at the indicated times in the presence or absence of 1 μM OSU8-1 were measured by an EP170.7 cell assay. Four independent experiments were carried out and a typical time course of the level of EGFR ligands is shown. Each bar is the average of quadruplicate values. (b) Tyrosine phosphorylation of EGFR in wounded keratinocytes. Keratinocytes, cultured in 10-cm dishes, were stimulated by tip-scraping and incubated for the indicated times in the presence or absence of 1 μM OSU8-1. Wounded keratinocytes were lysed and subjected to immunoprecipitation with EGFR antibodies followed by SDS-PAGE and Western blotting. Half of the immunocomplex was used for the detection of phosphotyrosine with PY-20 antibody, and the other half for the detection of EGFR protein with EGFR antibody.
Characterization of EGFR Ligands Produces by Keratinocytes in Response to Wounding
To investigate the identity of the shed EGFR ligands and their origin at early times after wounding, Northern blot analyses, inhibition of de novo protein synthesis by cycloheximide, and neutralization experiments were carried out. In the early phase after wounding (0–6 h), little if any enhancement of HB-EGF, AR, or TGF-α gene expression was observed (Fig. 4 a). Cycloheximide failed to abrogate the EGFR activation induced by wounding (Fig. 4 b). These experiments indicated that the EGFR ligands were shed from transmembrane forms that were preexisting in the cells. HB-EGF neutralizing antibody No. 197 suppressed the wound-induced activation of EGFR by ∼70% (Fig. 4 b), suggesting that HB-EGF constitutes a major portion of the shed EGFR ligands in the keratinocyte conditioned medium.
Figure 4.
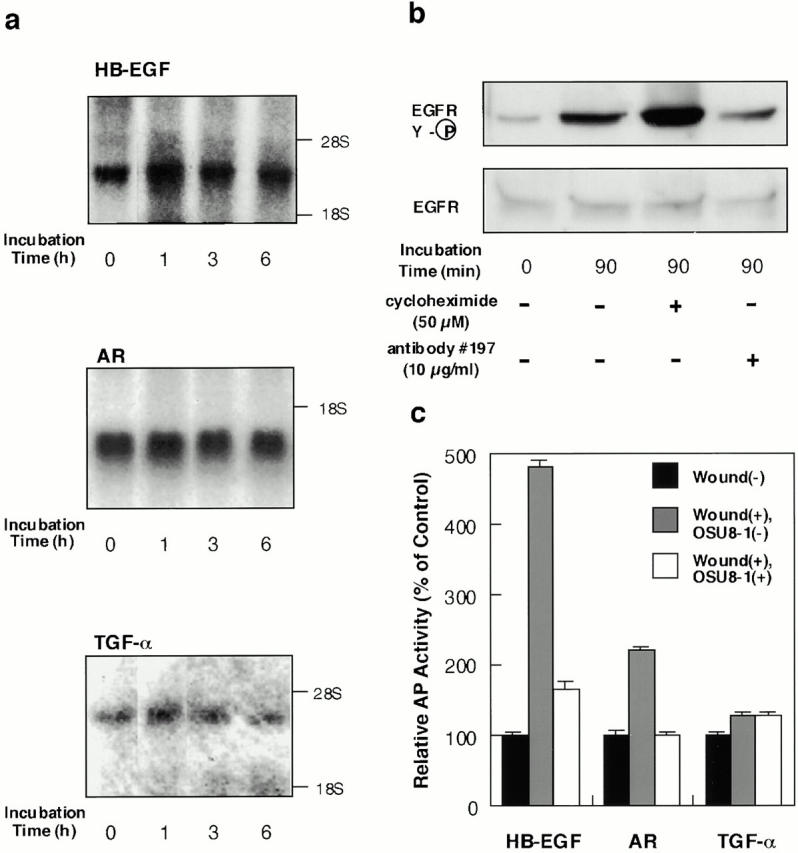
Characterization of EGFR-ligand shedding in keratinocytes. (a) Gene expression of EGFR ligands in wounded keratinocytes. After incubation of keratinocytes for the indicated time after wounding, total RNA was extracted and analyzed by Northern blotting. Sequential hybridization using the same membrane is shown. HB-EGF (2.4 kb), AR (1.4 kb), and TGF-α (4.8 kb) messages were detected. (b) Effect of cycloheximide and HB-EGF neutralizing antibody No. 197 on wound-induced EGFR activation in cultured keratinocytes. Keratinocytes were stimulated by tip-scraping in the presence or absence of cycloheximide (50 μM) or HB-EGF neutralizing antibody No. 197 (10 μg/ml). After incubating for the indicated times, the cells were lysed and subjected to immunoprecipitation with EGFR antibodies, fractionation by SDS-PAGE, and Western blotting. Half of the immunocomplex was used for the detection of tyrosine phosphorylation with phosphotyrosine antibody PY-20, and the other half for detection of EGFR protein with EGFR antibody. Cycloheximide (50 μM) did not abrogate the wound-induced tyrosine phosphorylation of EGFR. On the other hand, antibody No. 197 (10 μg/ml) suppressed wound-induced tyrosine phosphorylation of EGFR by 70%. (c) Quantitative analyses of EGFR ligand shedding. Stable transfectants of HaCat cells expressing nearly equal amounts of AP-tag HB-EGF, AP-tag AR, or AP-Tag TGF-α were incubated with or without 1 μM OSU8-1 for 60 min after wounding. AP activities in the conditioned media were measured. Each bar is the average of triplicate values.
To confirm which EGFR ligands are most susceptible to shedding after wounding, stable transfectants of HaCat, a keratinocyte cell line, which express almost equal amounts of AP-tag HB-EGF, AP-tag AR, or AP-tag TGF-α were subjected to a wound assay. The levels of soluble AP-tag HB-EGF, AP-tag AR, and AP-tag TGF-α in the conditioned medium were elevated 4.8-, 2.2-, and 1.3-fold, respectively, by wound stimuli (Fig. 4 c). These results again suggest that the transmembrane form of HB-EGF represents the major target for wound-induced EGFR ligand processing, in that it is 3.2- and 12.7-fold more susceptible to this processing than the precursors for AR and TGF-α, respectively. 1 μM of OSU8-1 was effective at inhibiting the shedding of AP-tag HB-EGF and also AP-tag AR, but had no effect on wound-induced AP-tag TGF-α shedding (Fig. 4 c).
Shedding of EGFR Ligands Is Essential for Keratinocyte Migration Induced by Wound Stimuli
Cultured keratinocytes grew and spread into a wound area during a 3-d incubation as shown in Fig. 5. To confirm the involvement of EGFR signaling in the wound-induced growth and migration of these keratinocytes, EGFR neutralizing antibody No. 425 or a tyrosine kinase inhibitor specific for EGFR (tyrphostin AG1478) were introduced into the wounded keratinocyte cultures. Cells treated with antibody No. 425 or AG1478 were both suppressed in terms of proliferation (to approximately 10–15% of the control) and migration (Fig. 5; see also Fig. 7 b). We also employed an EGFR-Fc fusion protein to neutralize all EGFR ligands released by the keratinocytes, and achieved a 70% inhibition of migration (Fig. 6 a). These data indicate that EGFR activation and the subsequent molecular signaling play an essential role in the wound-induced growth and migration of keratinocytes. The addition of 1 μM OSU8-1 also significantly suppressed cell migration to a level comparable to that seen with antibody No. 425 and AG1478 (Fig. 5). In contrast, growth of the cells was not suppressed to the extent observed for No. 425 and AG1478 (only ∼50% inhibition as shown in Fig. 7 b). Colony growth in the nonscraped area of the cultured keratinocytes was observed in the presence of OSU8-1, again suggesting that this compound is more effective in inhibiting cell migration than cell growth. Cells treated with both 20 ng/ml of human recombinant HB-EGF and 1 μM of OSU8-1 showed superior growth and migration into the scraped area, compared with OSU8–treated cells and control cells (Fig. 5). On the other hand, the addition of HB-EGF to wounded keratinocytes that were also treated with No. 425 or AG1478 had no effect on cell growth or migration (data not shown). These data suggest that OSU8-1 does not interfere directly with EGFR activation and its downstream signaling pathway.
Figure 5.
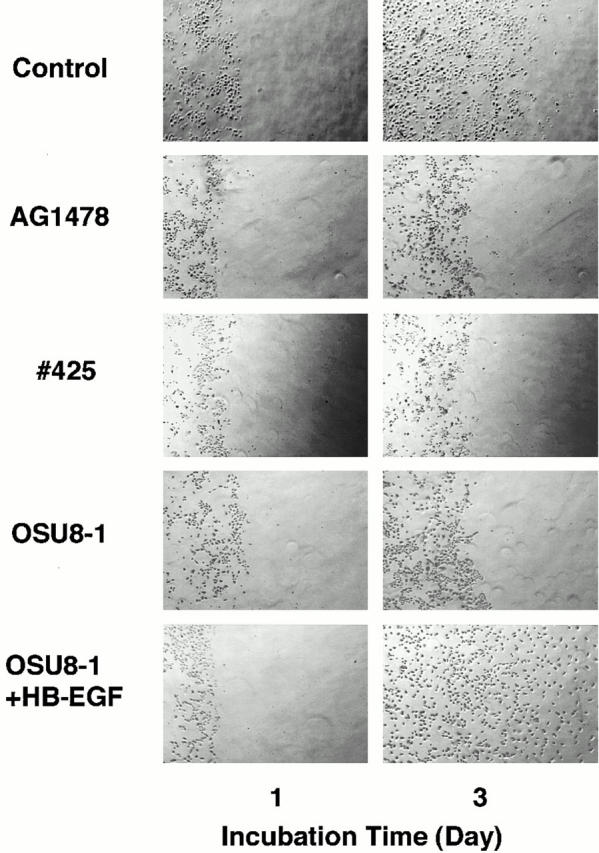
Evaluation of OSU8-1 and EGFR inactivation on keratinocyte migration. A portion of the keratinocytes were removed from tissue culture plates by scraping, and the remaining cells were cultured for 3 d in the absence or presence of EGFR neutralizing antibody No. 425 (10 μg/ml), EGFR kinase–specific inhibitor AG1478 (30 nM), OSU8-1 (1 μM), or both OSU8-1 (1 μM) and HB-EGF (20 ng/ml). The same area of each dish was monitored microscopically (40×) at day 0 and day 3.
Figure 7.
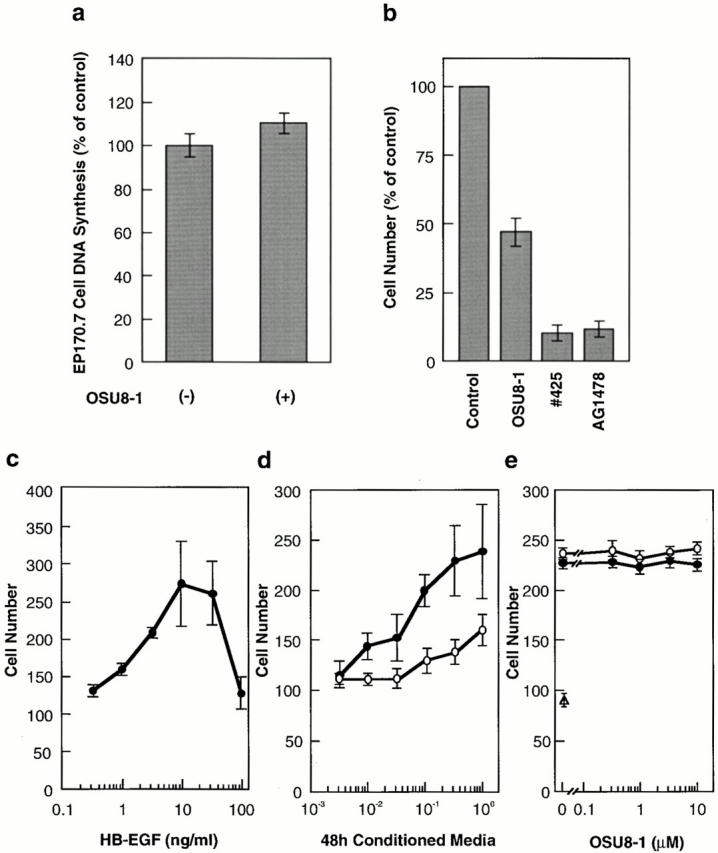
Quantitative evaluation of the effects of OSU8-1 and EGFR inactivation on keratinocyte growth and migration. (a) Effect of 1 μM OSU8-1 on juxtacrine activity of EGFR ligands in cultured keratinocytes. Juxtacrine activity was measured as described in Materials and Methods. (b) The effect of OSU8-1, antibody No. 425 and AG1478 on the growth of keratinocytes. Keratinocytes were seeded at a density of 105 cells/well in 6-well plates and incubated for 3 d in the absence or presence of OSU8-1 (1 μM), antibody No. 425 (10 μg/ml), or AG1478 (30 nM). (a and b) Values represent the average of triplicate experiments. (c) Stimulation of keratinocyte migration by HB-EGF. The ability of recombinant HB-EGF to stimulate keratinocyte migration was tested using a Boyden chamber. (d) Stimulation of keratinocyte migration by conditioned medium. Serially diluted, 48-h conditioned medium obtained from wounded keratinocytes was added to the bottom wells of Boyden chambers and tested for its ability to stimulate keratinocyte migration. Open circles, conditioned medium generated in the presence of 1 μM OSU8-1; closed circles, conditioned medium obtained without 1 μM OSU8-1. (e) Effect of OSU8-1 on the migration response of keratinocytes induced by conditioned medium from wounded keratinocyte cultures. The indicated concentrations of OSU8-1 were added to the top (open circles) and the bottom (closed circles) wells of a Boyden chamber containing wounded keratinocyte conditioned medium in the bottom wells. An open triangle shows migrated cell number obtained using fresh medium in the bottom wells as a control. (c–e) Each point is the average of quadruplicate measurements.
Figure 6.
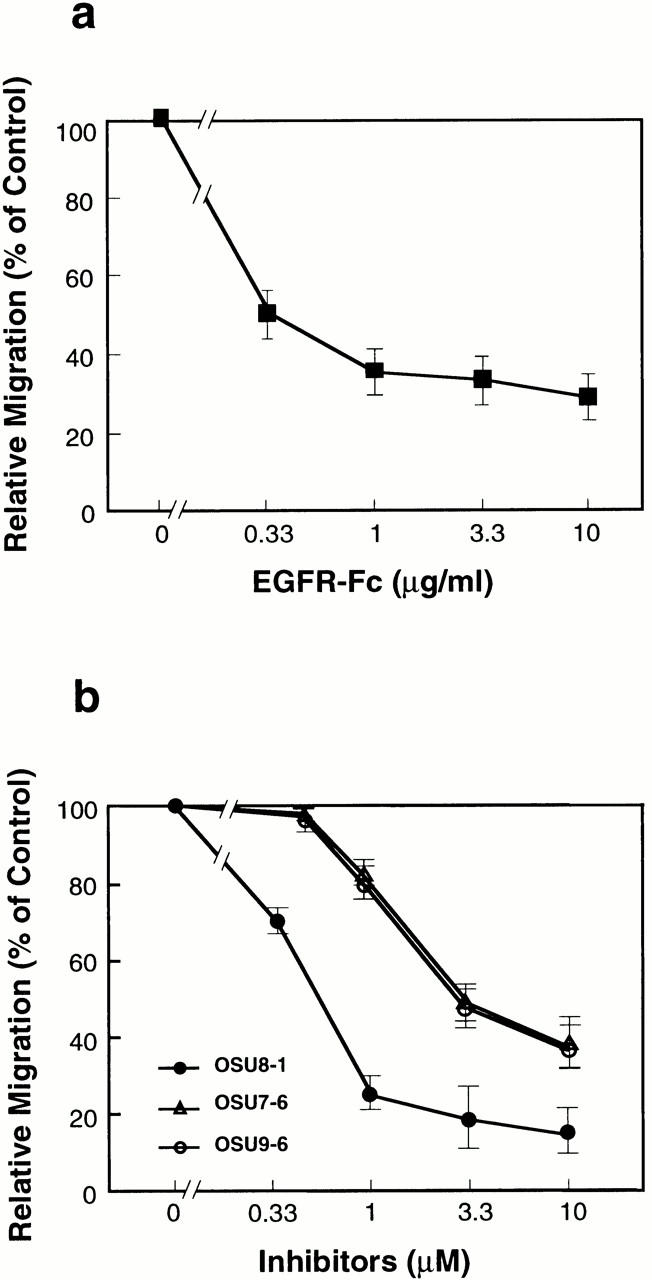
Dose effects of EGFR-Fc (a) and OSU8-1, OSU7-6, and OSU9-6 (b) on keratinocyte migration after wounding. Each point is the average of quadruplicate measurements. Migration was evaluated by the distance between the furthest migrated cell and the scraped edge.
OSU8-1 has a broad spectrum of inhibitory activity for MMPs (Table ). Thus, the inhibitory effects of OSU8-1 on wounded keratinocyte migration could conceivably reflect the suppression of other MMP-dependent events besides EGFR ligand shedding. To clarify the importance of EGFR ligand shedding in the migration of the keratinocytes, we further employed two other inhibitors with almost the same activities for blocking EGFR-ligand shedding, but distinguishable activities with regard to the MMPs, as shown in Table . OSU7-6 showed inhibitory activities with IC50s of 0.35, 2.0, and 0.4 nM for MMP-1, MMP-3, and MMP-9, respectively, which were comparable to those of OSU8-1. In contrast, OSU9-6 showed extremely low activities for MMP-1, MMP-3, and MMP-9, with IC50s of 10, 10, and >1 μM, respectively. Nonetheless, OSU7-6 and OSU9-6 were equally effective at inhibiting cell migration in a dose-dependent manner, blocking migration nearly 70% relative to the control at 10 μM concentration (Fig. 6 b).
Since the growth of keratinocytes has been shown to be mediated by juxtacrine stimulation in addition to auto/paracrine signals (Inui et al. 1997), we next addressed the issue of whether OSU8-1 is capable of interfering with the juxtacrine growth activity of EGFR ligands on the cell surface of keratinocytes in culture. As shown in Fig. 7 a, OSU8-1 actually slightly upregulated the juxtacrine growth activity of keratinocytes, a finding which could be due to the accumulation of membrane-anchored EGFR ligands produced in the presence of OSU8-1. In unwounded keratinocyte cultures, OSU8-1 inhibited 50% of the cell growth, a significantly less effective inhibition than that seen with No. 425 and AG1478 (Fig. 7 b). Taken together, these results are consistent with a model wherein OSU8-1 suppresses cell growth mediated by soluble EGFR ligands, with cell growth in the presence of OSU8-1 accounted for by juxtacrine stimulation.
To quantify more directly the inhibitory effect of OSU8-1 on keratinocyte autocrine motility, the migratory activity of keratinocyte conditioned medium collected 48 h after wounding in the presence or absence of OSU8-1 was measured using a Boyden chamber. As a positive control, recombinant HB-EGF was tested and shown to be a potent stimulator of keratinocyte migration in this assay, with a maximal stimulation at 10 ng/ml (Fig. 7 c). Stimulation of keratinocyte migration was also obtained using the conditioned medium (Fig. 7 d). With undiluted conditioned medium, the stimulation was comparable to that of recombinant HB-EGF at 10 ng/ml. However, conditioned medium collected in the presence of OSU8-1 was ∼30-fold less potent at promoting migration, indicating that OSU8-1 greatly reduced the production of autocrine motility factor (Fig. 7 d). To exclude unexpected effects of OSU8-1 itself on cell migration, various concentrations of OSU8-1 were added to the conditioned medium prepared from wounded keratinocytes, and the mixtures were tested for the migration activity. As shown in Fig. 7 e, OSU8-1 had no effect on the migration of keratinocytes induced by the conditioned medium of wounded keratinocytes, suggesting that OSU8-1 does not interrupt the intracellular signaling cascade of cell migration.
Effect of OSU8-1 on Cutaneous Wound Healing In Vivo
Under conditions of wounding, keratinocytes migrate from hair follicles and the wound edge towards the center of the wound. Given the effects of OSU8-1 described above on keratinocyte migration in vitro in response to wound stimuli, we therefore investigated the effects of OSU8-1 on cutaneous wound healing in mice. Keratin staining of wound tissue sections from wounds treated with vehicle solution alone revealed the migration of keratinocytes into the wounded site. Regenerated epithelium and granulation tissue was observed in the upper dermis at day 6 after wounding (n = 15, Fig. 8 a). In contrast, wound tissue sections from wounds treated with 10 mM of OSU8-1 showed essentially no tissue regeneration or repair of the epidermis (Fig. 8 b). It is noteworthy that the migration of keratinocytes into the wounded site was completely blocked in all of the OSU8-1– treated wounds (n = 10; Fig. 8 b). The application of HB-EGF at 5 μg/ml in addition to OSU8-1 restored keratinocyte migration, resulting in healing wounded sites (n = 5; Fig. 8 c). These results indicate that the production of the soluble forms of EGFR ligands represents a critical event in the process of wound healing in vivo.
Figure 8.
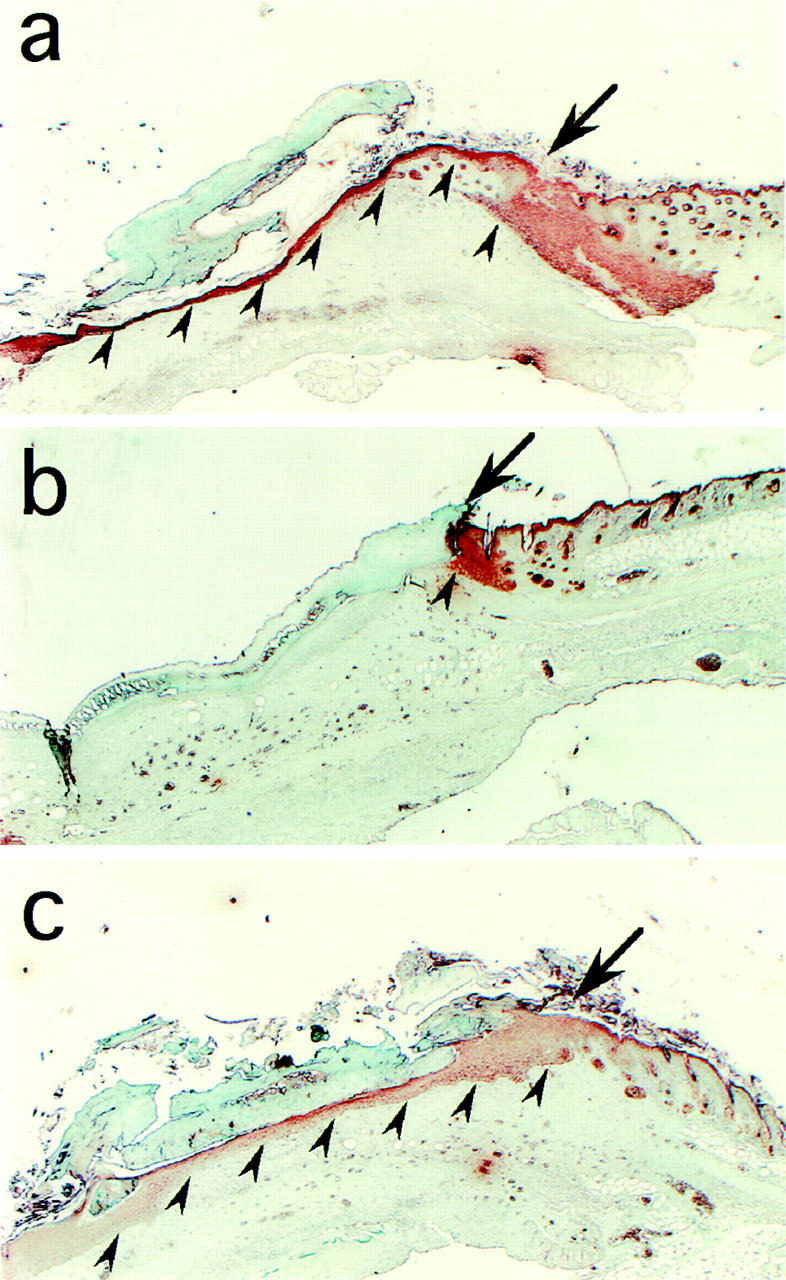
Effect of OSU8-1 on wound healing in vivo. Histology of excised mouse skin on day 6 after wounding, without or with 10 mM OSU8-1 treatment. Keratinocytes were stained with antikeratin/cytokeratin antibody. (a) A typical tissue section from the control wounds (n = 15). Keratinocytes are observed to have migrated from the edge of the wound, spreading under the crust and covering the wound site. Arrowheads indicate the base of the keratinocyte layer. The arrow marks the edge of the wound. (b) A typical tissue section from an OSU8-1–treated wound (n = 10). Both keratinocyte migration and the regeneration of the epidermis were greatly inhibited. (c) A typical tissue section from a wound treated with a cocktail of OSU8-1 and HB-EGF (5 μg/ml; n = 5). Both keratinocyte migration and the regeneration of the epidermis were completely restored.
Discussion
In primary keratinocyte cultures and skin explant models, it has been reported that keratinocyte migration and proliferation are key events and are predominantly mediated by autocrine EGFR activation (Stoll et al. 1997). Constitutive gene expression and protein production of EGFR ligands such as HB-EGF, AR, and TGF-α from keratinocytes also have been demonstrated in vivo and in vitro (Coffey et al. 1987; Cook et al. 1991; Hashimoto et al. 1994; Inui et al. 1997). However, the mechanisms, by which the endogenous ligands of EGFR are regulated after wounding, and their precise roles in the wound healing process are unclear. To address these questions, we focused on the molecular events associated with three EGFR ligands (HB-EGF, TGF-α, and AR) and EGFR activation in an early phase after wounding.
Production of EGFR ligands and EGFR activation by wound stimuli in cultured keratinocytes occurred coordinately and showed a biphasic pattern. The prolonged activation of EGFR is not in agreement with the previously reported time course for transient activation and downregulation of EGFR when stimulated by exogenous ligands, and may, therefore, be a physiological property of autocrine EGFR activation. The biphasic production of the shed EGFR ligands and EGFR activation could be accounted for if the first peak represents the local induction triggered by wound stimuli, whereas the second peak represents induction regulated by an intracellular signaling cascade (Fan and Derynck 1999; Gechtman et al. 1999). The fact that three types of EGFR autocrine ligands, namely, HB-EGF, TGF-α, and AR, have been reported to be expressed on the keratinocyte cell surface in membrane-anchored forms (Inui et al. 1997) suggested that the shedding of these ligands might be triggered by wound stimuli. Quantitative analyses of the wound-inducible shedding of EGFR ligands, using an AP-tag assay, revealed that HB-EGF represents the factor which is most susceptible among these three ligands for wound-induced processing. It is interesting to speculate that the difference in susceptibility for shedding among the three ligands might reflect the independent physiological significance of each factor.
We identified an inhibitor, OSU8-1, which effectively inhibits HB-EGF, AR, and TGF-α shedding, and characterized its effects on keratinocytes in vitro and in vivo. The addition of OSU8-1 markedly prevented the shedding of EGFR ligands induced by wound stimuli and EGFR activation in the early phase after wounding, resulting in the inhibition of keratinocyte migration. However, OSU8-1 was less effective at inhibiting cell proliferation. Based on the findings that the EGFR neutralizing antibody and the EGFR kinase inhibitor AG1478 inhibited both cell migration and proliferation, and that OSU8-1 did not abrogate the juxtacrine activity of keratinocytes, we hypothesize that the inhibition of EGFR ligand shedding by OSU8-1 abrogates auto/paracrine stimulation by soluble EGFR ligands, which is critical for keratinocyte migration, but that proliferation is less affected as it can be triggered by juxtacrine stimulation, which is mediated by membrane-anchored EGFR ligands.
The biological significance of the juxtacrine and auto/paracrine activities of HB-EGF has been poorly characterized. However, we recently reported that the juxtacrine activity of HB-EGF specifically evoked an antiapoptotic effect in a rat hepatoma cell line AH66tc and that transmembrane HB-EGF, therefore, functioned as a cell survival factor (Miyoshi et al. 1997). Cell survival in response to HB-EGF juxtacrine stimulation also has been shown in the case of kidney epithelial cells (Takemura et al. 1997). Differences in the biological effects evoked by juxtacrine and auto/paracrine activities are likely to reflect the existence of unique molecules that are recruited to each signaling pathway. It has been reported that mice harboring a knockout of Stat3 in their keratinocytes showed a significant retardation of cutaneous wound healing as reflected in a lack of keratinocyte migration. However, the growth response of the keratinocytes in these mice to several growth factors appeared to be normal (Sano et al. 1999). Consistent with these observations, we found that specific activation of Stat3 was evoked by EGFR ligand shedding in cultured keratinocytes (data not shown), suggesting that the ectodomain shedding of EGFR ligands could trigger intercellular signaling pathways unique for auto/paracrine and juxtacrine stimulation.
Analyses of the effects of OSU8-1 on cutaneous wound healing in vivo revealed that wounded tissue sections treated with 10 mM OSU8-1 showed essentially no tissue regeneration and repair at day 6 after wounding. In particular, there was a marked lack of keratinocyte migration, as compared with cutaneous healing in control mice. Administration of recombinant HB-EGF, in addition to OSU8-1, restored the migration of keratinocytes in wounded sites. Therefore, we conclude that the shedding of EGFR ligands represents a crucial event in the migration of keratinocytes to the wound site in vivo. The present findings may also have implications not only for our understanding of the molecular mechanism involved in wound healing, but also for other tissue remodeling processes such as trophoblast invasion, inflammatory diseases, and, in particular, tumor invasion.
Recently, it has been reported that the broad spectrum inhibition of MMPs by a synthetic inhibitor (galardin) in plasminogen-deficient mice completely arrested wound healing, suggesting an essential role for matrix-degrading proteases in wound healing (Lund et al. 1999). Since galardin, like OSU8-1, is a peptide-based zinc-chelating hydroxamate, it might inactivate not only the MMP family, but also the ADAM (a disintegrin and a metalloprotease) family involved in EGFR ligand shedding. Thus, we speculate that the inhibition of wound healing reported by Lund et al. 1999 might, in part, have been due to the inhibition of EGFR ligand shedding.
Tumor necrosis factor–α converting enzyme (TACE) represents an additional protein involved in ectodomain shedding. Its gene has been cloned (Black et al. 1997; Moss et al. 1997) and disrupted (Peschon et al. 1998), resulting in skin and eye defects. This phenotype resembles that of TGF-α knockout mice, suggesting that TACE might function widely as a shedding enzyme beyond its effects on tumor necrosis factor–α processing. TACE is now classified in the ADAM family, which is comprised of at least 31 members. Our most recent studies of inhibitors for EGFR ligand shedding indicate that >1 shedding protease could be involved in the shedding of EGFR ligands (Endo, T., and S. Higashiyama, unpublished data). The identification of shedding enzymes for many types of cell-surface proteins, including receptors and ligands, would be a key step in understanding the normal and pathological tissue remodeling processes described above.
Recently, molecular mechanism in the ectodomain shedding of EGFR ligands including HB-EGF and TGF-α have been vigorously studied, showing the involvement of MMP-3 (Suzuki et al. 1997), meltrin-γ/MDC9/ADAM9 (Izumi et al. 1998), and the MAP kinase cascade (Fan and Derynck 1999; Gechtman et al. 1999). Further, it also has been shown that the ectodomain shedding of HB-EGF is a crucial step in the transactivation of the EGFR via G-protein–coupled receptors (Prenzel et al. 1999). Taken together with our results herein, it is clear that the ectodomain shedding of EGFR ligands is a regulated molecular event involved in different types of physiological and pathological phenomena.
In conclusion, wound stimuli trigger EGFR ligand shedding, which represents an essential step in the cutaneous wound healing process. OSU8-1 inhibits the shedding of EGFR ligands, resulting in the strong inhibition of wound healing in vitro and in vivo. It is clear that shedding enzymes and their involvement in wound healing are deserving of further investigation.
Acknowledgments
We greatly acknowledge Dr. J.A. Abraham (Scios Inc.) for editing the manuscript, and Drs. S. Sano and J. Takeda (Osaka University) for helpful discussion and comments.
Footnotes
Abbreviations used in this paper: ADAM, a disintegrin and metalloprotease; AP, alkaline phosphatase; AR, amphiregulin; ECL, enhanced chemiluminescence; EGFR, epidermal growth factor receptor; EGFR-Fc, epidermal growth factor receptor–immunoglobulin G-Fcγ fusion protein; HB-EGF, heparin-binding EGF-like growth factor; MMP, matrix metalloprotease; TACE, tumor necrosis factor–α converting enzyme; TGF-α, transforming growth factor–α; TPA, 12-O-tetradecanoylphorbol-13-acetate.
References
- Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F., Castner B.J., Stocking K.L., Reddy P., Srinivasan S. A metalloproteinase disintegrin that releases tumor-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Blotnick S., Peoples G.E., Freeman M.R., Eberlein T.J., Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblastsdifferential production and release by CD4+ and CD8+ T cells. Proc. Natl. Acad. Sci. USA. 1994;91:2890–2894. doi: 10.1073/pnas.91.8.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden S. The chemotactic effect of mixtures of antibodies and antigens on polymorphonuclear leukocytes. J. Exp. Med. 1962;115:435–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann R., Lindquist P.B., Nagashima M., Kohr W., Lipari T., Napier M., Derynck R. Transmembrane TGF-α precursors activate EGF/TGF-α receptors. Cell. 1989;56:691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- Chen C.A., Okayama H. Calcium phosphate-mediated gene transfera highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffey R., Jr., Derynck R., Wilcox J.N., Bringman T.S., Goustin A.S., Moses H.L., Pittelkow M.R. Production and auto-induction of transforming growth factor-α in human keratinocytes. Nature. 1987;328:817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Cohen, S. 1964. Isolation and biological effects of an epidermal growth-stimulating protein. National Cancer Institute Monograph 13. Bethesda, MD. 13–27. [PubMed]
- Cook P.W., Mattox P.A, Keeble W.W., Pittelkow M.R., Plowman G.D., Shoyab M., Adelman J.P., Shippley G.D. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol. Cell. Biol. 1991;11:2547–2557. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Roberts A.B., Winkler M.E., Chen E.Y., Goeddel D.V. Human transforming growth factor-αprecursor structure and expression in E. coli . Cell. 1984;38:287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Fan H., Derynck R. Ectodomain shedding of TGF-α and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO (Eur. Mol. Biol. Organ.) J. 1999;24:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechtman Z., Alonson J.L., Raab G., Ingber D.E., Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the ref/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J. Biol. Chem. 1999;274:28828–28835. doi: 10.1074/jbc.274.40.28828. [DOI] [PubMed] [Google Scholar]
- Goishi K., Higashiyama S., Klagsbrun M., Nakano N., Umata T., Ishikawa M., Mekada E., Taniguchi N. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factorconversion from juxtacrine to paracrine growth factor activity. Mol. Biol. Cell. 1995;6:967–980. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Tojo A., Muroya K., Hashimoto Y., Hattori S., Nakamura S., Takenawa T., Yazaki Y., Shibuya M. Epidermal growth factor-receptor mutant lacking the autophosphorylation sites induces phosphorylation of Shc protein and Shc-Grb2/ASH association and retains mitogenic activity. Proc. Natl. Acad. Sci. USA. 1994;91:167–171. doi: 10.1073/pnas.91.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Higashiyama S., Asada H., Hashimura E., Kobayashi T., Sudo K., Nakagawa T., Damm D., Yoshikawa K., Taniguchi N. Heparin-binding EGF-like growth factor is an autocrine growth factor for human keratinocytes. J. Biol. Chem. 1994;269:20060–20066. [PubMed] [Google Scholar]
- Higashiyama S., Abraham J.A., Miller J., Fiddes J.C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J.A., Klagsbrun M. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migrationdependence on interactions with cell surface heparan sulfate. J. Cell Biol. 1993;122:933–940. doi: 10.1083/jcb.122.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S., Iwamoto R., Goishi K., Raab G., Taniguchi N., Klagsbrun M., Mekada E. The membrane protein CD9/DRAP27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J. Cell Biol. 1995;128:929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S., Higashiyama S., Hashimoto K., Higashiyama M., Yoshikawa K., Taniguchi N. Possible role of coexpression of CD9 with membrane-anchored heparin-binding EGF-like growth factor and amphiregulin in cultured human keratinocyte growth. J. Cell. Physiol. 1997;171:291–298. doi: 10.1002/(SICI)1097-4652(199706)171:3<291::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Hirata M., Hasuwa H., Iwamoto R., Umata T., Miyado K., Tamai Y., Kurisaki T., Sehara-Fujisawa A., Ohno S., Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund L.R., Romer J., Bugge T.H., Nielsen B.S., Frandsen T.L., Degen J.L., Stephens R.W., Dano K. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO (Eur. Mol. Biol. Organ.) J. 1999;17:4645–4656. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikovsky M., Breuing K., Liu P.Y., Eriksson E., Higashiyama S., Farber P., Sasse J., Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc. Natl. Acad. Sci. USA. 1993;90:3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healingaiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Massague J., Pandiella A. Membrane-anchored growth factors. Annu. Rev. Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- Miyoshi E., Higashiyama S., Nakagawa T., Hayashi N., Taniguchi N. Membrane-anchored heparin-binding epidermal growth factor-like growth factor acts as a tumor survival factor in a hepatoma cell line. J. Biol. Chem. 1997;272:14349–14355. doi: 10.1074/jbc.272.22.14349. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Jin S.L., Milla M.E., Bickett D.M., Burkhart W., Carter H.L., Chen W.J., Clay W.C., Didsbury J.R., Hassler D. Cloning of a disintegrin metalloproteinase that processes precursor tumor-necrosis factor-alpha. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Massague J. Cleavage of the membrane precursor for transforming growth factor alpha is a regulated process. Proc. Natl. Acad. Sci. USA. 1991;88:1726–1730. doi: 10.1073/pnas.88.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon J.J., Slack J.L., Reddy P., Stocking K.L., Sunnarborg S.W., Lee D.C., Russell W.E., Castner B.J., Johnson R.S., Fitzner J.N. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Prenzel N., Zwick E., Daub H., Leserer M., Abraham R., Wallasch C., Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Sano S., Itami S., Takeda K., Tarutani M., Yamaguchi Y., Miura H., Yoshikawa K., Akira S., Takeda J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO (Eur. Mol. Biol. Organ.) J. 1999;17:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y., Christofori G., Hanahan D., Ono Y., Sasada R., Igarashi K., Folkman J. Betacellulina mitogen from pancreatic β cell tumors. Science. 1993;259:1604–1614. doi: 10.1126/science.8456283. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G.D., McDonald V.L., Bradley J.G., Todaro G.J. Structure and function of human amphiregulina member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Stoll S., Garner W., Elder J. Heparin-binding ligands mediate autocrine epidermal growth factor receptor activation in skin organ culture. J. Clin. Invest. 1997;100:1271–1281. doi: 10.1172/JCI119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Raab G., Moses M.A., Fernandez C.A., Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J. Biol. Chem. 1997;272:31730–31737. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- Takemura T., Kondo S., Homma T., Sakai M., Harris R.C. The membrane-bound form of heparin-binding epidermal growth factor-like growth factor promotes survival of cultured renal epithelial cells. J. Biol. Chem. 1997;272:31036–31042. doi: 10.1074/jbc.272.49.31036. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Komurasaki T., Uchida D., Takayama Y., Isobe T., Okuyama T., Hanada K. Epiregulin, a novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J. Biol. Chem. 1995;270:7495–7500. doi: 10.1074/jbc.270.13.7495. [DOI] [PubMed] [Google Scholar]
- Tsuboi R., Chong-ming S., Daniel B.R., Ogawa H. A wound healing model using healing-impaired diabetic mice. J. Dermatol. 1992;19:673–675. doi: 10.1111/j.1346-8138.1992.tb03757.x. [DOI] [PubMed] [Google Scholar]
- Wong S., Winchell L.F., McCune B.K., Earp H.S., Texido J., Massaague J., Herman B., Lee D.C. The TGF-α precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989;56:495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]