Abstract
In yeast, assembly of exocytic soluble N-ethylmaleimide–sensitive fusion protein (NSF) attachment protein receptor (SNARE) complexes between the secretory vesicle SNARE Sncp and the plasma membrane SNAREs Ssop and Sec9p occurs at a late stage of the exocytic reaction. Mutations that block either secretory vesicle delivery or tethering prevent SNARE complex assembly and the localization of Sec1p, a SNARE complex binding protein, to sites of secretion. By contrast, wild-type levels of SNARE complexes persist in the sec1-1 mutant after a secretory block is imposed, suggesting a role for Sec1p after SNARE complex assembly. In the sec18-1 mutant, cis-SNARE complexes containing surface-accessible Sncp accumulate in the plasma membrane. Thus, one function of Sec18p is to disassemble SNARE complexes on the postfusion membrane.
Keywords: NSF, membrane fusion, SNAREs, exocyst, Sec1
Introduction
The secretory pathway carries newly synthesized integral membrane and secretory proteins from the ER to the cell surface. This pathway involves a series of vesicle budding and fusion events to carry cargo forward, and it is coordinated with a retrograde transport pathway that serves to recycle membrane components and soluble resident proteins. Each stage of transport involves several steps. First, proteins are collected into a transport compartment that buds from a donor membrane. Second, the transport compartment is transported to an acceptor site. Finally, the transport compartment is tethered to an appropriate target organelle, and the two membranes fuse.
Molecular dissection of membrane trafficking in yeast began with the identification of temperature-sensitive sec mutants defective in secretion (Novick and Schekman 1979; Novick et al. 1980). In 10 complementation groups of these sec mutants, there is an accumulation of 100-nm secretory vesicles containing fully processed invertase (Novick et al. 1981). The proteins encoded by the corresponding late-acting SEC genes were therefore deduced to function in the fusion of post-Golgi secretory vesicles with the plasma membrane. Additional proteins required for exocytic fusion are general factors that also function at several other membrane transport steps, including transport through the early secretory pathway (Novick et al. 1981).
Yeast secretory vesicles derived from the trans-Golgi network are transported along actin cables to exocytic sites at the tips of newly emerging buds and at mother–daughter necks in dividing cells (Finger and Novick 1998). Mutations that inhibit vesicle transport result in secretory vesicle accumulation in the mother cell rather than in the bud. One protein required for vesicle transport is Sec2p, the nucleotide exchange factor for Sec4p (Walch-Solimena et al. 1997). Activated GTP-Sec4p is thought to promote myosin (Myo2)-dependent motility of secretory vesicles to exocytic sites (Govindan et al. 1995; Schott et al. 1999).
After secretory vesicles have been transported to the bud, a complex of proteins known as the exocyst is required for an event leading to fusion with the plasma membrane. The exocyst contains six late-acting Sec proteins, Sec3p, Sec5p, Sec6p, Sec8p, Sec10p, and Sec15p, and two other proteins essential for exocytosis, Exo70p and Exo84p (TerBush et al. 1996; Guo et al. 1999a). One exocyst component, Sec15p, can bind to GTP-Sec4p on secretory vesicles (Guo et al. 1999b). Another exocyst component, Sec3p, is localized at exocytic sites even in the absence of membrane traffic (Finger et al. 1998). Thus, assembly of the exocyst may tether secretory vesicles to the plasma membrane. The interaction of at least two exocyst components, Sec15p and Exo70p, with activated GTPases suggests that the exocyst may also be a regulator of secretion that integrates cellular signaling pathways (Adamo et al. 1999; Guo et al. 1999b; Robinson et al. 1999).
Soluble N-ethylmaleimide–sensitive fusion protein (NSF) attachment protein receptors (SNAREs) are required at the membrane fusion step of most, if not all, intracellular transport events (Ferro-Novick and Jahn 1994; Rothman and Warren 1994). For fusion to occur, a trans-SNARE complex must assemble between SNARE proteins integrated in the two fusing membranes (Nichols et al. 1997; Weber et al. 1998). SNARE proteins within a single membrane can form cis-SNARE complexes (Otto et al. 1997; Swanton et al. 1998; Ungermann et al. 1998a). These cis-complexes may represent intermediates in the fusion mechanism (Price et al. 2000a), nonproductive intermediates or remnants of prior fusion events. The SNAREs for yeast exocytosis are Sncp on secretory vesicles and Ssop and Sec9p on the plasma membrane (Aalto et al. 1993; Protopopov et al. 1993; Brennwald et al. 1994). The Snc proteins are encoded by two functionally redundant genes: SNC1 and SNC2. Similarly, SSO1 and SSO2 are functionally redundant genes that encode Sso proteins. There are a large number of proteins in the SNARE family, and individual family members are concentrated on distinct classes of transport vesicles and organelles within the cell (Ferro-Novick and Jahn 1994; Rothman and Warren 1994). This diversity suggested that SNAREs may regulate the specificity of membrane fusion. Current evidence, however, indicates that the interactions between SNARE proteins are promiscuous (Gotte and von Mollard 1998; Grote and Novick 1999; Yang et al. 1999).
Structural and biochemical data suggest that trans-SNARE complexes catalyze the merger of lipid bilayers during intracellular membrane fusion. SNARE proteins assemble into a four-stranded, parallel α-helical bundle with two or more transmembrane segments protruding from its COOH terminus (Hanson et al. 1997; Lin and Scheller 1997; Sutton et al. 1998). Topological similarity between the structures of assembled SNARE complexes and the fusion active conformation of viral fusion proteins supports the model that SNARE complexes are the fusion proteins for intracellular fusion (Skehel and Wiley 1998; Hughson 1999). In fact, membrane fusion has been reconstituted with purified SNARE proteins incorporated into liposomes (Weber et al. 1998). However, it has also been reported that disassembly of trans-SNARE complexes does not prevent subsequent membrane fusion (Coorssen et al. 1998; Ungermann et al. 1998b).
Sec1p, another factor required for exocytosis, binds to assembled exocytic SNARE complexes, but not to free Ssop (Carr et al. 1999). Green fluorescent protein (GFP)-Sec1p is concentrated at sites of secretion, and this localization correlates with the abundance of SNARE complexes in two mutant strains: sec4-8, a SNARE complex–assembly mutant, and sec18-1, a SNARE complex–disassembly mutant. Based on these observations, we proposed that Sec1p stimulates fusion after SNARE complex assembly or regulates the fidelity of SNARE complex interactions (Carr et al. 1999). In contrast to yeast Sec1p, a rat brain Sec1p homologue has been proposed to regulate SNARE complex assembly because it binds with nanomolar affinity to the plasma membrane SNARE syntaxin, but not to assembled SNARE complexes (Pevsner et al. 1994; Yang et al. 2000). Since loss of function point mutations in ROP, a Drosophila Sec1p homologue, can either enhance or inhibit synaptic vesicle fusion, it is possible that Sec1 proteins have both positive and negative regulatory roles in membrane fusion (Wu et al. 1998). One of these functions may be the transmission of signals from Rab proteins since an activating mutation in Sly1p, the Sec1 homologue for ER to Golgi transport in yeast, suppresses a deletion of the Rab GTPase Ypt1p (Dascher et al. 1991; Ossig et al. 1991).
Sec18p, the yeast homologue of NSF, binds to SNARE complexes in the presence of its partner Sec17p (Sollner et al. 1993b; Ungermann et al. 1998a). A temperature-sensitive mutation in Sec18p results in inhibition of membrane transport at several stages of the secretory pathway after shifting to 37°C (Graham and Emr 1991). NSF has ATP-dependent SNARE complex–disassembly activity in vitro (Sollner et al. 1993a). It was originally proposed that disassembly of SNARE complexes might trigger fusion by exposing the amphipathic helicies of v- and t-SNAREs and thereby destabilizing the opposing membranes. More recently, Sec18p/NSF has been shown to act at a priming stage before membrane fusion in homotypic vacuolar fusion, chromaffin granule exocytosis, and synaptic transmission (Banerjee et al. 1996; Mayer et al. 1996; Kawasaki et al. 1998; Littleton et al. 1998; Schweizer et al. 1998; Xu et al. 1999). Experiments using an in vitro assay for vacuolar fusion suggest that disassembly of intravacuolar cis-SNARE complexes by Sec18p frees SNARE proteins to assemble into trans-SNARE complexes bridging two vacuoles (Ungermann et al. 1998a). Thus, after NSF is inactivated, there is an activity-dependent delay before membrane fusion is blocked that may correspond to the fusion of membranes containing previously primed SNAREs (Kawasaki et al. 1998; Littleton et al. 1998; Schweizer et al. 1998; Sanyal et al. 1999; Xu et al. 1999). In addition to its interaction with SNAREs, NSF also has an ATPase-independent activity necessary for fusion of postmitotic Golgi vesicles (Muller et al. 1999).
We have quantified the binding of Ssop to Sncp in sec mutant strains in order to incorporate SNARE complex assembly and disassembly into the sequence of events occurring in the later stages of the exocytic pathway. Our results suggest that Sec2p and the exocyst are required before SNARE complex assembly, whereas Sec1p acts after SNARE complex assembly. In addition, we have found that cis-SNARE complexes containing Sncp in the plasma membrane accumulate in a sec18-1 mutant. This result demonstrates that SNARE complexes are disassembled by Sec18p after membrane fusion.
Materials and Methods
Strains and Growth Conditions
The strains used in this study are listed in Table . The snc1Δ snc2Δ strain NY2201 is a spontaneous revertant of JG8 (Protopopov et al. 1993; David et al. 1998) that grows on YPD and no longer carries the GAL1p-TSNC1 balancer plasmid. The HA-SSO2, myc-SEC1, GFP-SEC1, and Gal1p-SNC2-HA strains in sec mutant backgrounds were constructed by standard genetic crosses from previously described strains (Abeliovich et al. 1998; Carr et al. 1999; Grote and Novick 1999). Although the expression level of GFP-Sec1p is similar to wild-type in the temperature-sensitive exocyst mutants, expression of GFP-SEC1 as the sole copy of SEC1 slightly lowers the restrictive temperature. Cells expressing hemagglutinin (HA)-Snc2p from the GAL1 promotor were grown first in YP/2% raffinose/0.75% galactose overnight and then shifted for 1 h to YPD. Under these conditions, the cells grow rapidly, and the expression level of HA-Snc2p is similar to the expression level of the Snc proteins in wild-type cells (Abeliovich et al. 1998).
Table 1.
Strains Used in this Study
| Strain | Genotype |
|---|---|
| NY13 | MATα ura3-52 |
| NY2201 | MATα snc1::URA3 snc2::ADE8 sup ura3 ade8 leu2 trp1 his |
| NY130 | MATα sec2-41 ura3-52 |
| NY402 | MATα sec5-25 ura3-52 |
| NY7 | MATα sec6-4 ura3-52 |
| NY47 | MATα sec8-6 ura3-52 |
| NY410 | MATα sec8-9 ura3-52 |
| NY61 | MATα sec10-2 ura3-52 |
| NY64 | MATα sec15-1 ura3-52 |
| NY3 | MATα sec1-1 ura3-52 |
| NY431 | MATα sec18-1 ura3-52 |
| NY1642 | MATα snc1::URA3 HA-SNC2 (Gal1p-HA-SNC2-LEU2) ura3-52 leu2-3,112 |
| NY1643 | MATα snc1::URA3 HA-SNC2 (Gal1p-HA-SNC2-LEU2) sec18-1 ura3-52 leu2-3,112 |
| NY1699 | MATα sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 |
| NY2214 | MATα sec3-2 sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 |
| NY2215 | MATα sec5-24 sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 |
| NY2216 | MATα sec6-4 sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 |
| NY2217 | MATα sec8-6 sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 |
| NY2218 | MATα sec8-9 sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 |
| NY2219 | MATα sec10-12 sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 |
| NY2220 | MATα sec15-1 sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 |
| NY2222 | MATα sec2-41 sec1::SEC1-GFP-URA3 leu2-3,112 ura3-52 his4-619 |
| NY1689 | MATα sec1::SEC1(MYC)3URA3 ura3-52 leu2-3,112 trp1 his3-Δ200 |
| NY2227 | MATα sec3-2 sec1::SEC1(MYC) 3 URA3 ura3-52 |
| NY2228 | MATα sec5-24 sec1::SEC1(MYC) 3 URA3 ura3-52 |
| NY2223 | MATα sec6-4 sec1::SEC1(MYC) 3 URA3 ura3-52 |
| NY2226 | MATα sec8-9 sec1::SEC1(MYC) 3 URA3 ura3-52 |
| NY2224 | MATα sec10-2 sec1::SEC1(MYC) 3 URA3 ura3-52 |
| NY2224 | MATα sec15-1 sec1::SEC1(MYC) 3 URA3 ura3-52 |
Quantitative SNARE Complex Coimmunoprecipitation Assay
Lysis, immunoprecipitation, and Western blotting conditions have been described previously (Grote and Novick 1999). In brief, 30 A 600 units of yeast cells were collected and washed with ice-cold TAF (20 mM Tris, pH 7.5, 20 mM NaN3, 20 mM NaF) to deplete intracellular ATP and arrest membrane traffic. A cleared lysate was prepared in HKDNE buffer (20 mM Hepes, 150 mM KCl, 1 mM DTT, 0.5% IGPAL [NP-40], 1 mM EDTA) by homogenization with zirconium beads in a mini bead-beater (BioSpec Products). Sncp was immunoprecipitated using anti-Sncp serum, and the coprecipitating Ssop was detected on a Western blot with biotinylated anti-Ssop antibodies and streptavidin-HRP. The amounts of Sncp and Ssop in each lysate were routinely examined by Western blotting, and their expression level in all of the sec mutant strains was equal to their expression level in wild-type yeast. Band intensities were quantified by densitometry using a scanning densitometer (Bio-Rad Laboratories) and NIH Image software. A standard curve was prepared from dilutions of the lysate to identify the linear range of the assay and to permit calculation of the percentage of total Ssop bound to Sncp. Procedures for the anti-HA and anti-myc immunoprecipitations have also been described previously (Carr et al. 1999).
Secretion Rate of 35S-labeled Proteins
Secretion of 35S-labeled proteins was measured by a modification of the method of Gaynor and Emr 1997. For each sample, 1.5 A 600 units of yeast cells grown to early log phase in synthetic complete medium without methionine were resuspended in 400 μl of methionine-free medium supplemented with 150 μCi 35S-ProMix (Amersham Pharmacia Biotech) and 0.06 mg/ml BSA, and labeled continuously at 37°C or or 38°C, or for 5 min at 25°C as indicated. The chase medium, when indicated, was synthetic complete medium supplemented with 7 mg/ml methionine, 4 mg/ml cysteine, and 0.06 mg/ml BSA prewarmed to 37°C. At the indicated times, cells were removed by centrifugation for 5 s in a microfuge. 300 μl medium was transferred to an ice-cold tube containing 30 μl of 500 mM NaN3, 500 mM NaF. Any remaining cells were removed by a second microfuge spin for 1 min at 14,000 g, and 300 μl of the supernatant was transferred to a tube containing 20 μl 100% TCA, 1 mg/ml deoxycholate and incubated for at least 30 min on ice. The TCA-precipitated proteins were washed twice with acetone at −20°C, air dried, and resuspended in Laemmli sample buffer. The total cell lysates were prepared by TCA precipitation and acetone washing of cells, followed by homogenization with glass beads in Laemmli sample buffer. Samples were boiled for 5 min, electrophoresed on an 8% acrylamide gel, and exposed to a phosphor screen for quantitation using a Storm® system (Molecular Dynamics).
GFP-Sec1p Fluorescence
Strains expressing GFP-Sec1p were grown overnight at 25°C in selective medium, and then for 2 h in 5 ml YPD to a final concentration of 0.2–0.5 A 600. Samples grown at 25°C, or after a 10-min shift to 37°C, were washed and fixed using the methanol/acetone method, as described previously (Carr et al. 1999). Variability in the number of cells with localized GFP-Sec1p is likely due to the labile nature of SNARE complexes, because washing the cells under ATP-depletion conditions before fixation helps to reduce disassembly of SNARE complexes, and this step is required to ensure detection of polarized localization (Carr et al. 1999). A small volume of fixed cells was pressed very flat before viewing. This step was required to visualize GFP-Sec1p localization simultaneously in individual cells. Images were obtained on a ZEISS Axiophot 2 microscope equipped with a Quantix CCD camera (Photometrics), a fluorescein filter (FITC, excitation 480 nm, emission 535 nm, dichroic BS 505), 100× objective (1.3 N.A.), and IPLab software. Cells were counted positive for polarized localization of GFP-Sec1 if fluorescence was detected in the bud of small budded cells or at the mother–daughter neck of large budded cells. Percent localization is calculated as 100 times the number of positives divided by the total number of cells counted.
Cell Fractionation
20 A 600 units of sec18-1 cells were shifted to 37°C for 10 min, chilled to 4°C, and lysed with acid-washed glass beads in 600 μl detergent-free IP buffer (20 mM Hepes, pH 7.4, 150 mM KCl, 1 mM DTT, 1 mM EDTA, 1 mM PMSF, 1 μM pepstatin A). The lysate was cleared of cellular debris by centrifugation for 30 s at 1,000 g and then fractionated into pellet and supernatant fractions by centrifugation for 10 min at 10,000 g. NP-40 was added to the supernatant to a final concentration of 0.5%. The pellet was resuspended in 600 μl complete IP buffer with 0.5% NP-40. A 30-μl aliquot of each sample was reserved to examine the intracellular distribution of Sncp and Ssop. The remainder was immunoprecipitated with anti-Sncp antibody.
Surface Iodination
Cell-surface proteins were iodinated by a modification of the method of Payne and Schekman 1989. In brief, 20 A 600 units of cells were resuspended in 300 μl PBS, 1 mM EDTA, incubated with three iodobeads (Pierce Chemical Co.) and 300 μCi Na125I for 15 min on ice, washed twice with PBS/EDTA, then processed for immunoprecipitation.
Results
Exocytic SNARE Complex Coimmunoprecipitation
To measure exocytic SNARE complexes, Ssop bound to Sncp was detected by probing a Western blot of an anti-Sncp immunoprecipitate with antibodies against Ssop (Grote and Novick 1999). The amount of Ssop in the immunoprecipitate was quantified by densitometry and compared with a standard curve prepared by diluting total cell lysate to calculate the percentage of total Ssop bound to Sncp. Only 0.7% of the Ssop from wild-type (NY13) cells grown at 25°C coprecipitates with Sncp (data not shown). This small percentage is consistent with expectations for a complex that assembles transiently during exocytosis, especially considering that Ssop is distributed over the entire plasma membrane (Brennwald et al. 1994) whereas fusion occurs only at specific exocytic sites. The coimmunoprecipitation assay is specific for SNARE complexes that assemble during yeast exocytosis by three criteria. First, if the assay is performed with a snc deletion strain (NY2201), there is a 20-fold reduction in the amount of Ssop in the precipitate. Second, exocytic SNARE complexes must exist in the yeast cells before lysis because HA-Snc2p and Myc-Sso1p do not coprecipitate if the tagged proteins are expressed in different populations of cells that are lysed together (Carr et al. 1999). Third, there is a significant reduction in the amount of Ssop bound to Sncp if transport through the early secretory pathway is inhibited by temperature-sensitive sec mutations that interfere with budding from the ER or Golgi complex (Grote and Novick 1999).
The amount of Ssop bound to Sncp is influenced by the growth rate. If wild-type cells are grown in glycerol (a nonfermentable carbon source) instead of glucose, their doubling time increases from 2.1 to 7.3 h and there is a 60% reduction in the amount of Ssop bound to Sncp (data not shown). This effect is likely to result from a reduction in the secretion rate, because a major function of the secretory pathway is to deliver membrane and cell wall components needed for growth. Because of the positive correlation between the amount of exocytic SNARE complexes and the growth rate, mutant strains that grow slowly at their permissive temperature were avoided in this study.
Exocytic SNARE Complex Assembly Requires Vesicle Transport
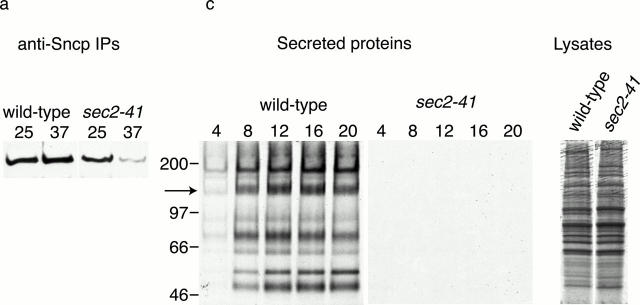
We have previously reported that exocytic SNARE complexes do not assemble at 37°C in sec4-8 mutant yeast. Therefore, we proposed that the Sec4p Rab-GTPase acts upstream of SNARE complex assembly (Grote and Novick 1999). To determine whether Sec4p must be in its active, GTP-bound conformation to promote SNARE complex assembly, we measured the binding of Ssop to Sncp in the sec2-41 mutant. In this strain, deletion of a COOH-terminal targeting domain from Sec2p results in Sec2p mislocalization, and thereby prevents Sec2p from activating Sec4p (Nair et al. 1990; Walch-Solimena et al. 1997; Elkind et al. 2000). An advantage of the sec2-41 strain is that, unlike sec4-8, the cells divide at the same rate as wild-type cells and have wild-type levels of SNARE complexes at 25°C. After shifting sec2-41 cells to 37°C for 10 min, there was an 80% reduction in the amount of Ssop coprecipitated with Sncp compared with wild-type cells at 37°C or sec2-41 cells at 25°C (Fig. 1, a and b). This observation suggests that Sec2p acts upstream of SNARE complex assembly.
Figure 1.
SNARE complex assembly and secretion in sec2 mutant yeast. (a and b) SNARE complex assembly. Wild-type (NY13) and sec2-41 (NY130) yeast were grown to early log phase at 25°C. An aliquot of each strain was shifted to 37°C for 10 min. Ssop coimmunoprecipitating with Sncp from a detergent-solubilized lysate was observed by Western blotting (a) and quantified by densitometry (b). The steady-state amount of SNARE complexes in wild-type cells at 25°C was defined as 100%. (c and d) Secretion rate. Cells were grown at 25°C, pelleted, and resuspended in [35S]methionine labeling medium prewarmed to 37°C. At the indicated times (in minutes), cells were pelleted from an aliquot and media proteins were collected by TCA precipitation. The media proteins and 5% of a total cell lysate from the 20-min time point were run on a 5% polyacrylamide gel and detected by autoradiography (c). A 16-h exposure for the secreted proteins and a 30-min exposure for the total cell lysates are presented. Secretion of the 150-kD protein (marked with an arrow in c) was quantified using a PhosphorImager (d).
We next compared the secretion rates of wild-type and sec2-41 cells at 37°C to examine the relationship between the reduction in SNARE complex levels and the rate of exocytosis (Fig. 1c and Fig. d). It is well established that secretion of invertase is significantly reduced in all of the sec mutants (Novick et al. 1980). However, secretion was measured after 1 h at 37°C in these experiments. We have used an [35S]methionine labeling assay to quantify the secretion rate during the first 16 min after shifting to 37°C because phenotypes observed at short times after shifting to the restrictive temperature are more likely to be a direct consequence of the mutation. In wild-type cells, release of 35S-proteins into the medium was clearly observed 4 min after addition of labeling medium prewarmed to 37°C (Fig. 1 c). The rate of secretion was linear for the next 12 min, and then reached a plateau, possibly due to depletion of [35S]methionine precursors (Fig. 1 d). By extrapolating from the secretion curve, we calculate that <4 min is required for [35S]methionine uptake from the medium, protein synthesis, and transit of several proteins through the entire secretory pathway in wild-type yeast at 37°C. In contrast to wild-type cells, almost no secretion was observed from sec2-41 cells even though there was no difference between the levels of total protein synthesis in the two strains. The observation that both secretion and SNARE complex levels are reduced at an early time point after shifting to 37°C argues against the possibility that the reduction in SNARE complexes levels is an indirect effect of the sec2-41 mutation. Since secretory vesicles are not transported to sites of secretion in the sec2-41 mutant (Walch-Solimena et al. 1997), we propose that GTP-Sec4p–dependent transport of vesicles to fusion sites is essential before SNARE complexes can assemble. However, we cannot exclude the possibility that Sec4p has an additional activity that activates SNARE complex activity more directly.
The Exocyst Has a Function before SNARE Complex Assembly
To determine whether the exocyst is required for SNARE complex assembly, binding of Ssop to Sncp was measured in several temperature-sensitive exocyst mutant alleles. There was a clear reduction in the amount of Ssop bound to Sncp after 10 min at 37°C in sec5-24, sec6-4, and sec15-1. However, near wild-type levels of Ssop remained bound to Sncp in sec3-2, sec8-9, and sec10-2. These results suggested that components of the exocyst might function both before and after SNARE complex assembly. To examine this interpretation in more detail, we measured the amount of Ssop bound to Sncp at 3, 10, and 30 min after shifting to 38°C and compared these results with the severity of the secretion block (Fig. 2). A transient twofold increase in the amount of Ssop bound to Sncp was observed in the wild-type strain at the 3-min time point (Fig. 2 a). This temporary increase in the abundance of SNARE complexes may reflect an increase in the secretion rate resulting from the temperature shift. To emphasize the effects of mutations in the exocyst components (rather than general effects of the temperature shift), the data on Ssop binding to Sncp in the exocyst mutant strains are expressed as a percentage of the amount of Ssop bound to Sncp in wild-type cells at each time point (Fig. 2 b). In addition, the amount of SNARE complexes present at the 10-min time point is compared with the secretion rate between 4 and 12 min after the addition of [35S]methionine at 38°C (Fig. 2 c).
Figure 2.
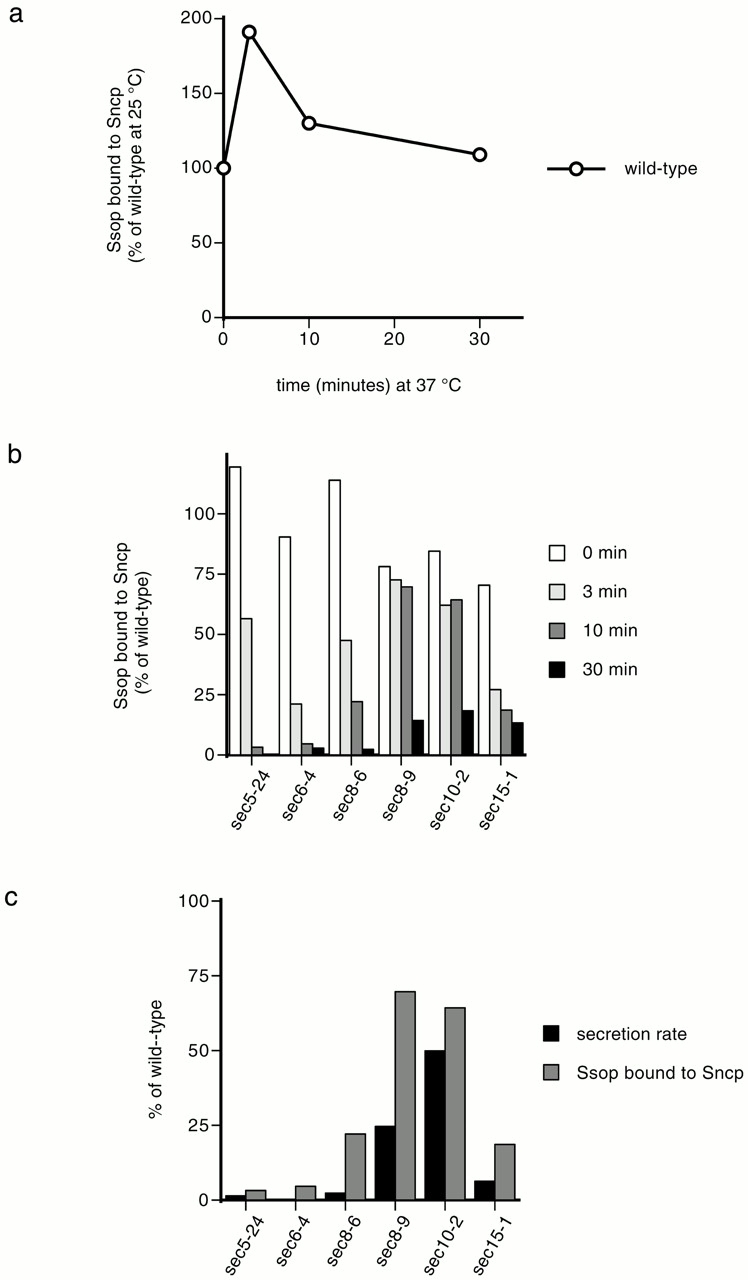
SNARE complex assembly and secretion in exocyst mutant yeast. (a) Variation in SNARE complex amounts with time at 38°C. Wild-type (NY13) cells grown at 25°C were incubated at 38°C for the indicated times. Coimmunoprecipitation of Ssop with Sncp was quantified as described in the legend to Fig. 1 b. The amount of Ssop bound to Sncp at 25°C was defined as 100%. (b) SNARE complex assembly. sec5-24 (NY402), sec6-4 (NY7), sec8-6 (NY47), sec 10-2 (NY61), and sec15-1 (NY64) cells grown at 25°C were incubated at 38°C for the indicated times. The amount of Ssop bound to Sncp in the wild-type at each time point was defined as 100%. (c) Positive correlation between SNARE complex assembly and the secretion rate. Release of 35S-p150 from cells labeled with [35S]methionine at 38°C was quantified by PhosphorImager as described in the legend to Fig. 1 d. The rate of secretion from 4 to 12 min after [35S]methionine addition is plotted. In all strains tested, the secretion rate was constant after the 4 min required for biosynthesis and transport of 35S-labeled proteins through the secretory pathway. The secretion rate in wild-type cells at 38°C was defined as 100%. The amount of Ssop bound to Sncp after 10 min at 38°C (from b) is also displayed to facilitate comparisons.
The results show a reduction in SNARE complex levels after 30 min at 38°C in all of the mutant strains. However, at this late time point, indirect effects resulting from an absence of flux through the secretory pathway may complicate the interpretation of the results. At the 3- and 10-min time points, reduced binding of Ssop to Sncp was observed in the sec5-24, sec6-4, and sec15-1 mutants and also in sec8-6, which is a more tightly blocked SEC8 allele than sec8-9. Only a 25% reduction in the abundance of SNARE complexes was observed in the sec10-2 mutant at the early time points. However, the secretion rate in sec10-2 was reduced by only 50%. Thus, there is a positive correlation between the reduction in the abundance of SNARE complexes at the 10-min time point and the severity of the secretion block (Fig. 2 c). sec3-2 was excluded from this analysis because its secretion rate was reduced by <20% 16 min after shifting to 37°C (data not shown). We conclude that Sec5p, Sec6p, Sec8p, and Sec15p are required before SNARE complex assembly. No firm conclusions can be made concerning the time of action of Sec3p, Sec10p, or the remaining exocyst components, Exo70p and Exo84p, because alleles with a fast-acting, conditional secretory block are not available.
Sec1p Functions after SNARE Complex Assembly
To evaluate the effect of Sec1p on SNARE complex levels, the amount of Ssop bound to Sncp and the secretion rate was measured after shifting sec1-1 mutant cells to 37°C. The percentage of Ssop bound to Sncp in the sec1-1 mutant was similar to wild-type for the first 10 min and then declined to 30% of wild-type levels after 30 min at 37°C (Fig. 3 a). By contrast, secretion was inhibited by >95% within 4 min after shifting to 37°C (Fig. 3 b). We conclude that Sec1p is not likely to be required for SNARE complex assembly because secretion was blocked but SNARE complexes remained assembled at the 10-min time point.
Figure 3.
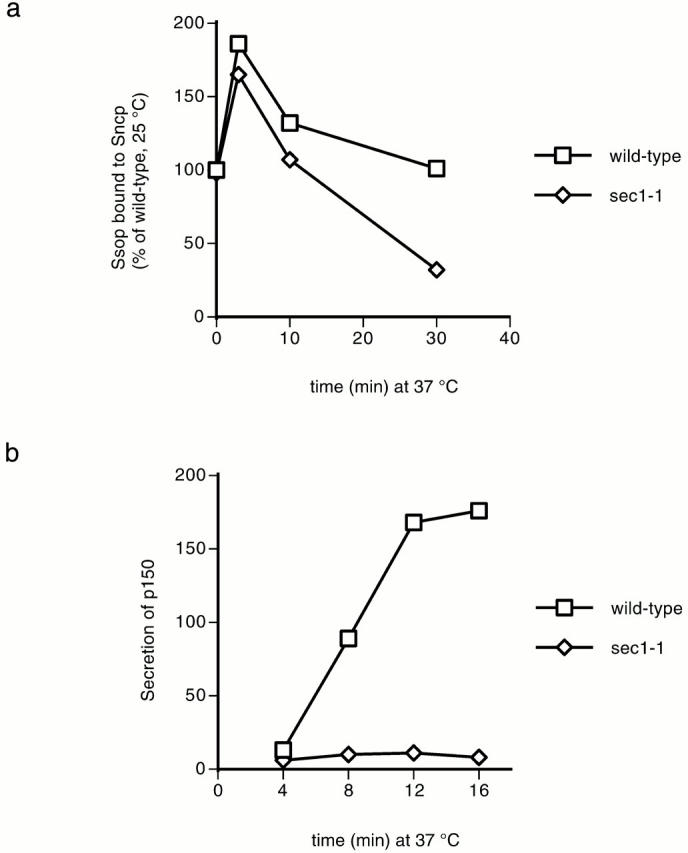
SNARE complex assembly and secretion in sec1 mutant yeast. (a) SNARE complex assembly. Wild-type (NY13) and sec1-1 (NY3) cells grown at 25°C were shifted to 37°C for the indicated times. Coimmunoprecipitation of Ssop with Sncp was quantified as described in the legend to Fig. 1 b. (b) Secretion. The rate of 35S-p150 secretion was calculated as described in the legend to Fig. 2 c.
Sec1p Binding to Ssop and the Polarized Localization of GFP-Sec1p Correlate with the Abundance of SNARE Complexes in sec Mutant Cells
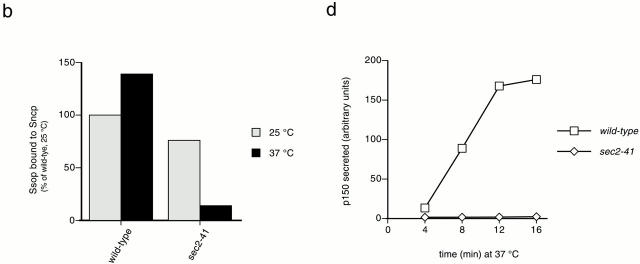
We have previously reported that Sec1p from yeast lysates binds to preassembled SNARE complexes but not to free Ssop (Carr et al. 1999). To address the possibility that other Sec proteins are required for the interaction between Sec1p and SNARE complexes, we used immunoprecipitation to examine this interaction in sec mutants incubated at 37°C for 10 min. For these experiments, epitope-tagged HA-Sso2 or myc-Sec1 proteins were immunoprecipitated with monoclonal anti-HA or anti-myc antibodies. To test for Sec1p binding to Ssop, we probed for Sec1p in the HA-Sso2p immunoprecipitates (Fig. 4 a) and probed for Ssop in the myc-Sec1 immunoprecipitates (Fig. 4 b). We also measured the abundance of SNARE complexes in each mutant by probing for Sncp in the HA-Sso2p immunoprecipitates and by probing for Ssop in an anti-Sncp immunoprecipitate from the myc-Sec1p lysates. Both precipitations were specific, because neither Ssop nor Sec1p was present in immunoprecipitations from untagged control strains. Except for the sec1-1 strain, there was a positive correlation between the amounts of Sec1p and Sncp bound to Ssop. Thus, the association of Sec1p with Ssop appears to be limited by the abundance of SNARE complexes in these sec mutants. The reduced binding of mutant Sec1-1p from the sec1-1 strain may result either from the reduced abundance of Sec1-1p in the lysate or because the mutant Sec1-1p is defective in SNARE binding.
Figure 4.
Binding of Sec1p to SNARE complexes in sec mutant strains. (a) Coimmunoprecipitation of Sec1p and Sncp with HA-Sso2p. Wild-type and sec mutant strains expressing HA-Sso2p and an untagged wild-type control were grown at 25°C and shifted to 37°C for 10 min. Sec1p and Sncp coprecipitating with HA-Sso2p in anti-HA immunoprecipitates were observed by Western blotting. Note that the strains in this experiment were shifted to 37°C rather than to 38°C as in Fig. 2. This lower temperature is partially permissive for SNARE complex assembly in the sec8-6 mutant strain. (b) Coimmunoprecipitation of Ssop with myc-Sec1p and Sncp. Wild-type and sec mutant strains expressing myc-Sec1p and an untagged wild-type control were grown at 25°C and shifted to 37°C for 10 min. Ssop coprecipitating with myc-Sec1p in anti-myc immunoprecipitates and coimmunoprecipitating with Sncp in anti-Sncp precipitates was observed by Western blotting.
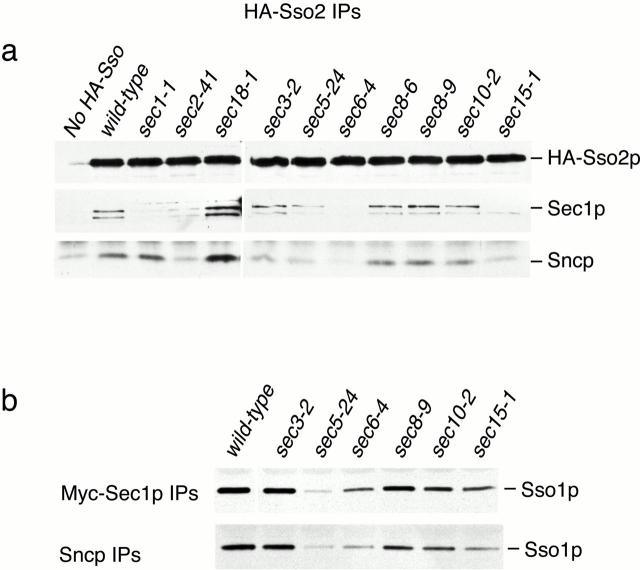
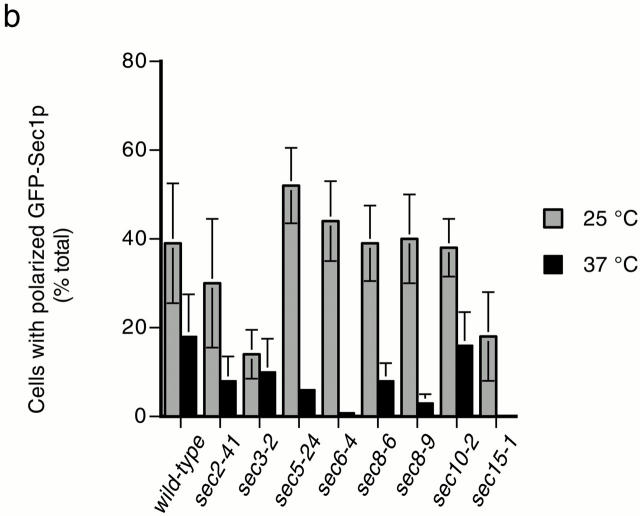
We also previously reported a positive correlation between SNARE complex abundance and the concentration of GFP-Sec1p at exocytic sites (Carr et al. 1999). Here, we examine the localization of GFP-Sec1p in additional sec mutants (Fig. 5). In wild-type cells, GFP-Sec1p is concentrated at the tips of newly emerging buds and at mother–daughter necks in cells undergoing cytokinesis (Fig. 5 a); polarized GFP-Sec1p localization is not detected in cells in the isotropic growth phase. To quantify the fluorescence results, the percentage of cells with GFP-Sec1p fluorescence at bud tips or mother–daughter necks was calculated (Fig. 5 b). The percentages shown are based on detectable polarized localization of GFP-Sec1p. Thus, they do not reflect differences in the intensity of polarized fluorescence, which are difficult to quantify (compare wild-type and sec3-2 cells at 25°C; both are images scored positive for localization). At 25°C, 30–50% of wild-type cells displayed polarized localization of GFP-Sec1p. After a 10-min shift to 37°C, localization is detected in ∼20% of the cells, and the intensity is slightly diminished. GFP-Sec1p has a polarized distribution at 25°C in all of the sec mutants except sec15-1 and sec3-2. The reduced localization in sec15-1 at 25°C correlates with a slight reduction in the number of SNARE complexes under these conditions and may simply reflect the higher threshold for detection of the GFP-Sec1p signal. In sec3-2, GFP-Sec1p is present at bud tips and necks, but is also observed in a diffuse distribution throughout the entire cell. Since there is normal expression of GFP-Sec1p and a wild-type level of SNARE complexes in sec3-2, this results suggests that Sec3p may be required for the concentration of SNARE complexes at exocytic sites. However, the lack of polarized GFP-Sec1p localization may also be explained by a negative genetic interaction between the sec3-2 and GFP-SEC1 alleles (see Materials and Methods). In the remainder of the sec mutants, the extent of polarized GFP-Sec1p localization generally correlates with the abundance of assembled SNARE complexes. Accordingly, after 10 min at 37°C, GFP-Sec1p remains polarized in sec10-2, but is depolarized in the vesicle transport mutant sec2-41 and in the remaining exocyst mutants: sec5-24, sec6-4, sec8-6, and sec8-9. We conclude from these results that SNARE complex assembly is required for polarized localization of GFP-Sec1p.
Figure 5.
Localization of GFP-Sec1p in sec mutants. (a) Fluorescent images of GFP-Sec1p localization in wild-type (SEC+, NY1696), sec2-41 (NY2222), sec3-2 (NY2214), sec 5-24 (NY2215) sec6-4 (NY2216), sec8-6 (NY2217), sec8-9 (NY2218), sec10-2 (NY2219), and sec15-1 (NY2220) cells at 25°C and after a 10-min incubation at 37°C. Cells were fixed and viewed by epifluorescence microscopy. (b) Quantitation of GFP-Sec1p localization in wild-type and sec mutant cells. The average of duplicate experiments (except for wild-type, which was done in triplicate) is followed by a range that reflects the variability between experiments performed on different days. At 25°C, the percentage of cells with polarized GFP-Sec1 was as follows: wild-type, 39 ± 13% (n = 826); sec2-41, 30 ± 14% (n = 569); sec3-2, 14 ± 6% (n = 753); sec5-24, 52 ± 9% (n = 409); sec6-4, 44 ± 9% (n = 232); sec8-6, 39 ± 9% (n = 708); sec8-9, 40 ± 10% (n = 410); sec10-2, 38 ± 7% (n = 396); and sec15-1, 18 ± 10% (n = 351). At 37°C, the percentage of cells with polarized GFP-Sec1 was as follows: SEC+, 18 ± 5% (n = 1,046); sec2-41, 8 ± 7% (n = 722); sec3-2, 10 ± 8% (n = 945); sec5-24, 6 ± 0.15% (n = 462); sec6-4, 0.8 ± 0.25% (n = 403); sec8-6, 8 ± 4% (n = 816); sec8-9, 3 ± 2% (n = 419); sec10-2, 16 ± 8% (n = 307); and sec15-1, 0 (n = 554).
Sec18p Disassembles SNARE Complexes after Fusion
To examine the role of Sec18p in yeast exocytic fusion, Ssop binding to Sncp was measured in a sec18-1 mutant strain. The mutant Sec18-1p has a glutamate to glutamine substitution that inhibits ATP hydrolysis by the D1 ATPase domain and consequently inhibits SNARE complex disassembly (Steel et al. 1999). A threefold increase was observed in the amount of Ssop bound to Sncp within 30 s after shifting sec18-1 cells to 37°C (Fig. 6 a). Importantly, in contrast to the transient increase and later decline in SNARE complex levels observed in wild-type cells, there was no decrease in SNARE complex levels at later time points in the sec18-1 mutant, consistent with the biochemical evidence for a defect in SNARE complex disassembly. The observation that there is no further increase in SNARE complex assembly after the first 30 s at 37°C is consistent with the possibility that SNARE proteins must be primed by Sec18p for assembly into trans-SNARE complexes (Ungermann et al. 1998a). However, further SNARE complex assembly is also expected to be inhibited in the sec18-1 mutant as a consequence of the block in transit through the Golgi complex (Grote and Novick 1999).
Figure 6.
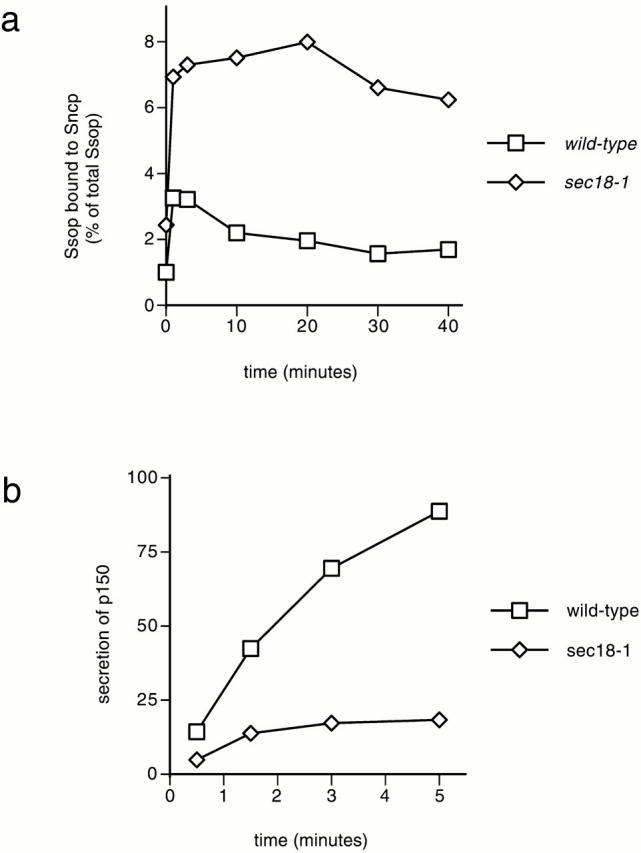
SNARE complex accumulation and secretion in sec18-1 mutant yeast. (a) Kinetics of SNARE complex accumulation. Wild-type (NY13) and sec18-1 (NY431) cells were grown at 25°C. The amount of Ssop bound to Sncp was quantified at the indicated times after shifting to 37°C as in the legend to Fig. 1 b. (b) Onset of the secretion block at 37°C. Cells were labeled with [35S]methionine for 5 min at 25°C, pelleted, and resuspended in 37°C chase medium. Aliquots were collected at the indicated times, and secreted 35S-p150 was quantified as in the legend to Fig. 1 d.
In an attempt to determine whether Sec18p functions before or after the late-acting Sec proteins, SNARE complex levels were measured in double mutant strains created by crossing sec18-1 to other temperature-sensitive sec mutants. An increase in Ssop binding to Sncp after shifting to 37°C was observed in every double mutant tested, suggesting that Sec18p acts upstream of the other Sec proteins in the SNARE complex assembly–disassembly cycle. However, a trivial explanation for these results is that Sec18-1p is inactivated more rapidly than the other mutant Sec proteins.
To measure the rate of secretion in sec18-1 cells during the first 5 min after shifting to 37°C, cells were pulse labeled with [35S]methionine for 6 min at 25°C, pelleted, and resuspended in 37°C chase medium (Fig. 6 b). Labeling at 25°C allows 35S-protein to populate the secretory pathway before imposing the secretion block. The amount of 35S-protein secreted by sec18-1 cells in the first 30 s at 37°C was reduced by 50% compared with wild-type. Secretion of 35S-protein continued at a reduced rate from 30 to 90 s after shifting to 37°C, and then reached a plateau at 3 min. Thus, both secretion and SNARE complex disassembly are inhibited in the sec18-1 mutant after shifting to 37°C. However, at early time points, there appears to be a more complete inhibition of SNARE complex disassembly than of membrane fusion. These results are consistent both with the relatively rapid inhibition (<1 min) of intra-Golgi transport reported for sec18-1 and the activity-dependent delay observed for NSF phenotypes in other systems (Graham and Emr 1991; Kawasaki et al. 1998; Littleton et al. 1998; Schweizer et al. 1998; Sanyal et al. 1999).
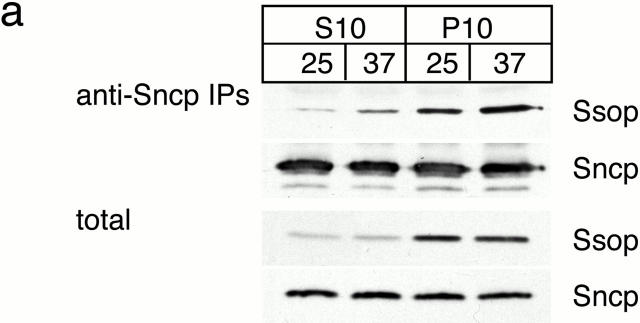
To determine the site of action of Sec18p, we examined where SNARE complexes accumulate in the sec18-1 mutant. Cis-SNARE complexes have been reported between synaptobrevin and syntaxin in synaptic vesicles (Otto et al. 1997). To test for cis-SNARE complexes in yeast secretory vesicles, sec18-1 yeast maintained at 25°C or shifted to 37°C for 10 min were lysed in detergent-free buffer and separated into 10,000-g pellet and supernatant fractions (Fig. 7 a). At 10,000 g, most secretory vesicles remain in the supernatant, but the plasma membrane and docked secretory vesicles pellet (Goud et al. 1988). Sncp was present in both pellet and supernatant fractions as expected (Protopopov et al. 1993). Ssop, which is concentrated on the plasma membrane (Aalto et al. 1993; Brennwald et al. 1994), was primarily found in the pellet fraction, but ∼5% of the Ssop remained in the supernatant. This small pool of Ssop may represent newly synthesized Ssop in transit to the plasma membrane, vesiculated fragments of the plasma membrane, or Ssop that has recycled via endocytosis. Sncp was immunoprecipitated from both fractions to determine the localization of SNARE complexes. As expected, there was an increase in the amount of Ssop bound to Sncp after shifting to 37°C. Most of the Ssop coprecipitating with Sncp was in the pellet fraction, but there was also a small amount of Ssop bound to Sncp in the supernatant fraction. Nevertheless, the amount of SNARE complexes in the supernatant increased less than twofold after shifting to 37°C. Therefore, we conclude that exocytic SNARE complexes are primarily associated with the plasma membrane, and that the SNARE complexes that accumulate in sec18-1 are not enriched in free secretory vesicles.
Figure 7.
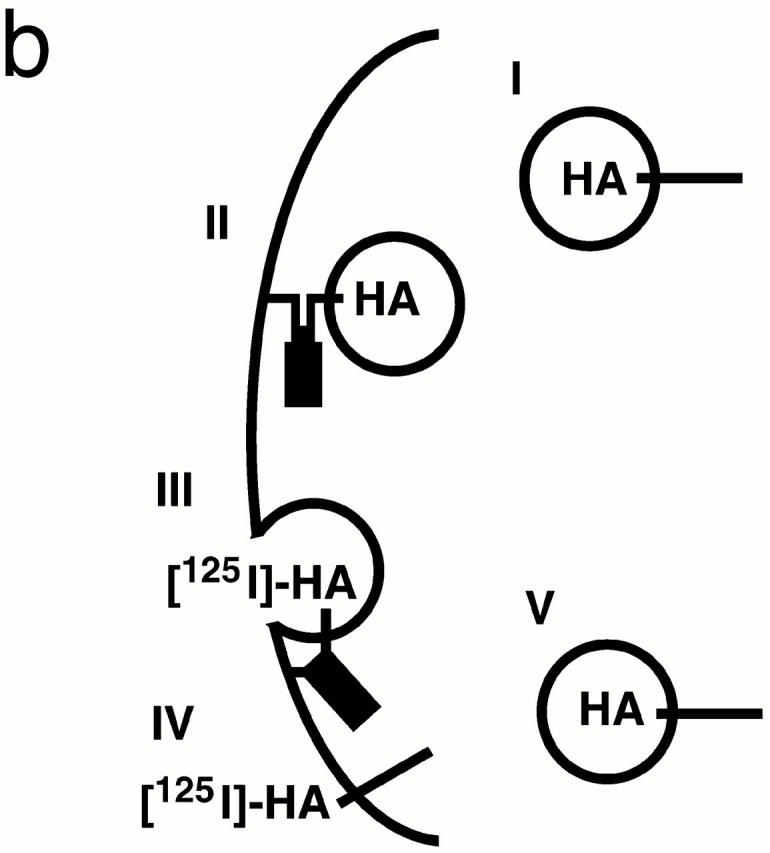
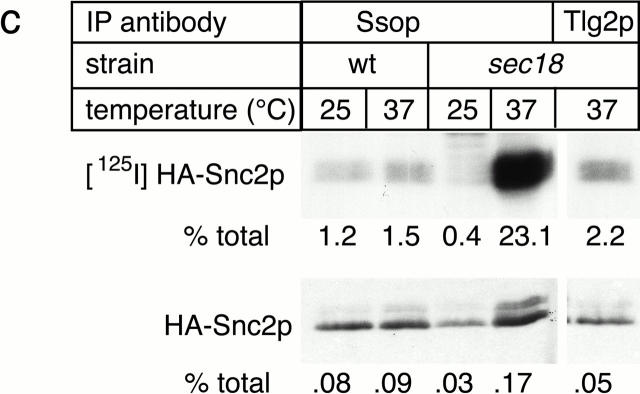
SNARE complex accumulation on the plasma membrane of sec18-1 cells. (a) Subcellular fractionation of SNAREs and SNARE complexes. sec18-1 (NY431) cells were maintained at 25°C or shifted to 37°C for 10 min, lysed in detergent-free buffer, and then fractionated into 10,000-g pellet (P) and supernatant (S) fractions. Sncp was immunoprecipitated from each fraction. An immunoblot from the total and anti-Sncp immunoprecipitated fractions was probed for Sncp and Ssop. Exposure times were varied to emphasize the distribution of Sncp and Ssop between pellet and supernatant fractions. (b) Snc2-HA in postfusion cis-SNARE complexes is accessible to surface iodination. In this model illustrating the final stages of the secretory pathway, secretory vesicles (I) dock to the plasma membrane (II) and fuse (III). After fusion, Snc2-HA on the plasma membrane (IV) recycles via endocytosis (V). Snc2-HA can be surface labeled in cis-SNARE complexes (III) but not in trans-SNARE complexes (II). (c) Coprecipitation of surface-iodinated HA-Sncp with Ssop. Aliquots of HA-Sncp expressing wild-type (NY1642) and sec18-1 (NY1643) cells were either maintained at 25°C or shifted to 37°C for 10 min. The cells were then surface labeled with 125I at 4°C, lysed, and immunoprecipitated with antibodies to Ssop and Tlg2p. Coprecipitating 125I–HA-Sncp (upper panel) was quantified by PhosphorImager analysis and compared with the total amount of 125I–HA-Sncp in an anti-HA immunoprecipitate. HA-Sncp immunoreactivity detected by Western blotting (lower panel) was quantified by densitometry and compared with the total HA-Snc2p immunoreactivity in the lysate.
The SNARE complexes that pellet at 10,000 g might be either trans-SNARE complexes between the plasma membrane and docked secretory vesicles or cis-SNARE complexes where the transmembrane anchors of both Sncp and Ssop are in the plasma membrane. To address this issue, a surface iodination assay was used to quantify the amount of cis-SNARE complexes. For this experiment, COOH-terminally HA-tagged Sncp was expressed in wild-type and sec18-1 mutant cells. The triple-HA tag includes nine tyrosines that are accessible to surface iodination only if HA-Sncp is on the plasma membrane (Fig. 7 b).
To test the specificity of iodination for HA-Sncp on the cell surface, iodination reactions were performed on intact cells and cells that had been homogenized with glass beads before labeling. Despite the accessibility of vesicular HA-Snc2p in the homogenized cells, the total amount of 125I incorporated into HA-Snc2p was reduced 20-fold in the homogenized cells compared with the intact cells due to competition from cytosolic proteins (data not shown). Thus, even if 125I+ crossed the plasma membrane of a small number of broken cells during the iodination procedure, labeling of vesicular HA-Snc2p would be minimal. Therefore, iodination is specific for surface-accessible HA-Sncp.
As previously observed for unlabeled cells (Fig. 6 a), there was a modest increase (fivefold) in the percentage of total HA-Snc2p bound Ssop when the sec18-1 strain was incubated at 37°C for 10 min before surface iodination (Fig. 7 c). Strikingly, there was a 50-fold increase in the amount of 125I–HA-Snc2p bound to Ssop (Fig. 7 c). Therefore, we conclude that cis-SNARE complexes on the plasma membrane are enriched in sec18-1 cells at 37°C. Conversely, under permissive conditions, we surmise that trans-SNARE complexes between Sncp in secretory vesicles and Ssop on the plasma membrane are enriched over cis-SNARE complexes on the plasma membrane because there are 50-fold fewer cis-SNARE complexes, but the total amount of SNARE complexes (cis plus trans) is reduced by only fivefold.
As a control for the surface iodination experiment, a second immunoprecipitate was collected from the lysate of sec18-1 cells shifted to 37°C using anti-Tlg2p antibodies. Tlg2p is an Ssop homologue that binds to Sncp in vivo and is localized primarily on endosomal and trans-Golgi membranes (Abeliovich et al. 1998). 10-fold less 125I–HA-Snc2p was bound to Tlg2p than to Ssop, consistent with the primary localization of Tlg2p on intracellular membranes (Fig. 7 c). However, 2% of the 125I–HA-Snc2p coprecipitated with Tlg2p, suggesting that a fraction of the Tlg2p is located on the plasma membrane and capable of binding to Sncp under steady-state conditions. 125I–HA-Snc2p was not detected in a Tlg2p immunoprecipitate from sec18-1 cells maintained at 25°C (data not shown). Thus, a small amount of cis-SNARE complexes between Tlg2p and HA-Snc2p also accumulate on the plasma membrane after shifting sec18-1 cells to 37°C.
Discussion
Events Leading to Membrane Fusion
Assembly of exocytic SNARE complexes between Sncp, Ssop, and Sec9p is a tightly regulated process dependent on functions provided by Sec2p and several components of the exocyst. Sec2p, the nucleotide exchange factor for the Sec4p GTPase, is required for transport of vesicles along actin microfilaments into the bud (Walch-Solimena et al. 1997). Thus, a failure to transport secretory vesicles to exocytic sites can explain the SNARE complex assembly defect in sec2-41 cells. The heterooligomeric structure of the exocyst suggests that it has multiple functions, but the activity most likely to be required for SNARE complex assembly is vesicle tethering. Vesicle tethering has also been proposed as a precondition for SNARE complex assembly in ER to Golgi transport and homotypic vacuole fusion (Sapperstein et al. 1996; Mayer and Wickner 1997; Cao et al. 1998). Tethering by the exocyst may be mediated by the binding of Sec15p to Sec4p on secretory vesicles (Guo et al. 1999b) and the subsequent assembly of Sec15 with Sec3p on the plasma membrane at sites of secretion (Finger et al. 1998). The instability of the exocyst observed in exocyst mutant strains (TerBush et al. 1996) may interfere with tethering and thereby prevent SNARE complex assembly. Conversely, increasing SNARE assembly by overexpressing a component of the SNARE complex may reduce the requirement for tethering and thereby explain the suppression of several exocyst mutants by Ssop overexpression (Aalto et al. 1993). The reduction in SNARE complex levels in exocyst mutants indicates that the exocyst acts upstream of SNARE complex assembly, but does not provide evidence for a physical interaction between the exocyst and SNARE proteins. Although binding of the rat neuronal homologues of the exocyst and Ssop has been reported (Kee et al. 1997), we have been unable to detect a similar interaction between the analogous yeast proteins (Grote, E., and D. TerBush, unpublished observation).
Proteins in the Sec1 family have been proposed to regulate SNARE complex assembly because the rat brain Sec1p homologue binds to syntaxin, an Ssop homologue, but not to an assembled SNARE complex (Pevsner et al. 1994; Yang et al. 2000). If Sec1p is essential for SNARE complex assembly, we would expect to find a reduction in the amount of Ssop bound to Sncp in the sec1-1 mutant as we found in the sec2-41 and exocyst mutants. Instead, we observed wild-type levels of SNARE complexes after secretion was blocked. Thus, if Sec1p is required for SNARE complex assembly, there must be a simultaneous block in SNARE complex disassembly such that assembled SNARE complexes are trapped for a period of time after Sec1-1p inactivation. The possibility that Sec1p has two activities is consistent with the observation from Drosophila that different ROP alleles have opposing effects on synaptic vesicle exocytosis (Wu et al. 1998).
Since yeast Sec1p binds to SNARE complexes but not to free Ssop, we favor the alternative model that Sec1p acts exclusively after SNARE complex assembly (Carr et al. 1999). However, an accumulation of SNARE complexes might be expected if fusion is blocked after SNARE complex assembly. We can suggest two possible explanations for the lack of complex accumulation in the sec1-1 mutant. Either the block in membrane fusion indirectly inhibits further SNARE complex assembly, or trans-SNARE complexes continue to assemble and disassemble in a futile cycle that is independent of fusion. Although our assay for SNARE complex abundance does not distinguish between static and dynamic SNARE complexes, the possibility that trans-SNARE complexes can be disassembled by Sec18p without membrane fusion is supported by observations in the in vitro vacuolar fusion system (Ungermann et al. 1998b).
The steps in the mechanism of membrane fusion that occur after SNARE complex assembly have only recently come under investigation. Experiments with purified SNARE proteins incorporated into liposomes suggest that a SNARE complex is the minimal machinery required to catalyze membrane fusion (Weber et al. 1998). However, even after preassembly of trans-SNARE complexes in this system, the half-time of fusion is longer than the time required for transport of proteins through the entire yeast secretory pathway (Parlati et al. 1999). Thus, Sec1p or other SNARE complex binding proteins may stimulate the rate of fusion in vivo. Stimulation may involve alterations in the mode of interaction between SNARE proteins or “zippering up” a partially assembled SNARE complex towards the membrane-proximal COOH terminus of the α-helical bundle (Hanson et al. 1997; Chen et al. 1999; Xu et al. 1999). A similar activity has been proposed for synaptotagmin I, a Ca2+-dependent regulator of synaptic vesicle exocytosis that also binds to assembled SNARE complexes (Davis et al. 1999; Leveque et al. 2000).
Sec18p and SNARE Complex Recycling
Sec18p/NSF has been proposed to directly stimulate membrane fusion (Rothman and Warren 1994) or, alternatively, to prime SNARE proteins for subsequent assembly into trans-SNARE complexes (Ungermann et al. 1998a). To identify the site of Sec18p action in yeast, we determined where SNARE complexes accumulate in the sec18-1 mutant. Our data show that SNARE complexes are predominately associated with large membranes that pellet at 10,000 g, both before and after inactivating Sec18p. This result is consistent either with a trans-SNARE complex linking secretory vesicles to the plasma membrane or a cis-SNARE complex within the plasma membrane. Experiments in the comatose (NSF) mutant of Drosophila have produced conflicting results concerning the site of SNARE complex accumulation. One group reported that SNARE complexes accumulate in a 15,000-g supernatant fraction enriched in synaptic vesicles (Littleton et al. 1998), whereas a second group demonstrated that SNARE complexes are associated with large membranes that sediment more rapidly than synaptic vesicles on a glycerol gradient (Tolar and Pallanck 1998). In more recent experiments on rat synaptic terminals, highly stable SNARE complexes were detected in a 15,000-g pellet containing the plasma membrane, but not in rapidly purified synaptic vesicles (Leveque et al. 2000). In agreement with the latter two groups, we conclude that SNARE complexes are associated with docked vesicles and/or the plasma membrane.
We used surface iodination of HA-Snc2p to mark cis-SNARE complexes on the plasma membrane. Our results revealed a 50-fold increase in the abundance of cis-SNARE complexes between HA-Snc2p and Ssop when sec18-1 cells were shifted to 37°C. Since the amount of total SNARE complexes (cis plus trans) increased by only 5-fold, the ratio of cis-complexes on the plasma membrane to total complexes increased 10-fold. Assuming that all of the SNARE complexes from the 37°C cells are cis-SNARE complexes, a maximum of 10% of the SNARE complexes can be cis-complexes before the temperature shift. Therefore, our results suggest a shift in SNARE complexes from docked secretory vesicles to the plasma membrane upon Sec18p inactivation, and also imply that >90% of the SNARE complexes recovered from wild-type cells are trans-complexes. We used the same protocol to explore the possibility that Sec1p also acts after fusion. The results showed a 2.9 ± 1.2–fold increase (n = 2) in the fraction of SNARE complexes containing 125I–Snc2-HA in sec1-1 cells after shifting to 37°C compared with the 10-fold increase observed in sec18-1 cells (our unpublished observation). Although we cannot rule out any postfusion role for Sec1p, the modest level of cis-SNARE complexes we detected excludes the possibility that Sec1p acts exclusively in SNARE complex disassembly. Exocytic SNARE complex assembly is dependent on upstream events mediated by Sec2p and the exocyst. Therefore, the cis-SNARE complexes that accumulate in the sec18-1 mutant are likely to be fusion remnants rather than complexes that spontaneously assembled between HA-Snc2 and Sso proteins that were present on the plasma membrane before the temperature shift. Thus, we conclude that once trans-SNARE complexes have assembled, disassembly by Sec18p is not required for the completion of fusion.
Postfusion disassembly of SNARE complexes is essential to maintain the steady-state localization of SNAREs within a cell. After fusion, Sncp recycles to the Golgi complex by endocytosis for subsequent rounds of secretion (Lewis et al. 2000; Grote et al. 2000), whereas Ssop and Sec9p remain on the plasma membrane. SNARE complexes must be disassembled before their constituent proteins can be segregated. Further support for the proposal that Sec18p/NSF facilitates SNARE recycling comes from the observation that NSF is concentrated at sites adjacent to active zones in the frog neuromuscular junction that may be specialized for endocytosis (Boudier et al. 1996; Roos and Kelly 1999). Because NSF/Sec18p is not required for the budding of primary endocytic vesicles from the plasma membrane (Hicke et al. 1997), endocytosis of SNARE complexes under conditions of limited NSF activity can explain the presence of t-SNAREs and SNARE complexes in coated vesicles and synaptic vesicles (Walch-Solimena et al. 1995; Otto et al. 1997; Swanton et al. 1998).
Since SNARE proteins are continually recycled, our conclusion that Sec18p disassembles cis-SNARE complexes after fusion is consistent with the current consensus that Sec18p/NSF functions at a priming step before fusion. In the case of homotypic fusion, there is no distinction between postfusion disassembly and prefusion priming because the individual SNARE proteins are not segregated into separate compartments before the next round of fusion (Price et al. 2000b). However, since Sec18p/NSF interacts with both SNARE complexes and free t-SNAREs (Sollner et al. 1993a; Hanson et al. 1995; Nichols et al. 1997), Sec18p/NSF may function at several stages in the cycle to maintain SNAREs in a primed state. An alternative possibility is that disassembly of SNARE complexes by NSF immediately after fusion produces primed SNAREs that are stabilized until the next fusion event.
It has been suggested that assembly of cognate SNARE pairs plays a role in mediating the specificity of vesicle targeting (Rothman and Warren 1994). For SNAREs to regulate the fidelity of vesicle targeting, there must be a mechanism to inactivate SNAREs that are mislocalized, including newly synthesized SNAREs in transit through the secretory pathway, v-SNAREs recycling from a target organelle back to a donor compartment, and t-SNAREs missorted due to insufficient NSF activity (Pfeffer 1996). Surprisingly, we have found a small amount of the endosomal/trans-Golgi t-SNARE Tlg2p bound to surface-accessible HA-Snc2p in sec18-1 cells at 37°C (Fig. 7 c). This result suggests that Tlg2p cycles through the plasma membrane, and more importantly, that Tlg2p is active for SNARE complex assembly even when present on the plasma membrane. The apparent lack of specificity in the location of Tlg2p activation does not support the hypothesis that SNAREs or SNARE-activating proteins are determinants of vesicle targeting.
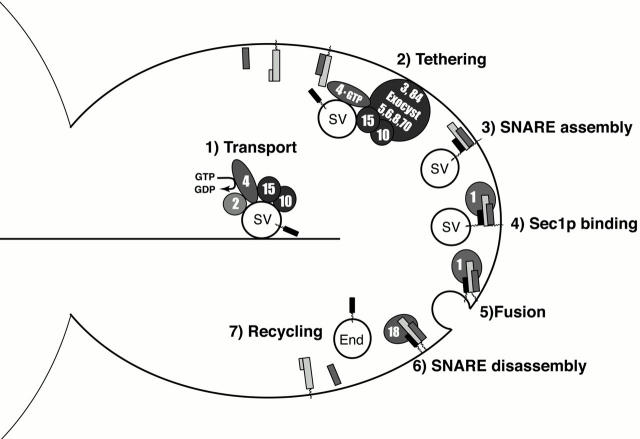
Analysis of the abundance of exocytic SNARE complexes in sec mutant yeast strains has allowed us to further refine the order of events in the secretory pathway (Fig. 8). In our model, SNARE complexes assemble after secretory vesicles are targeted to exocytic sites and tethered to the plasma membrane. Sec1p then binds to the assembled SNARE complexes, followed by membrane fusion. After fusion is complete, SNARE complexes are disassembled by Sec18p and the SNARE proteins are recycled. Although our experiments reveal the order of action of the Sec proteins, they do not address their precise biochemical activities. Thus, it will be interesting to discover how vesicle tethering and SNARE complex assembly are coordinated, and how the interaction between Sec1p and SNARE complexes stimulates membrane fusion.
Figure 8.
Model of the secretory pathway.
Acknowledgments
The authors thank Graham Warren for comments on the manuscript.
This work was supported in part by the National Institutes of Health.
Footnotes
Abbreviations used in this paper: GFP, green fluorescent protein; HA, hemagglutinin; NSF, N-ethylmaleimide–sensitive fusion protein; SNARE, soluble NSF attachment protein receptor.
References
- Aalto M.K., Ronne H., Keranen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeliovich H., Grote E., Novick P., Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J. Biol. Chem. 1998;273:11719–11727. doi: 10.1074/jbc.273.19.11719. [DOI] [PubMed] [Google Scholar]
- Adamo J.E., Rossi G., Brennwald P. The rho GTPase rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell. 1999;10:4121–4133. doi: 10.1091/mbc.10.12.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Barry V.A., DasGupta B.R., Martin T.F.J. N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J. Biol. Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- Boudier J.A., Charvin N., Boudier J.L., Fathallah M., Tagaya M., Takahashi M., Seagar M.J. Distribution of components of the SNARE complex in relation to transmitter release sites at the frog neuromuscular junction. Eur. J. Neurosci. 1996;8:545–552. doi: 10.1111/j.1460-9568.1996.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Brennwald P., Kearns B., Champion K., Keranen S., Bankaitis V., Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Cao X., Ballew N., Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr C.M., Grote E., Munson M., Hughson F.M., Novick P.J. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.A., Scales S.J., Patel S.M., Doung Y.C., Scheller R.H. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Coorssen J.R., Blank P.S., Tahara M., Zimmerberg J. Biochemical and functional studies of cortical vesicle fusionthe SNARE complex and Ca2+ sensitivity. J. Cell Biol. 1998;143:1845–1857. doi: 10.1083/jcb.143.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Ossig R., Gallwitz D., Schmitt H.D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell. Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D., Sundarababu S., Gerst J.E. Involvement of long chain fatty acid elongation in the trafficking of secretory vesicles in yeast. J. Cell Biol. 1998;143:1167–1182. doi: 10.1083/jcb.143.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.F., Bai J., Fasshauer D., Wolowick M.J., Lewis J.L., Chapman E.R. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- Elkind N.B., Walch-Solimena C., Novick P.J. The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles. J. Cell Biol. 2000;149:95–110. doi: 10.1083/jcb.149.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Finger F.P., Hughes T.E., Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Finger F.P., Novick P. Spatial regulation of exocytosislessons from yeast. J. Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor E.C., Emr S.D. COPI-independent anterograde transportcargo-selective ER to Golgi protein transport in yeast COPI mutants. J. Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M., von Mollard G.F. A new beat for the SNARE drum. Trends Cell Biol. 1998;8:215–218. doi: 10.1016/s0962-8924(98)01272-0. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N.C., Novick P.J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Govindan B., Bowser R., Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T.R., Emr S.D. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J. Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E., Novick P.J. Promiscuity in Rab-SNARE interactions. Mol. Biol. Cell. 1999;10:4149–4161. doi: 10.1091/mbc.10.12.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E., Vlacich G., Pypaert M., Novick P. A snc1 endocytosis mutantphenotypic analysis and supression by overproduction of dihydrosphingosine phosphate lyase. Mol. Biol. Cell. 2000;In press doi: 10.1091/mbc.11.12.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Grant A., Novick P. Exo84p is an exocyst protein essential for secretion J. Biol. Chem. 274 1999. 23558 23564a [DOI] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for sec4p, targeting secretory vesicles to sites of exocytosis EMBO (Eur. Mol. Biol. Organ.) J. 18 1999. 1071 1080b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P.I., Otto H., Barton N., Jahn R. The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin. J. Biol. Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- Hanson P.I., Roth R., Morisaki H., Jahn R., Heuser J.E. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Hicke L., Zanolari B., Pypaert M., Rohrer J., Riezman H. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol. Biol. Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson F.M. Membrane fusionstructure snared at last. Curr. Biol. 1999;9:R49–R52. doi: 10.1016/s0960-9822(99)80008-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki F., Mattiuz A.M., Ordway R.W. Synaptic physiology and ultrastructure in comatose mutants define an in vivo role for NSF in neurotransmitter release. J. Neurosci. 1998;18:10241–10249. doi: 10.1523/JNEUROSCI.18-24-10241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y., Yoo J.S., Hazuka C.D., Peterson K.E., Hsu S.C., Scheller R.H. Subunit structure of the mammalian exocyst complex. Proc. Natl. Acad. Sci. USA. 1997;94:14438–14443. doi: 10.1073/pnas.94.26.14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque C., Boudier J.A., Takahashi M., Seagar M. Calcium-dependent dissociation of synaptotagmin from synaptic SNARE complexes. J. Neurochem. 2000;74:367–374. doi: 10.1046/j.1471-4159.2000.0740367.x. [DOI] [PubMed] [Google Scholar]
- Lewis M.J., Nichols B.J., Prescianotto-Baschong C., Riezman H., Pelham H.R. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.C., Scheller R.H. Structural organization of the synaptic exocytosis core complex. Neuron. 1997;19:1087–1094. doi: 10.1016/s0896-6273(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Littleton J.T., Chapman E.R., Kreber R., Garment M.B., Carlson S.D., Ganetzky B. Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron. 1998;21:401–413. doi: 10.1016/s0896-6273(00)80549-8. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J. Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner W., Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Muller J.M., Rabouille C., Newman R., Shorter J., Freemont P., Schiavo G., Warren G., Shima D.T. An NSF function distinct from ATPase-dependent SNARE disassembly is essential for Golgi membrane fusion. Nat. Cell Biol. 1999;1:335–340. doi: 10.1038/14025. [DOI] [PubMed] [Google Scholar]
- Nair J., Muller H., Peterson M., Novick P. Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. J. Cell Biol. 1990;110:1897–1909. doi: 10.1083/jcb.110.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B.J., Ungermann C., Pelham H.R., Wickner W.T., Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossig R., Dascher C., Trepte H.H., Schmitt H.D., Gallwitz D. The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol. Cell. Biol. 1991;11:2980–2993. doi: 10.1128/mcb.11.6.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Hanson P.I., Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl. Acad. Sci. USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F., Weber T., McNew J.A., Westermann B., Sollner T.H., Rothman J.E. Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl. Acad. Sci. USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G.S., Schekman R. Clathrina role in the intracellular retention of a Golgi membrane protein. Science. 1989;245:1358–1365. doi: 10.1126/science.2675311. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Hsu S.C., Braun J.E., Calakos N., Ting A.E., Bennett M.K., Scheller R.H. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Pfeffer S.R. Transport vesicle dockingSNAREs and associates. Annu. Rev. Cell Dev. Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Price A., Seals D., Wickner W., Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein J. Cell Biol. 148 2000. 1231 1238a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A., Wickner W., Ungermann C. Proteins needed for vesicle budding from the Golgi complex are also required for the docking step of homotypic vacuole fusion J. Cell Biol. 148 2000. 1223 1229b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopov V., Govindan B., Novick P., Gerst J.E. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae . Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Robinson N.G., Guo L., Imai J., Toh E.A., Matsui Y., Tamanoi F. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 1999;19:3580–3587. doi: 10.1128/mcb.19.5.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J., Kelly R.B. The endocytic machinery in nerve terminals surrounds sites of exocytosis. Curr. Biol. 1999;9:1411–1414. doi: 10.1016/s0960-9822(00)80087-1. [DOI] [PubMed] [Google Scholar]
- Rothman J.E., Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Sanyal S., Basole A., Krishnan K.S. Phenotypic interaction between temperature-sensitive paralytic mutants comatose and paralytic suggests a role for N-ethylmaleimide-sensitive fusion factor in synaptic vesicle cycling in drosophila. J. Neurosci. 1999;19:RC47. doi: 10.1523/JNEUROSCI.19-24-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein S.K., Lupashin V.V., Schmitt H.D., Waters M.G. Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D., Ho J., Pruyne D., Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F.E., Dresbach T., DeBello W.M., O'Connor V., Augustine G.J., Betz H. Regulation of neurotransmitter release kinetics by NSF. Science. 1998;279:1203–1206. doi: 10.1126/science.279.5354.1203. [DOI] [PubMed] [Google Scholar]
- Skehel J.J., Wiley D.C. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell. 1998;95:871–874. doi: 10.1016/s0092-8674(00)81710-9. [DOI] [PubMed] [Google Scholar]
- Sollner T., Bennett M.K., Whiteheart S.W., Scheller R.H., Rothman J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion Cell. 75 1993. 409 418a [DOI] [PubMed] [Google Scholar]
- Sollner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J.E. SNAP receptors implicated in vesicle targeting and fusion Nature. 362 1993. 318 324b [DOI] [PubMed] [Google Scholar]
- Steel G.J., Laude A.J., Boojawan A., Harvey D.J., Morgan A. Biochemical analysis of the Saccharomyces cerevisiae SEC18 gene productimplications for the molecular mechanism of membrane fusion. Biochemistry. 1999;38:7764–7772. doi: 10.1021/bi990315v. [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer D., Jahn R., Brunger A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Swanton E., Sheehan J., Bishop N., High S., Woodman P. Formation and turnover of NSF- and SNAP-containing SNARE complexes occur on undocked, clathrin-coated vesicle-derived membranes. Mol. Biol. Cell. 1998;9:1633–1647. doi: 10.1091/mbc.9.7.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D.R., Maurice T., Roth D., Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Tolar L.A., Pallanck L. NSF function in neurotransmitter release involves rearrangement of the SNARE complex downstream of synaptic vesicle docking. J. Neurosci. 1998;18:10250–10256. doi: 10.1523/JNEUROSCI.18-24-10250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Nichols B.J., Pelham H.R., Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion J. Cell Biol. 140 1998. 61 69a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato K., Wickner W. Defining the functions of trans-SNARE pairs Nature. 396 1998. 543 548b [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C., Blasi J., Edelmann L., Chapman E.R., von Mollard G.F., Jahn R. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J. Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C., Collins R.N., Novick P.J. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Sollner T.H., Rothman J.E. SNAREpinsminimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wu M.N., Littleton J.T., Bhat M.A., Prokop A., Bellen H.J. ROP, the Drosophila Sec1 homolog, interacts with syntaxin and regulates neurotransmitter release in a dosage-dependent manner. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:127–139. doi: 10.1093/emboj/17.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Ashery U., Burgoyne R.D., Neher E. Early requirement for alpha-SNAP and NSF in the secretory cascade in chromaffin cells. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:3293–3304. doi: 10.1093/emboj/18.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Gonzalez L., Jr., Prekeris R., Steegmaier M., Advani R.J., Scheller R.H. SNARE interactions are not selective. Implications for membrane fusion specificity. J. Biol. Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- Yang B., Steegmaier M., Gonzalez L.C., Jr., Scheller R.H. nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]