Abstract
TRAPP, a novel complex that resides on early Golgi, mediates the targeting of ER-to-Golgi vesicles to the Golgi apparatus. Previous studies have shown that YPT1, which encodes the small GTP-binding protein that regulates membrane traffic at this stage of the secretory pathway, interacts genetically with BET3 and BET5. Bet3p and Bet5p are 2 of the 10 identified subunits of TRAPP. Here we show that TRAPP preferentially binds to the nucleotide-free form of Ypt1p. Mutants with defects in several TRAPP subunits are temperature-sensitive in their ability to displace GDP from Ypt1p. Furthermore, the purified TRAPP complex accelerates nucleotide exchange on Ypt1p. Our findings imply that Ypt1p, which is present on ER-to-Golgi transport vesicles, is activated at the Golgi once it interacts with TRAPP.
Keywords: exchange factor, small GTPase, secretion, ER-to-Golgi, tethering factor
Introduction
Proteins destined to traverse the secretory pathway are incorporated into transport vesicles that bud from a donor organelle to target and fuse with an acceptor compartment. In ER-to-Golgi traffic, a set of proteins have been identified (Ypt1p, Uso1p, TRAPP, and the Sec34p–Sec35p complex) that are specifically required for tethering/docking vesicles to the Golgi complex. Ypt1p is a member of the Ypt/Rab family of small GTP-binding proteins (Segev et al. 1988). Uso1p, a large 206-kD protein that contains a globular domain at its amino terminus and a coiled-coil domain at its carboxy terminus, tethers ER-to-Golgi vesicles to the Golgi apparatus (Barlowe 1997). Sec35p associates with Sec34p and several other proteins to form a multiprotein complex (Kim et al. 1999) that acts in conjunction with Uso1p (VanRheenen et al. 1999). TRAPP, a highly conserved novel complex that contains 10 subunits (Bet3p, Bet5p, Trs20p, Trs23p, Trs31p, Trs33p, Trs65p, Trs85p, Trs120p, and Trs130p), stably associates with the cis-Golgi complex where it acts before the assembly of the SNARE complex (Rossi et al. 1995; Sacher et al. 1998; Barrowman et al. 2000; Sacher et al. 2000).
Ypt/Rab proteins are molecular switches that cycle between GTP- and GDP-bound forms (Novick and Zerial 1997). This cycle is achieved by nucleotide exchange and GTP hydrolysis. Since the intrinsic rates of nucleotide exchange and GTP hydrolysis are low for most small GTP-binding proteins, accessory proteins are needed to stimulate these reactions. Guanine nucleotide exchange factors (GEFs) promote GDP dissociation and GTP uptake (reviewed in Martinez and Goud 1998), while GTPase-activating proteins stimulate GTP hydrolysis (reviewed in Scheffzek et al. 1998). In the case of Ypt1p, nucleotide exchange stimulated by a GEF is essential for its function (Jones et al. 1995). Ypt1p locked in its nucleotide-free state is a dominant negative inhibitior of ER-to-Golgi transport in vitro. Although previous studies have shown that Golgi membranes have a nucleotide exchange activity that acts on Ypt1p, this GEF has not been identified (Jones et al. 1998). In this report, we demonstrate that TRAPP binds to the nucleotide-free form of Ypt1p to accelerate nucleotide exchange on Ypt1p.
Materials and Methods
Media and Reagents
Cells were grown in YPD medium containing 1% yeast extract, 2% bactopeptone, and 2% glucose. All chemical reagents were obtained from Sigma-Aldrich unless otherwise noted.
Preparation of Mutant Lysates
Cell lysates were prepared from the following strains: SFNY 26-3A (MATa ura3-52), SFNY596 (MATa bet3-1 leu2-3, 112 ura3-52), SFNY713 (MATα GAL + ura3-52 leu2-3,112: (LEU2 bet5-1) his3Δ200 bet5Δ::HIS3), SFNY293 (MATα uso1-1 ura3 trp1 his7), SFNY691 (MATα sec34-2 ura3-52 leu2-3, 112), SFNY816 (MATa sec35-1 ura3-52), SFNY974 (MATa ura3-52 leu2-3, 112 his3Δ200 trs130Δ33::HIS3) referred to as trs130ts2 in the text, SFNY1019 (MATα ura2-52 trs31-1), and SFNY1040 (MATa ura3-52 trs85Δ::URA3). Wild-type and mutant cells were grown overnight at 25°C to OD599 = 1.0. The cells (600 OD599 units) were converted to spheroplasts (Kim, et al. 1999) and lysed in 6 ml of buffer A (20 mM Hepes, pH 7.2, 1 mM DTT, protease inhibitor cocktail). Unbroken cells were removed during a 3-min centrifugation at 450 g and the supernatant (total lysates) was assayed.
In Vitro Binding Assay
Glutathione-S-transferase (GST)-Ypt1p and GST-Ypt51p were purified from 1.5L of Escherichia coli (DH5α). Expression was induced for 2.5 h by the addition of IPTG (1 mM). The fusion protein was bound to a 2-ml bed volume of glutathione-agarose (Amersham Pharmacia Biotech) according to the manufacturer's protocol and stored in buffer I (50 mM Hepes, pH 7.2, 100 mM NaCl, 1 mM DTT, 1 mM EDTA, 0.5 mM MgCl2). Nucleotide was removed or exchanged on 200 μg of GST-Ypt1p or GST-Ypt51p (∼4.1 nmol of Ypt protein assuming molecular mass of ∼48,000 for the fusion protein) in 500 μl of buffer I with a threefold molar excess of either GDP or GTPγS. To prepare the nucleotide-free forms of GST-Ypt1p and GST-Ypt51p, 10 mM EDTA was used in place of MgCl2 in buffer I. The beads were rotated at room temperature for 1 h and then washed two times with 1 ml of buffer II (50 mM Hepes, pH 7.2, 100 mM NaCl, 1 mM DTT, 5 mM MgCl2). To rebind GDP to the nucleotide-free form of GST-Ypt1p, the beads were washed two times with 1 ml of buffer I and incubated for 1 h with a threefold molar excess of GDP in 500 μl of buffer I. The beads were then washed two times with 1 ml of buffer II and immediately incubated with a yeast lysate as described below.
A lysate from wild-type yeast (SFNY26-3A) was prepared by converting 7500 OD599 units of cells to spheroplasts during a 1-h incubation at 37°C (Sacher et al. 2000) and lysing the cells in 140 ml of 20 mM Hepes, pH 7.2, with a Wheaton dounce homogenizer. The lysate was centrifuged at 25,000 rpm in a 70Ti rotor (Beckman) for 1 h and protein was measured by the Bradford assay. The lysate was then dialyzed overnight against buffer II. A total of 500 mg of lysate was incubated with GST-Ypt1p or GST-Ypt51p, prepared as described above, for 2 h at 4°C. The beads were then transferred to an Eppendorf tube following a 3-min centrifugation at 1500 g and washed two times with 1 ml of buffer II (±GDP or GTPγS as required) and once with 1 ml of buffer II containing 250 mM NaCl. The beads were boiled in 150 μl of SDS-PAGE sample buffer and fractionated on an SDS-12.5% polyacrylamide gel. Western blots were probed with antibodies against the TRAPP subunits as described in the legend to Fig. 1.
Figure 1.
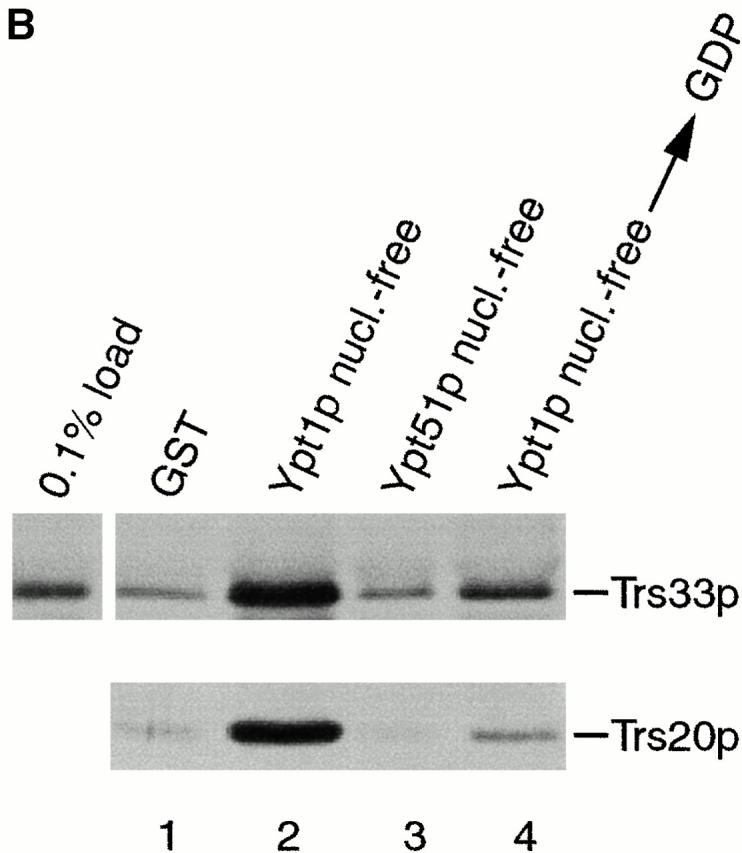
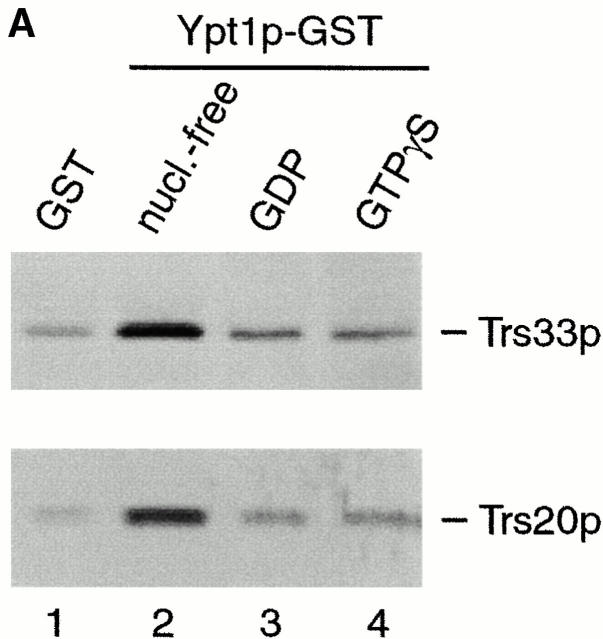
TRAPP subunits bind preferentially to the nucleotide-free form of Ypt1p. (A) A yeast lysate was incubated with agarose beads containing either GST (lane 1) or GST-Ypt1p (lanes 2–4) as described in the Materials and Methods. Before the incubation, Ypt1p was either stripped of nucleotide (nucleotide-free, lane 2) or loaded with GDP (lane 3) or GTPγS (lane 4). The beads were washed and the bound proteins were eluted by boiling in SDS-PAGE sample buffer. The eluate was then fractionated on a SDS–12.5% polyacrylamide gel, and Western blot analysis was performed by the enhanced chemiluminescence method using anti-Trs33p antibody at 1:2,500 dilution (top) or anti-Trs20p antibody at 1:1,000 dilution (bottom). (B) A yeast lysate was incubated with agarose beads containing either GST (lane 1), GST-Ypt1p (lanes 2 and 4), or GST-Ypt51p (lane 3). Before the incubation, Ypt1p and Ypt51p were stripped of nucleotide (nucleotide-free, lanes 2 and 3) or stripped of nucleotide and allowed to rebind GDP (lane 4). The beads were processed as above. The amount of Trs33p present in 0.1% of the lysate that was incubated with the beads is shown.
TRAPP Purification
TRAPP was purified from 300 OD599 units of cells. SFNY904 (MATα ura3-52 bet3Δ::URA3 leu2-3, 112 BET3-protein A::LEU2 L-A-o) was used for the purification because it contained protein A–tagged Bet3p and was free of the L-A virus (L-A-o). The L-A virus coat protein, gag, is a common contaminant in the purification (see Sacher et al. 2000). To purify TRAPP, cells were converted to spheroplasts, lysed in 3 ml of buffer B (150 mM KCl, 20 mM Hepes, pH 7.2, 2 mM EDTA, 1% Trition X-100, 0.5 mM DTT, protease inhibitor cocktail), and the unbroken cells were removed as described above. The lysate was centrifuged at 14,000 g for 10 min and the supernatant (10 mg/ml of protein) was incubated with 150 μl of a 50% slurry of IgG–Sepharose (Amersham Pharmacia Biotech) for 4 h. The beads were washed three times with 3 ml of release buffer (20 mM Hepes, pH 7.2, 5 mM MgCl2, 1 mM DTT, 0.75 mM GTP, 0.75 mMGDP, 1 mg/ml BSA) or uptake buffer (20 mM Hepes, pH 7.2, 5 mM MgCl2, 1 mM EDTA, 1 mM ATP, 1 mM DTT, 1 mg/ml BSA) and then divided into six equal aliquots. Samples were centrifuged and the supernatant was aspirated. The beads were resuspended in 8 μl of release or uptake buffer and assayed for nucleotide exchange activity. As a control, extract prepared from SFNY823 (MATa ura3-52 L-A-o), which does not contain tagged Bet3p, was incubated with IgG–Sepharose beads in the same way as extract prepared from SFNY904. The beads were processed as described above.
The fold purification of TRAPP was determined by estimating the amount of Trs33p on beads and comparing it to the amount of Trs33p in the lysate. Western blot analysis using the enhanced chemiluminescence method and a standard curve of known amounts of recombinant Trs33p was used to determine the concentration of Trs33p. Previous studies have shown that the components of TRAPP are present in approximately equimolar amounts (Sacher et al. 2000). With this information we were able to calculate the picomoles of TRAPP per mg of protein in the lysate versus the picomoles of TRAPP per mg of protein on the beads. The amount of protein on beads was determined by eluting the washed beads for 5 min at room temperature with 0.2 M glycine (pH 2.8). No protein was detected from untreated beads that were washed in wash buffer and eluted. Protein was measured by the Bradford assay. The method of purification described here results in a preparation that is ∼12% pure. Comparision of the amount of protein on the beads after incubation with tagged and untagged lysates indicates that the contaminants are not binding in a TRAPP-specific manner.
GDP–GTP Exchange Assay
GDP displacement activity was monitored essentially as described by Jones et al. 1998. Six histidine tagged (His6)-Ypt1p, expressed and purified from E. coli by the protocol of Du et al. 1998, was preloaded by incubating 2 μM of the purified protein with 7.25 μM of [8,5′,-3H]GDP (34 Ci/mmol; NEN Life Science Products) in preloading buffer (20 mM Hepes, pH 7.2, 5 mM EDTA, 1 mM DTT) for 15 min at 30°C. At the end of the incubation, MgCl2 was added to a final concentration of 10 mM. The dissociation assay was initiated by the addition of [3H]GDP-Ypt1p (200 nM final concentration) to release buffer in the presence of cell lysates (4 mg/ml) or an aliquot of IgG–Sepharose beads prepared as described above. The reactions (20–30 μl) were performed at 30°C for varying periods of time and terminated by the addition of 1 ml of ice cold stop buffer (20 mM Tris, pH 8.0, 25 mM MgCl2). The reaction mix was filtered on a nitrocellulose filter, washed three times with 3 ml of stop buffer, and the amount of label bound to Ypt1p was measured by scintillation counting (Jones et al. 1998). The protocol for assaying GTP uptake was the same with the following exceptions. Ypt1p at a final concentration of 200 nM was incubated with an aliquot of IgG–Sepharose beads, prepared as described above, and 400 nM of [35S]GTPγS (1,000 Ci/mmol; NEN Life Science Products), that was diluted to a specific activity of 25 Ci/mmol. Samples were incubated for the indicated periods of time at room temperature.
The increase in specific exchange activity that resulted as a consequence of the TRAPP purification was determined by estimating the [3H]GDP release activity/mg of protein in the lysate and comparing it to the [3H]GDP release activity/mg of protein on the beads.
Results
TRAPP Binds to the Nucleotide-free Form of Ypt1p
We previously noted interactions between YPT1 and the genes encoding two subunits of TRAPP, BET3 and BET5 (Rossi et al. 1995; Jiang et al. 1998). These genetic interactions suggested that Ypt1p and TRAPP may physically interact with each other. To address this possibility, in vitro binding experiments were performed with purified TRAPP. TRAPP was purified, using a protein A–tagged Bet3p fusion protein (see Materials and Methods), by absorbing the complex onto IgG–Sepharose beads as described previously (Sacher et al. 2000). Binding of Ypt1p to beads containing TRAPP, but not control beads that lack TRAPP, was observed when the beads were incubated with a clarified yeast lysate (data not shown). To determine the nucleotide state of the Ypt1p that bound to TRAPP, this small GTP-binding protein was fused to the carboxy terminus of GST (glutathione-S-transferase) and immobilized on glutathione Sepharose beads. GST–Ypt1p was then stripped of nucleotide (nucleotide-free form) or pre-loaded with either GDP (Ypt1p–GDP) or GTPγS, a nonhydrolyzable analogue of GTP (Ypt1p–GTP). As a control, GST alone was used. The beads were incubated with a lysate prepared from wild-type yeast cells and then probed with antibodies to TRAPP subunits to determine the binding of the different forms of Ypt1p. When Ypt1p was preloaded with either GDP or GTPγS (Fig. 1 A, lanes 3 and 4, respectively) small amounts of TRAPP, comparable to background levels (lane 1), bound to beads. However, when Ypt1p was first stripped of nucleotide, binding of TRAPP above background was observed (lane 2). Other tethering factors acting at this stage of the secretory pathway (Uso1p and Sec34p), did not bind to the nucleotide-free form of Ypt1p (data not shown).
To assess the specificity of the interaction between Ypt1p and TRAPP, we examined the interaction of TRAPP with another small GTPase, Ypt51p/Vps21p. Ypt51p/Vps21p mediates membrane traffic on the endosomal and/or vacuolar sorting pathways (Horazdovsky et al. 1994; Singer-Krüger et al. 1994). A GST–Ypt51p fusion protein was expressed in E. coli, purified on glutathione Sepharose beads and stripped of nucleotide. The beads were then incubated with a yeast lysate, washed, and probed with antibodies to TRAPP subunits. As seen in Fig. 1 B, the binding of TRAPP to GST–Ypt51p in its nucleotide-free state was comparable to the GST control (compare lanes 1 and 3), whereas binding to the nucleotide-free form of GST-Ypt1p was much stronger (compare lanes 2 and 3). We estimate that ∼0.4% of the total amount of TRAPP in the lysate bound to GST-Ypt1p in its nucleotide-free state (Fig. 1 B, compare 0.1% load with lane 2).
If the removal of nucleotide from Ypt1p irreversibly denatures the protein, binding of TRAPP to the nucleotide-free form of Ypt1p could be the result of a nonspecific interaction. To address this possibility, Ypt1p was stripped of nucleotide and then allowed to rebind GDP before it was added to the binding assay. GST–Ypt1p treated in this way showed a greatly reduced ability to bind TRAPP (Fig. 1 B, compare lanes 2 and 4). Thus, the interaction between the nucleotide-free form of Ypt1p and TRAPP is specific and not due to a nonspecific effect of irreversibly denaturating Ypt1p. These results indicate that Ypt1p and TRAPP physically interact with each other, and that TRAPP preferentially binds to the nucleotide-free state of Ypt1p.
An Extract Prepared from the bet3-1 Mutant Fails to Stimulate [3H]GDP Release from Ypt1p
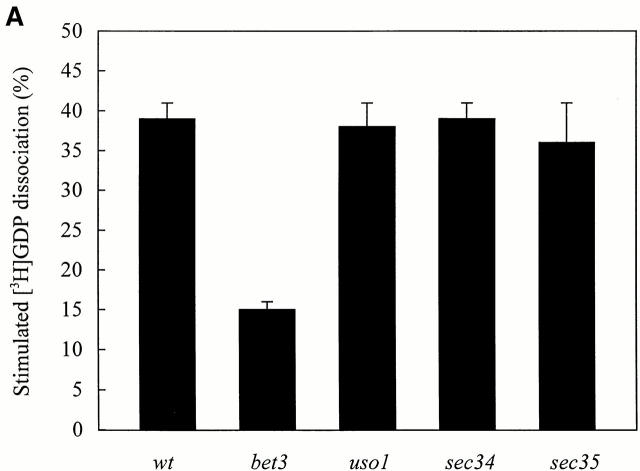
Guanine nucleotide exchange factors (GEF) bind with a higher affinity to the nucleotide-free form of the GTPase than to other forms of the small GTP-binding protein (Lai et al. 1993). The interaction between TRAPP and Ypt1p in its nucleotide-free state suggested that TRAPP may provide guanine nucleotide exchange activity for Ypt1p. To begin to address this possibility, we prepared extracts from various temperature-sensitive (ts) mutants that fail to tether vesicles to the Golgi and then tested their ability to displace GDP from Ypt1p. A mutant that harbors mutations in one of the TRAPP subunits, bet3-1, as well as mutants defective in other components of the tethering machinery, uso1-1, sec34-2, and sec35-1 were examined. Crude cell extracts of wild-type and the mutants were incubated at 30° or 37°C (restrictive temperature) with recombinant Ypt1p preloaded with [3H]GDP. The intrinsic rate of [3H]GDP displacment from Ypt1p was measured in the presence of BSA. Extracts of uso1-1, sec34-2, and sec35-1 stimulated [3H]GDP release from Ypt1p as well as wild type at both 30° and 37°C (Fig. 2a and Fig. b), indicating that exchange activity was not defective in these mutants. In contrast, the bet3-1 mutant was partially defective in the rate of [3H]GDP release from Ypt1p at 30°C (Fig. 2 A) and activity was almost completely abolished at 37°C (Fig. 2 B). These findings imply that TRAPP may displace GDP from Ypt1p.
Figure 2.
[3H]GDP release from Ypt1p in the presence of cell lysates prepared from ts mutants that block vesicle tethering. His6-Ypt1p preloaded with [3H]GDP was incubated with cell lysates for 10 min at 30°C (A) or 37°C (B) as described in the Materials and Methods. Lysates assayed at 37°C were pre-incubated for 5 min at 37°C immediately before the reaction was performed. The amount of [3H]GDP bound to His6-Ypt1p was measured using a filter binding assay (Jones et al. 1998). The data are expressed as the percent of radioactivity released from Ypt1p relative to the amount bound before the incubation. The average intrinsic rate of [3H]GDP release from Ypt1p was measured in the presence of BSA (4 mg/ml) and the value obtained (13.0 ± 2% at 30°C; 26.7 ± 4% at 37°C) was subtracted as background. The numbers reported are the average of three separate experiments.
TRAPP Stimulates Guanine Nucleotide Exchange on Ypt1p
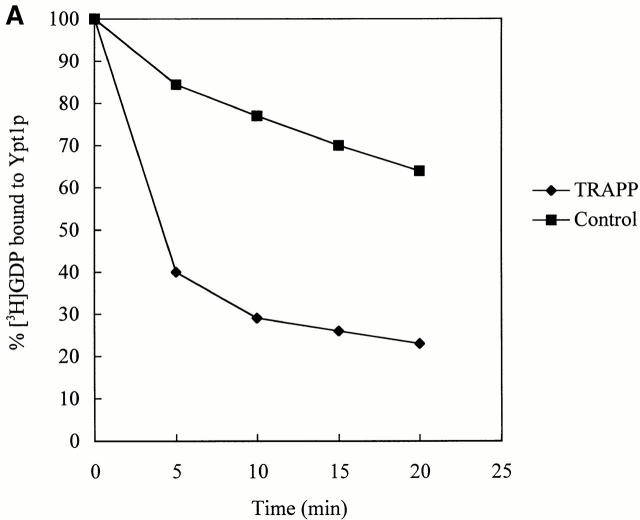
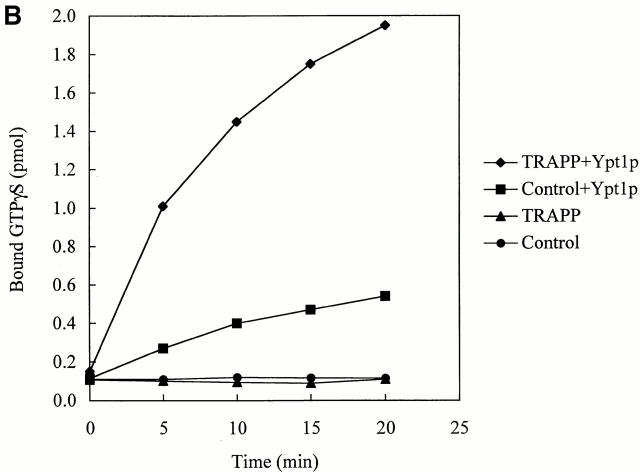
If TRAPP is an exchange factor for Ypt1p, it should stimulate the uptake of GTP onto Ypt1p as well as displace GDP. Because we were unable to measure GTP uptake in yeast extracts, we could not assess if bet3-1 was defective for this activity. Therefore, we purified the TRAPP complex and measured the ability of the purified complex to stimulate GTP uptake by Ypt1p. A strain (SFNY904; Sacher et al. 2000) in which the sole copy of Bet3p was fused at its carboxy terminus to protein A was used for the purification. Lysates prepared from SFNY904, as well as a strain that does not contain the fusion protein (SFNY823), were treated with IgG–Sepharose as described in the Materials and Methods. The beads were washed and incubated with Ypt1p and [35S]GTPγS or Ypt1p preloaded with [3H]GDP. Prebound [3H]GDP dissociated from Ypt1p with an intrinsic rate and [35S]GTPγS bound with a similar intrinsic rate (Fig. 3 A and B). Purified TRAPP potently stimulated both of these rates when compared with the controls. The uptake of [35S]GTPγS in the reaction was dependent on Ypt1p and not other small GTP-binding proteins that may have co-purified with TRAPP (Fig. 3 B, compare TRAPP ± Ypt1p). In addition, the stimulation of nucleotide exchange onto Ypt1p was found to be dependent on the concentration of TRAPP (data not shown). Since small GTPases purified from E. coli are in a complex with GDP (Poe et al. 1985), the uptake of [35S]GTPγS onto Ypt1p is a reflection of nucleotide exchange.
Figure 3.
TRAPP stimulates guanine nucleotide exchange on Ypt1p. (A) To measure [3H]GDP dissociation from Ypt1p, His6-Ypt1p was preloaded with [3H]GDP and then incubated for various times at 30°C with IgG–Sepharose beads containing TRAPP or lacking it (see Control). The beads were prepared as described in the Materials and Methods. For each time point, the data are expressed as the percent of label bound to Ypt1p compared with the zero time point. (B) To measure the uptake of [35S]GTPγS onto Ypt1p, IgG–Sepharose beads with TRAPP or without it (see control) were incubated at room temperature in the absence or presence of His6-Ypt1p plus [35S]GTPγS. Radioactivity bound to Ypt1p was measured at the indicated periods of time.
The binding of TRAPP to beads resulted in a 478-fold purification of the complex (0.23 pmol of TRAPP/mg of protein in the lysate verses 110 pmol of TRAPP/mg of protein on beads). When assayed in the presence of 3 pmol of [3H]GDP-Ypt1p, the specific exchange activity on the beads (70 pmol/min/mg) increased by 438-fold compared with the lysate (0.16 pmol/min/mg). The finding that the fold purification of TRAPP is approximately the same as the increase in the specific exchange activity, implies that TRAPP must be the exchange factor for Ypt1p. Our findings also indicate that TRAPP acts catalytically, as 0.55 pmol of TRAPP in the exchange assay displaced 2.81 pmol of GDP in 5 min at 30°C from 4.5 pmol of [3H]GDP-Ypt1p. This number is likely to be an underestimate of the catalytic activity of TRAPP, as we have found that the specific exchange activity of lysates is 2.5-fold lower when Bet3p is fused to protein A.
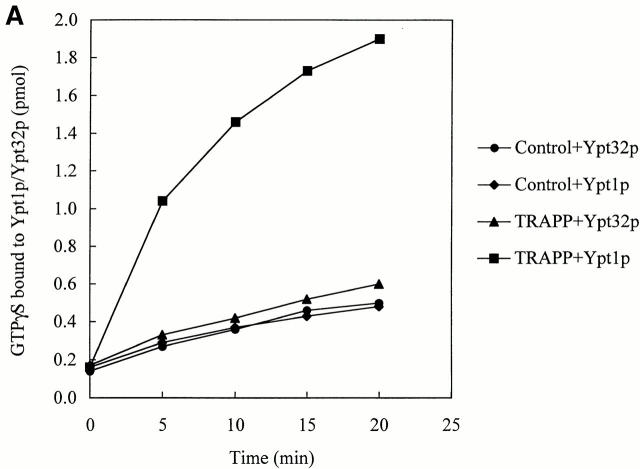
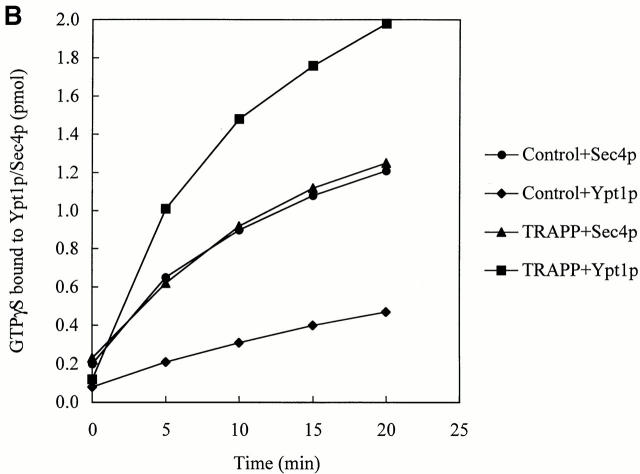
We addressed the substrate specificity of TRAPP, relative to other Rabs, and found that it did not stimulate nucleotide exchange on Ypt32p (Fig. 4 A) and Sec4p (Fig. 4 B). These small GTPases are required for the budding of vesicles from the trans-Golgi and for post-Golgi membrane traffic in yeast, respectively (Salminen and Novick 1987; Benli et al. 1996; Jedd et al. 1997). Thus, guanine nucleotide exchange onto Ypt1p is potently and specifically catalyzed by TRAPP.
Figure 4.
TRAPP does not stimulate the uptake of [35S]GTPγS onto Ypt32p or Sec4p. Equal amounts of His6-Ypt32p (A), or His6-Sec4p (B) and His6-Ypt1p were incubated with [35S]GTPγS at room temperature with IgG–Sepharose beads, containing TRAPP or lacking it (see Control) exactly as described above for His6-Ypt1p. At the indicated periods of time, the radioactivity bound to protein was measured. Note that Sec4p has a high intrinsic exchange rate as previously reported (Kabcenell et al. 1990).
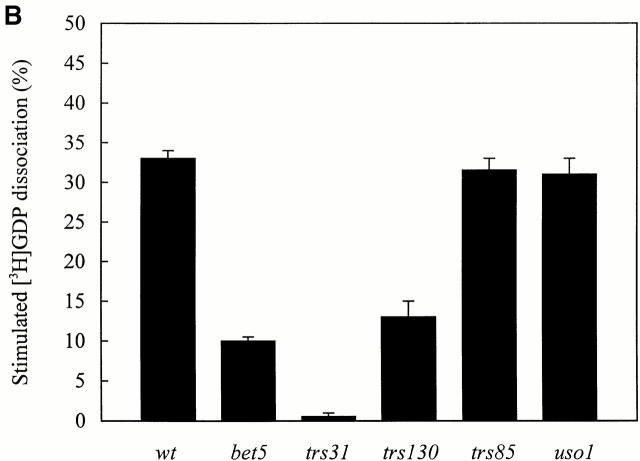
Extracts Prepared from Mutants with Defects in Different TRAPP Subunits Have a Reduced Ability to Stimulate the Release of [3H]GDP from Ypt1p
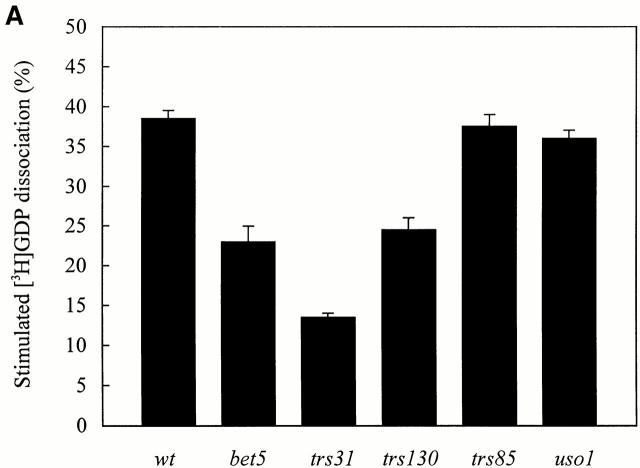
TRAPP contains 10 subunits (Sacher et al. 1998, Sacher et al. 2000), one of which may have exchange activity. Two approaches were used to assess this possibility. First, extracts were prepared from ts mutants (bet3-1, bet5-1, trs31-1, and trs130ts2) with defects in different TRAPP subunits and assayed for their ability to stimulate [3H]GDP release from Ypt1p. Second, recombinant forms of each of the 10 subunits were expressed in E. coli and assayed for exchange activity.
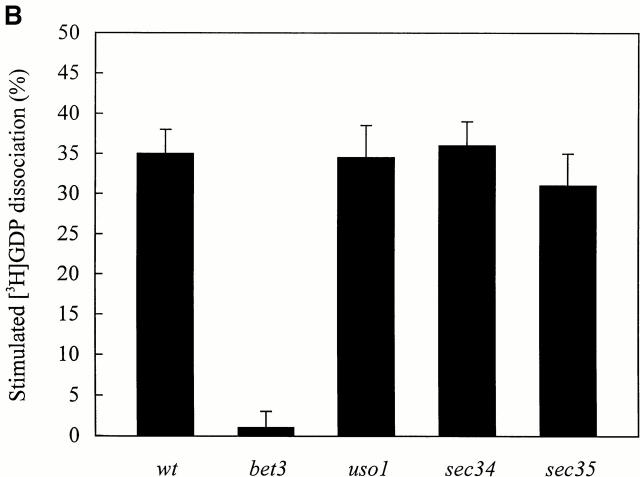
Wild-type and ts mutant extracts were incubated with Ypt1p that was preloaded with [3H]GDP and assayed for release activity at 30° and 37°C as described above. As was found for bet3-1, extracts prepared from the bet5-1, trs31-1, and trs130ts2 mutants were partially defective for activity at 30°C (Fig. 5 A) and more dramatically affected at 37°C (Fig. 5 B). As a control, we also assayed a lysate prepared from trs85Δ. Whereas BET3, BET5, TRS31, and TRS130 are essential for the vegetative growth of yeast, TRS85 is dispensable (Sacher et al. 2000). As anticipated, the absence of Trs85p did not lead to a loss of activity at either 30°C (Fig. 5 A) or 37°C (Fig. 5 B).
Figure 5.
[3H]GDP release from Ypt1p in the presence of cell lysates prepared from ts mutants that are defective in different TRAPP subunits. His6-Ypt1p preloaded with [3H]GDP was incubated with cell lysates for 10 min at 30°C (A) or 37°C (B) as described in the Materials and Methods. Lysates assayed at 37°C were pre-incubated for 5 min at 37°C immediately before the reaction was performed. The average intrinsic rate of [3H]GDP release from Ypt1p was measured in the presence of BSA (4 mg/ml) and the value obtained (15.5 ± 2% at 30°C; 28.0 ± 2% at 37°C) was subtracted as background. The numbers reported are the average of two separate experiments.
Our analysis of lysates prepared from mutants with defects in TRAPP subunits suggests that the overall conformation of the complex may be important for exchange activity. Nonetheless, in search of the subunit that stimulates the exchange of nucleotide onto Ypt1p, we expressed recombinant forms of the different subunits in E. coli. Initially, each of the subunits were expressed with a six histidine tag (His6). However, as some of the subunits were not expressed well or were completely insoluble, we found it necessary to use other tags. Each subunit was then purified by affinity chromatography, using either nickel agarose (for His6-tagged subunits), amylose resin (MBP-tagged subunits), or glutathione Sepharose beads (for GST-tagged subunits) and assayed on beads or in solution. Since the experiments described above were performed with ∼400 ng of purified TRAPP, a minimum of 400 ng of each recombinant subunit was assayed for exchange activity. None of the subunits, however, displayed such an activity (data not shown).
Discussion
Ypt1p localizes to the ER, Golgi and ER-to-Golgi transport vesicles (Segev et al. 1988; Segev 1991; Lian and Ferro-Novick 1993; Cao and Barlowe 2000), whereas TRAPP persistently localizes to the Golgi (Sacher et al. 1998; Barrowman et al. 2000). Here we show that TRAPP preferentially binds to the nucleotide-free form of Ypt1p. Furthermore, purified TRAPP stimulates both GDP release and GTP uptake onto Ypt1p. These data indicate that TRAPP acts as a GEF for Ypt1p and imply that TRAPP acts upstream of this small GTP-binding protein.
The observation that TRAPP is the GEF for Ypt1p is consistent with an earlier report that described a specific exchange activity for Ypt1p in a P100 fraction that is enriched for Golgi membranes (Jones et al. 1998). Our findings are also consistent with in vitro transport studies showing that Ypt1p acts on the acceptor compartment (Bacon et al. 1989; Cao and Barlowe 2000). Since Ypt1p is present on ER-derived vesicles but not activated until it encounters TRAPP on the Golgi, we propose it is the GDP-bound, or inactive form, of Ypt1p that resides on this transport intermediate.
Exchange factors for other Rabs have been identified (Horiuchi et al. 1997; Walch-Solimena et al. 1997). Sec2p is the exchange factor for Sec4p, the closest yeast homologue of Ypt1p (Salminen and Novick 1987). The activation of Sec4p by Sec2p is coupled to the polarized delivery of vesicles to the growing bud tip that is the site for exocytosis (Walch-Solimena et al. 1997). Rabex-5 is the GEF for Rab5. It forms a complex with the Rab5 effector, Rabaptin-5. The Rabex-5/Rabaptin-5 complex is essential for endocytic membrane fusion (Horiuchi et al. 1997). Sec2p, Rabex 5, and its yeast homologue Vps9p (Hama et al. 1999), however, do not share homology with any of the 10 TRAPP subunits.
ts mutants in 4 of the 10 genes that encode TRAPP subunits (bet3-1, bet5-1, trs31-1, and trs130ts2) have been isolated (Rossi et al. 1995; Jiang et al. 1998; Sacher, M., manuscript in preparation). Extracts prepared from these mutants displayed ts defects in their ability to stimulate the release of GDP from Ypt1p. Some of these mutations disrupt the complex (bet3-1 and bet5-1), while others do not (trs130ts2) (Barrowman, J., and S. Ferro-Novick, unpublished observations). Thus, the conformation of TRAPP may be important for stimulating nucleotide exchange on Ypt1p. Consistent with this hypothesis, we have found that purified recombinant forms of each of the TRAPP subunits do not accelerate the release of GDP from Ypt1p or stimulate the uptake of GTP. Alternatively, one or more subunits may be required to stabilize the component(s) of the complex that has exchange activity. The observation that exchange activity is almost completely abolished in the bet3-1 and trs31-1 mutants implies that TRAPP is the major exchange factor for Ypt1p.
Previous findings have shown that a dominant mutation in SLY1 (SLY1-20), which encodes a soluble NSF attachment protein receptor–associated protein, can bypass the loss of YPT1 (Dascher et al. 1991). The finding that the loss of TRAPP cannot be bypassed by SLY1-20 (Barrowman et al. 2000) implies that, in addition to stimulating nucleotide exchange on Ypt1p, TRAPP has other roles. Nonetheless, the identification of TRAPP as the GEF for Ypt1p has begun to shed light on the molecular mechanism by which TRAPP tethers/targets vesicles to the Golgi.
Acknowledgments
We thank Li-Lin Du for the Ypt1p expression vector, purified Sec4p and Ypt32p, as well as many useful discussions. We also thank Lora Cavallo and Yueyi Zhang for constructing the trs31-1 and trs85Δ mutants, Birgit Singer-Krüger for the YPT-GST expression vectors, N. Barry Elkind for technical advice on nucleotide exchange assays, Li-Lin Du and Chave Carr for a critical reading of the manuscript, and Joyce Anquillare for assistance in the preparation of this manuscript.
W. Wang is supported as an Associate of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper: GEF, guanine nucleotide exchange factor; GST, glutathione-S-transferase; His6, six histidine tag; ts, temperature-sensitive.
References
- Bacon R.A., Salminen A., Ruohola H., Novick P., Ferro-Novick S. The GTP-binding protein Ypt1 is required for transport in vitrothe Golgi apparatus is defective in ypt1 mutants. J. Cell Biol. 1989;109:1015–1022. doi: 10.1083/jcb.109.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell. Biol. 1997;139:1097–1108. doi: 10.1083/jcb.139.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman J., Sacher M., Ferro-Novick S. TRAPP stably associates with the Golgi and is required for vesicle docking. EMBO J. 2000;19:862–869. doi: 10.1093/emboj/19.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benli M., Doring F., Robinson D. G., Yang X., Gallwitz D. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Cao X., Barlowe C. Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J. Cell Biol. 2000;149:55–65. doi: 10.1083/jcb.149.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Ossig R., Gallwitz D., Schmitt H.D. Identification and structure of four yeast genes (SLY1) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell Biol. 1991;11:872–875. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Collins R.N., Novick P.J. Identification of a Sec4p GTPase- activating protein (GAP) as a novel member of a Rab GAP family. J. Biol. Chem. 1998;273:3253–3256. doi: 10.1074/jbc.273.6.3253. [DOI] [PubMed] [Google Scholar]
- Hama H., Tall G. G., Horazdovsky B.F. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J. Biol. Chem. 1999;274:15284–15291. doi: 10.1074/jbc.274.21.15284. [DOI] [PubMed] [Google Scholar]
- Horazdovsky B.F., Busch G.R., Emr S.D. VPS21 encodes a rab5-like GTP binding protein that is required for the sorting of yeast vacuolar proteins. EMBO J. 1994;13:1297–1309. doi: 10.1002/j.1460-2075.1994.tb06382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H., Lippe R., McBride H.M., Rubino M., Woodman P., Stenmark H., Rybin V., Wilm M., Ashman K., Mann M., Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Jedd G., Mulholland J., Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 1997;5:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Scarpa A., Zhang L., Stone S., Feliciano E., Ferro-Novick S. A high copy suppressor screen reveals genetic interactions between BET3 and a new gene. Evidence for a novel complex in ER-to-Golgi transport. Genetics. 1998;149:833–841. doi: 10.1093/genetics/149.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Litt R.J., Richardson C.J., Segev N. Requirement of nucleotide exchange for Ypt1p GTPase mediated protein transport. J. Cell Biol. 1995;130:1051–1061. doi: 10.1083/jcb.130.5.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Richardson C.J., Litt R.J., Segev N. Identification of regulators for Ypt1p GTPase nucleotide cycling. Mol. Biol. Cell. 1998;9:2819–2837. doi: 10.1091/mbc.9.10.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabcenell A. K., Goud B., Northup J. K., Novick P. J. Binding and hydrolysis of guanine nucleotides by Sec4p, a yeast protein involved in the regulation of vesicular traffic. J. Biol. Chem. 1990;265:9366–9372. [PubMed] [Google Scholar]
- Kim D.W., Sacher M., Scarpa A., Quinn A.M., Ferro-Novick S. High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol. Biol. Cell. 1999;10:3317–3329. doi: 10.1091/mbc.10.10.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Boguski M., Broek D., Powers S. Influence of guanine nucleotides on complex formation between Ras and CDC25 proteins. Mol. Cell. Biol. 1993;13:1345–1352. doi: 10.1128/mcb.13.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J.P., Ferro-Novick S. Bos1p, an integral membrane protein of the endoplasmic reticulum to Golgi transport vesicles, is required for their fusion competence. Cell. 1993;73:735–745. doi: 10.1016/0092-8674(93)90253-m. [DOI] [PubMed] [Google Scholar]
- Martinez O., Goud B. Rab proteins. Biochim. Biophys. Acta. 1998;1404:101–112. doi: 10.1016/s0167-4889(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Novick P., Zerial M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Poe M., Scolnick E.M., Stein R.B. Viral harvey ras p21 expressed in Escherichia coli purifies as a binary one-to-one complex with GDP. J. Biol. Chem. 1985;260:3906–3909. [PubMed] [Google Scholar]
- Rossi G., Kolstad K., Stone S., Palluault F., Ferro-Novick S. BET3 encodes a novel hydrophilic protein that acts in conjunction with yeast SNAREs. Mol. Biol. Cell. 1995;6:1769–1780. doi: 10.1091/mbc.6.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M., Jiang Y., Barrowman J., Scarpa A., Burston J., Zhang L., Schieltz D., Yates J.R., III, Abeliovich H., Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M., Barrowman J., Schieltz D., Yates J.R., III, Ferro-Novick S. Identification and characterization of five new subunits of TRAPP. Eur. J. Cell Biol. 2000;79:71–80. doi: 10.1078/S0171-9335(04)70009-6. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Scheffzek K., Ahmadian M.R., Wittinghofer A. GTPase-activating proteinshelping hands to complement an active site. TIBS. 1998;23:257–262. doi: 10.1016/s0968-0004(98)01224-9. [DOI] [PubMed] [Google Scholar]
- Segev N. Mediation of the attachment or fusion step in vesicular transport by the GTP-binding Ypt1p protein. Science. 1991;252:1553–1556. doi: 10.1126/science.1904626. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding Ypt1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988;52:915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Singer-Krüger B., Stenmark H., Dusterhoft A., Philippsen P., Yoo J. S., Gallwitz D., Zerial M. Role of three rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J. Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen S.M., Cao X., Sapperstein S.S., Chiang E.C., Lupashin V.V., Barlowe C., Waters M.G. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J.Cell Biol. 1999;147:729–742. doi: 10.1083/jcb.147.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C., Collins R. N., Novick P. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]