Abstract
Bad is a distant relative of Bcl-2 and acts to promote cell death. Here, we show that Bad expression levels are greatly increased in thymocytes during apoptosis. We generated bad transgenic mice to study the action of upregulated Bad expression on T cell apoptosis. The T cells from these mice are highly sensitive to apoptotic stimuli, including anti-CD95. The numbers of T cells are greatly depleted and the processes of T cell development and selection are perturbed. We show that the proapoptotic function of Bad in primary T cells is regulated by Akt kinase and that Bad overexpression enhances both cell cycle progression and interleukin 2 production after T cell activation. These data suggest that Bad can act as a key regulator of T cell apoptosis and that this is a consequence of its upregulation after exposure to death stimuli.
Keywords: Bad, apoptosis, selection, cell cycle, Akt
Apoptosis plays a crucial role in the development and maintenance of an efficient immune system. The development of T cells in the thymus is characterized by the production of large numbers of immature thymocytes that are then subjected to stringent selection criteria. These immature thymocytes undergo random rearrangement of their T cell receptor genes and display the successfully rearranged protein products on their surfaces. Some of these cells are then positively selected based on the appropriate specificity of their T cell receptors and further differentiate (1). The remaining cells, up to 95% of the CD4+CD8+ T cell precursors, die by apoptosis, due to either negative selection, as a consequence of expressing self-reactive receptors, death by neglect, or as a result of the failure to receive any selection signals (2).
One of the first regulators of apoptosis to be identified was Bcl-2, which is now known to be part of a family of related proteins (3, 4). Overexpression of bcl-2 leads to the inhibition of cell death (5), although there are some apoptotic pathways that are unaffected by bcl-2 expression, such as the CD95-mediated pathway (6). The family of Bcl-2 related proteins can be divided into two groups based on their ability to promote or inhibit apoptosis. Promoters include Bax, Bak, Bik, etc., whereas inhibitors include such proteins as Bcl-2 itself, Bcl-xL, and Mcl-1 (7). The homology between family members differs greatly, although all members possess at least one of four motifs termed Bcl-2 homology (BH)1 domains (8). The physiological role of Bcl-2 as a regulator of apoptosis has been closely studied in the processes of T cell development and selection. Gain of function studies overexpressing a bcl-2 transgene in vivo show protection of immature thymocytes from a variety of death stimuli as well as increased numbers of mature T cells (9–13).
More recently discovered members of the Bcl-2 family are less well characterized than the founder member, Bcl-2. One such protein is Bad, which was first isolated on the basis of its interaction with Bcl-2 (14). Bad contains 204 amino acids and has only limited homology to other Bcl-2 family members. It is widely expressed in many tissues and in cell types (15). Bad was shown to interact selectively with Bcl-xL and to a lesser extent, Bcl-2, but not to form homodimers. Overexpression of Bad in an IL-3–dependent cell line showed it to be proapoptotic, acting by having an antagonistic effect on the death-repressing activity of Bcl-xL . However, the overexpression of Bad alone or with Bcl-2 did not have any significant effect, suggesting that the action of Bad is dependent on heterodimerization (14). Recent studies have indicated the presence of a BH3 domain in Bad that is essential for heterodimerization and apoptotic activity (16–18). Moreover, mutant Bcl-2 and Bcl-xL molecules that have lost the ability to bind Bax are still able to interact with Bad, suggesting a different mechanism of heterodimerization between Bad and other molecules (16, 18).
Bad is found in the cytosol and becomes phosphorylated on serine 112 or serine 136 after stimulation of an IL-3– dependent cell line by IL-3 (19, 20). Phosphorylation of Bad leads to its sequestration by an isoform of the 14-3-3 protein in the cytosol. Bad bound to 14-3-3 is inactive since it can no longer bind Bcl-xL, resulting in enhanced cell survival (19, 21). The kinase responsible for phosphorylation of at least one of the Bad serine residues has now been identified as Akt/PKB (22, 23). Akt is a serine/threonine kinase which acts downstream of the lipid kinase phosphatidylinositide-3-kinase (PI-3-K) (24). Akt phosphorylates Bad on a site that is required for its interaction with 14-3-3 (23). Datta et al. showed that this site is serine 136. They also demonstrated that Akt overexpression can prevent Bad-induced cell death of neurons, which suggests that Akt-dependent cell survival is mediated in part by phosphorylation of Bad (22).
To investigate the role of Bad in primary T cells, we examined Bad expression in thymocytes after exposure to apoptotic stimuli and found the level of Bad to be greatly upregulated. To examine the functional consequences of this Bad overexpression in more detail we generated mice expressing a bad transgene in their T cells. These mice have severely depleted numbers of T cells and the remaining T cells are highly sensitive to apoptotic stimuli such as γ-radiation, dexamethasone, and CD95. bad transgenic mice with a unique TCR on a recombination activation gene 1 (RAG-1)−/− background were found to have highly perturbed T cell selection. We demonstrate that the proposed regulation of Bad function by the PI-3-K/Akt pathway also operates in primary T cells. As a consequence of elevated Bad expression, Akt kinase activity is much higher in T cells from bad transgenic mice, these cells also show enhanced cell cycle progression and produce greater amounts of IL-2 in response to activation by anti-CD3. Our data highlight both the proapoptotic function of Bad within a physiological context and the consequences of Akt/Bad regulation for other processes such as cell cycle progression and cytokine production.
Materials and Methods
Generation of Transgenic Mice.
The hemagglutinin (HA) bad mouse cDNA (14) was cloned into the polylinker EcoRI site of the VA hCD2 expression cassette (25). The SalI-XbaI fragment containing the bad transgene was then purified and microinjected into CBA × C57 Bl/10 fertilized oocytes. Transgenic founder animals were identified by Southern blotting of DNA from tail biopsies and bred to C57 Bl/10 mice to generate lines that were maintained as heterozygotes.
Western Analysis.
Thymocyte whole cell lysates (107 cells per lane) were immunoblotted using enhanced chemiluminescence (Amersham Corp.) for detection. The goat polyclonal anti-Bad antibody (C20)-G was purchased from Santa Cruz Biotech., the mouse monoclonal anti-HA antibody (12CA5) from Boehringer Mannheim, and the anti-Tubulin antibody from SeroTec.
Apoptosis Assays and FACS® Analysis.
Single-cell suspensions were prepared from thymuses in complete medium (RPMI, 50 μM 2-ME, 10% FCS). 5 × 105 cells were plated either untreated or exposed to dexamethasone (5 μM, Sigma Chemical Co.), γ-irradiation (5 Gy), or anti-CD95 antibody (1 μg/ml, Jo2; PharMingen), and then incubated at 37°C. At each time point, duplicate aliquots were processed to determine the percentage of cells undergoing apoptosis. Cells were centrifuged at 1,200 rpm, washed once in PBS, and stained with propidium iodide in a hypotonic buffer at 4°C, then analyzed using flow cytometry as described in Nicoletti et al. (26). For FACS® analysis, single cell suspensions were prepared from thymuses/spleens in air-buffered IMDM and staining and analysis were carried out as previously described (27). LY294002 and wortmannin were purchased from Sigma Chemical Co. and both were dissolved in DMSO before use.
The following mAbs and second layer reagents were used: FITC-conjugated YTS169.4 (anti-CD8α) (28), phycoerythrin-conjugated anti-CD4 (Sigma Chemical Co.), biotin-conjugated KT11 (anti-Vβ11) (29), FITC-conjugated anti–TCR-δ (GL3), and streptavidin red 670 (GIBCO BRL).
Akt Kinase Assay.
The Akt kinase assay was carried out essentially as described in del Peso et al. (23). Akt was immunoprecipitated with polyclonal anti-Akt1 (Santa Cruz Biotech.), the immunocomplexes were collected with protein A–Sepharose beads, and Akt kinase activity was assayed by an in vitro kinase reaction containing [γ-32P]ATP and histone H2B (Boehringer Mannheim) as substrate. The products of the reaction were resolved by SDS-PAGE and transferred to nitrocellulose filters for quantification by PhosphorImaging (Molecular Dynamics).
Quantitation of T Cell IL-2 Production.
Single cell suspensions were prepared from lymph nodes and then purified using nonadhesive nylon wool. Purity was determined using flow cytometry. Cells were stained for CD3 and the percentage of T cells present was always >90%. Plates were precoated with serially diluted anti-CD3 antibody (2C11). 2 × 105 cells were plated on precoated wells in complete medium and incubated at 37°C for 48 h. Supernatant was then collected and incubated with IL-2–dependent CTLL cells (104/well) for 24 h at 37°C, and then 1 μCi [3H]thymidine was added per well and cultures were incubated for 12 h. Cultures were harvested using a Skatron Micro96 Harvester and the amount of [3H]thymidine incorporated was determined by scintillation counting on a Wallac 1205 Betaplate.
Results
Bad Is Greatly Upregulated during Thymocyte Apoptosis.
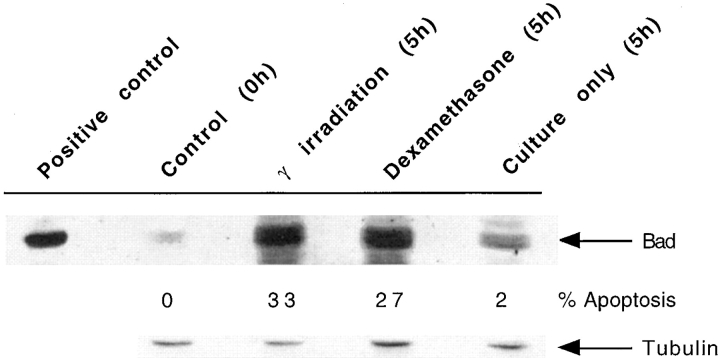
To study the action of Bad, we first determined the effect of apoptosis on Bad expression levels in thymocytes. Thymocytes were taken from C57 Bl/10 mice and cultured for 5 h, either unstimulated or after exposure to 10 Gy of γ-radiation or incubation with 5 μM dexamethasone. The level of apoptosis induced in the thymocytes was determined using flow cytometric analysis following cell lysis in a hypotonic buffer and DNA staining by propidium iodide (26). Equivalent numbers of thymocytes were loaded in each track for immunoblot analysis and the Western blot was probed with a polyclonal antibody specific for Bad. To determine the level of Bad in normal thymocytes, cells were frozen immediately after removal from the thymus (Control, 0 h in Fig. 1). As shown in Fig. 1, the level of Bad in normal thymocytes is very low, although there is a massive induction of Bad as thymocytes enter apoptosis particularly in response to strong apoptotic stimuli such as dexamethasone and γ-radiation. This suggests that Bad induction is concomitant with apoptosis in primary thymocytes and may well play an important regulatory role. To investigate this hypothesis further and to study the action of Bad in primary cells, we decided to establish lines of transgenic mice overexpressing Bad exclusively within their T cells.
Figure 1.
Upregulation of Bad expression during thymocyte apoptosis. Total cell lysate from 107 thymocytes per lane was resolved by SDS PAGE, Western blotted, and Bad expression detected by probing with anti-Bad antibody. Cell lysate was prepared from thymocytes immediately after removal of the thymus from C57 Bl/10 mice (Control, 0 h). Lysates were also prepared from thymocytes 5 h after γ-radiation, 5 h with 5 μM dexamethasone, or 5 h in culture alone. The percent apoptosis in the samples before cell lysate preparation is shown. The positive control for Bad expression is a thymocyte lysate from a bad transgenic mouse; equal numbers of cells were not added in this lane. Tubulin expression is a loading control to show that each track contains approximately equivalent amounts of protein.
Generation and Characterization of Mice Transgenic for bad.
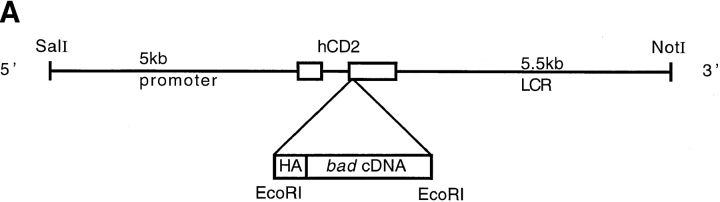
Mice were generated bearing a transgene with the mouse bad cDNA linked to a HA epitope under the control of the human CD2 promoter and locus control region (LCR) (Fig. 2 A). The bad transgene is targeted to the T cell lineage in both thymus and periphery (30, 31). The HA-tagged Bad protein produced by the transgene can be distinguished from the endogenous Bad protein using the mAb 12CA5 (32).
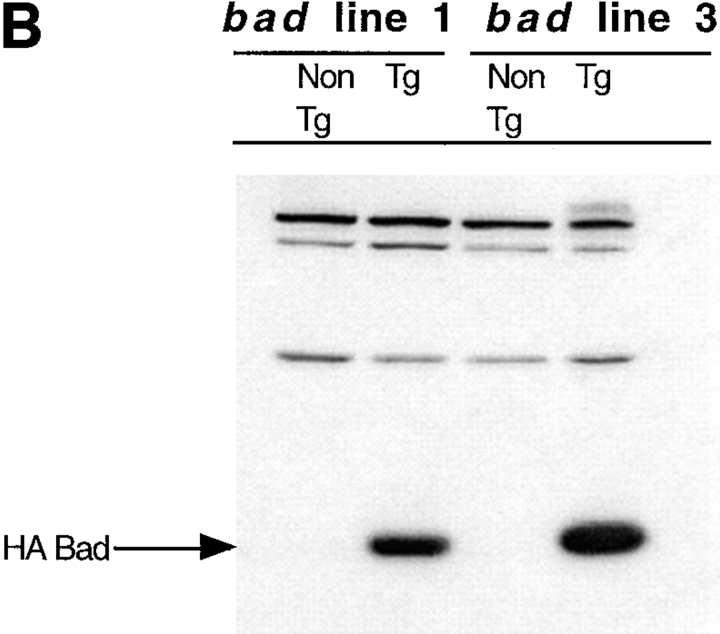
Figure 2.
bad transgene. (A) The mouse bad cDNA including a 5′ HA epitope was cloned into the EcoRI site of the human CD2 VA expression vector. The SalI-XbaI fragment was then isolated for microinjection. Western blot analysis of transgene expression in the two transgenic lines studied was carried out using total cell extract of thymocytes. Equal amounts of protein were loaded in each lane. (B) The blot was probed with the 12CA5 mAb against HA, to detect the presence of the transgene and (C) with a polyclonal anti-Bad antibody to compare the levels of endogenous and transgenic Bad expression.
The level of HA Bad expression in T cells was examined by Western blot analysis of total cell extracts prepared from the thymuses of two independent lines of transgenic mice. Equal amounts of protein were loaded in each lane and expression was detected with either the 12CA5 monoclonal (Fig. 2 B) or a polyclonal antibody against Bad (Fig. 2 C). HA Bad was only detected in thymocytes from transgenic mice (Fig. 2 B). In a replicate filter probed with the anti-Bad antibody, both endogenous Bad and transgenic HA Bad were detected (Fig. 2 C). Endogenous Bad expression is seen only in the thymocytes from nontransgenic mice. The highly expressed transgenic HA Bad is represented by the broader band seen in the transgenic lanes. The size of the transgenic protein is greater than that of the endogenous protein due to the additional 11 amino acids of the HA epitope. Therefore, we have established lines of transgenic mice that have upregulated levels of Bad expression in their T cells in a manner analogous to that found in thymocytes after exposure to apoptotic stimuli.
bad Transgenic Mice Have Depleted Numbers of T Cells.
We analyzed the bad transgenic mice to look at the consequences of overexpressing bad within their T cells. In two independent lines of bad transgenic mice, bad line 1 and bad line 3, the transgenic mice have greatly reduced numbers of thymocytes, up to 10-fold less, in comparison with nontransgenic littermates (Table I). Similarly, the numbers of mature T cells in the spleen are also significantly reduced in bad transgenic mice (data not shown). We then analyzed the effect of bad on the different populations of T cells in the thymus. Bad transgenic mice have greatly decreased percentages of both CD4−CD8+ and CD4+CD8− T cells compared to nontransgenic mice (Fig. 3 A). Similar analysis of splenocytes shows a substantial decrease in the proportion of mature single positive (SP) T cells present in the bad transgenic mice compared to controls (Fig. 3 B). Unlike for TCR-α/β T cells, the significance of apoptosis in TCR-γ/δ T cell development is unknown. We found that the percentage of γ/δ T cells relative to the percentage of α/β T cells in the CD4−CD8− thymocytes of bad transgenic mice was virtually unchanged relative to nontransgenic thymuses (Fig. 3 C). Similarly, the total number of TCR-γ/δ expressing cells in the thymus of bad transgenic mice is not significantly different. Therefore, the effect of the bad transgene on mature T cell production is restricted to TCR-α/β bearing T cells rather than TCR-γ/δ T cells.
Table I.
bad Transgenic Mice Have Decreased Numbers of Thymocytes
| Total thymocyte numbers | ||||||
|---|---|---|---|---|---|---|
| Line 1 (6 wk) | Line 3 (5 wk) | Line 1 (4 wk) | ||||
| bad transgenic | 7.3 × 107 | 2.4 × 107 | 2.7 × 107 | |||
| 2.6 × 107 | 2.8 × 107 | 3.8 × 107 | ||||
| Nontransgenic | 23 × 107 | 16 × 107 | 24 × 107 | |||
| 17 × 107 | 13 × 107 | 17 × 107 | ||||
Figure 3.
bad transgenic mice have decreased numbers of mature α/β T cells. (A) FACS® analysis of CD4 vs. CD8 expression of thymocytes and (B) splenocytes from bad transgenic and nontransgenic littermates. Cells were stained with specific anti-CD4 and anti-CD8 antibodies. The subpopulations of CD8+CD4+, CD4+CD8−, and CD4−CD8+ T cells are gated and their percentages given. (C) Histogram indicating the percentage of TCR-γ/δ expressing cells within the CD4−CD8− thymocytes of bad transgenic and nontransgenic littermates. The numbers in parentheses indicate the total number of TCR-γ/δ expressing cells in each thymus.
Bad Accelerates Apoptosis in a Manner Rescuable by Bcl-2.
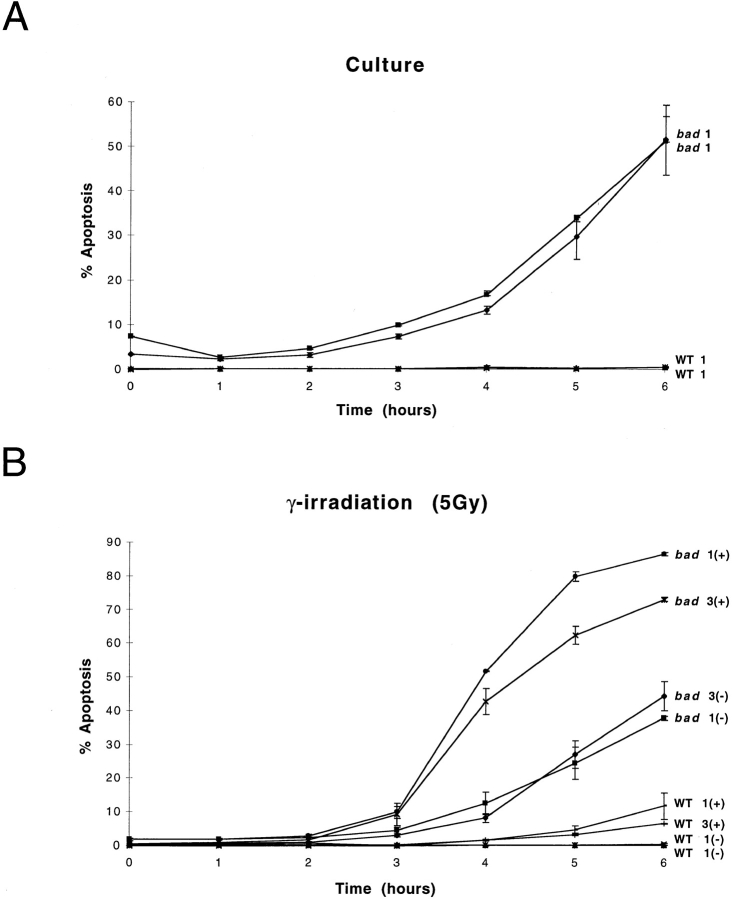
We investigated the effect of bad overexpression in response to apoptotic stimuli in T cells from bad transgenic mice. We used several stimuli that have been shown to induce apoptotic cell death. These included stimuli blocked by Bcl-2, i.e., γ-radiation and dexamethasone as well as CD95-induced apoptosis which is not blocked by Bcl-2 but can be blocked by Bcl-xL (33, 34). Thymocytes from bad transgenic mice and nontransgenic littermates were treated with apoptotic stimuli, samples harvested at various time points, and the amount of apoptosis determined by propidium iodide staining of DNA followed by flow cytometry analysis (26). Previous studies have shown that overexpression of Bad alone in a cell line has no effect on apoptosis (14). However, we show that thymocytes from bad transgenic mice placed in culture have significantly increased levels of apoptosis by as early as 4 h (Fig. 4 A). After 6 h of incubation there is >10-fold increase in the proportion of cells undergoing apoptosis compared with the nontransgenic mice. Therefore, overexpression of bad alone is able to greatly accelerate the rate of apoptosis of thymocytes in culture.
Figure 4.
bad transgenic mice exhibit increased levels of apoptosis. (A) Total thymocytes from bad and nontransgenic (WT) littermates were cultured in vitro for 6 h. Duplicate samples were analyzed from each mouse at each time point indicated. Each value represents the mean ± range of the duplicate determinations. Two individual mice per phenotype are represented. The (+) indicates the presence of the apoptotic stimulus and the (−) indicates its absence. The numbers 1 and 3 refer to mice from the independent bad transgenic lines 1 and 3, respectively. Similar studies were carried out after treatment with either (B) 5 Gy γ-irradiation, (C) 5 μM dexamethasone, or (D) anti-CD95 antibody, together with untreated controls. Similar results were obtained in three independent experiments.
We tested various apoptotic stimuli to determine the action of Bad in different apoptosis pathways. γ-radiation induces p53-dependent apoptosis through DNA damage, and thymocytes in particular are highly sensitive to this form of damage (35). The level of apoptosis in bad transgenic and nontransgenic thymocytes was determined after treatment with 5 Gy of γ-radiation. By 4 h after irradiation, <5% of nontransgenic thymocytes are apoptotic as opposed to >50% of bad transgenic thymocytes (Fig. 4 B). Dexamethasone-induced, p53-independent apoptosis was also tested. The bad transgenic thymocytes were also significantly more sensitive to dexamethasone-induced apoptosis than nontransgenic thymocytes (Fig. 4 C). Therefore, overexpression of bad sensitizes primary T cells to the Bcl-2–dependent pathways of apoptosis induced by γ-radiation and dexamethasone.
CD95-mediated apoptosis has been known to occur via a different pathway to that of DNA damage and glucocorticoid-induced apoptosis (36) and has been shown to be independent of both Bcl-2 (6) and Bax (27). Thymocytes were treated with 1 μg/ml of anti-CD95 antibody (Jo2) and the level of apoptosis determined at various time points. Anti-CD95 treatment clearly accelerates apoptosis in bad transgenic thymocytes (Fig. 4 D). Therefore, it appears that Bad is also capable of acting in a Bcl-2–independent apoptotic pathway. This may well be due to its interaction with Bcl-xL since Bcl-xL is known to block CD95-induced apoptosis in primary T cells (33, 34).
Bad has been shown previously to interact with Bcl-2 (14). Therefore, we tested whether overexpression of bcl-2 in T cells could rescue the proapoptotic effect of bad overexpression. We crossed bcl-2 transgenic mice to bad transgenic mice and determined the amount of apoptosis after in vitro culture of thymocytes from the resulting mice. The presence of the bcl-2 transgene in bad transgenic mice lead to a partial rescue in the level of apoptosis upon culture of thymocytes from mice containing only the bad transgene (Fig. 5). However, the bcl-2 transgene did not restore the number of thymocytes to wild-type levels, which may be due to the level of the transgenic Bcl-2 protein being insufficient to bind all the transgenic HA Bad protein or the Bad–Bcl-2 interaction being too weak to overcome the effects of bad overexpression (14).
Figure 5.
bcl-2 expression partially rescues thymocytes from the proapoptotic effects of bad. Total thymocytes from bad, bcl-2, and bad/bcl-2 heterozygous double transgenic mice were cultured in vitro over 8 h and samples processed at 4-h intervals to determine the percentage of apoptotic cells.
Bad Perturbs T Cell Selection.
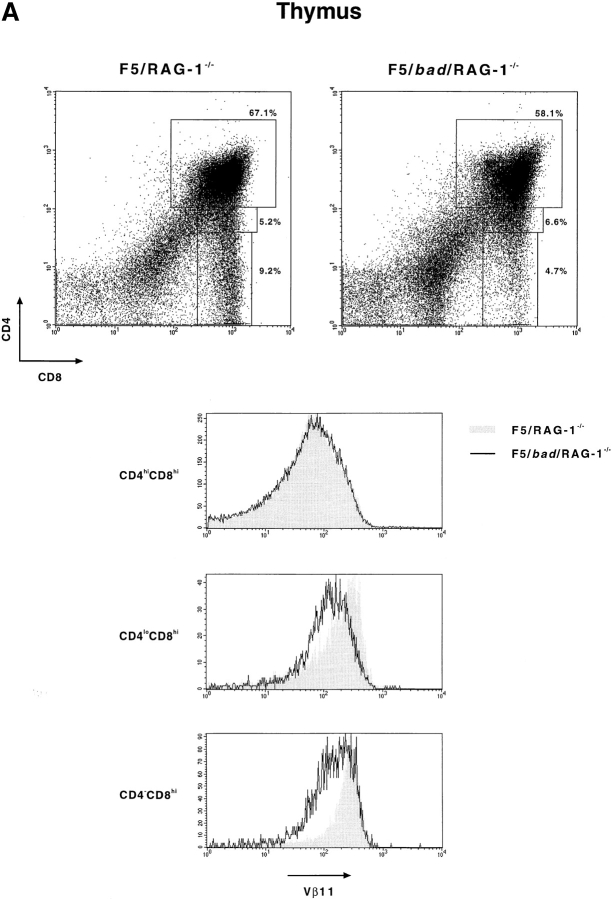
To further analyze the action of Bad on T cell development we generated mice containing the bad transgene, an F5 TCR transgene (37) on the RAG-1−/− background (38) as well as being homozygous for the H-2b MHC haplotype. The F5 TCR recognizes an influenza virus nucleoprotein–derived peptide in the context of MHC class I H-2Db (39). These mice allow the action of bad on the selection of a unique TCR specificity to be examined in the absence of endogenous TCR gene rearrangements.
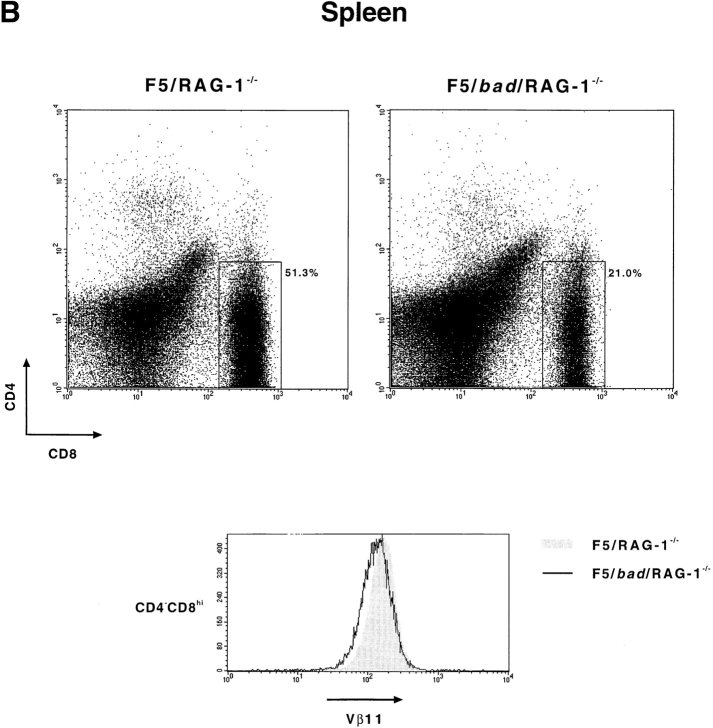
As shown in Fig. 6 A and Table II, overexpression of bad causes a dramatic decrease in both total thymic cellularity and mature T cell production. Thymocytes and splenocytes were analyzed after staining with antibodies against CD4, CD8, and Vβ11 (which recognizes the F5 TCR β chain) (Fig. 6). The presence of bad in the F5/RAG-1−/− mice results in a decrease in total thymocyte numbers from 19.8 × 107 to 5.4 × 107 and a reduction in CD4−CD8hi mature T cell numbers >10-fold (Table II). This is also reflected in a decrease in the percentage of CD4−CD8hi T cells produced (Fig. 6 A). In the thymus of F5/RAG-1−/− mice, TCR expression is upregulated (Vβ11hi) during the transition from CD4hiCD8hi, through CD4loCD8hi to CD4−CD8hi thymocytes, as shown by the shaded region in Fig. 6 A (37). However, the presence of the bad transgene greatly diminishes this upregulation, particularly in the transitional CD4loCD8hi population (Fig. 6 A). The effect on mature T cells is also seen in the periphery, where the percentage of CD4−CD8hi cells is reduced by more than twofold in the bad transgenic mice (Fig. 6 B). It should be noted that the level of Vβ11 expression on CD4−CD8hi peripheral T cells is similar in bad transgenic and nontransgenic mice. This indicates that the action of Bad is during selection and those T cells which pass this point are similar in transgenic and nontransgenic mice. We conclude that Bad, as a result of its proapoptotic action, can directly affect the process of T cell selection.
Figure 6.
bad perturbs T cell selection. Thymocytes and splenocytes were isolated from F5/bad/RAG-1−/− and F5/RAG-1−/− mice, and subsequently stained with antibodies specific for CD8, CD4, and Vβ11. (A) Dot plots showing CD8 vs. CD4 expression on thymocytes with the percentages of each subpopulation indicated. Vβ11 expression in the gated regions, CD8+CD4+, CD8hiCD4lo, and CD8+CD4−, is shown in the histograms. The shaded region represents F5/RAG-1−/− mice and the solid line represents F5/bad/RAG-1−/− mice. (B) Similarly for splenocytes from mice of the same genotypes, CD4 vs. CD8 expression is shown in the dot plots with a gate on the CD8+CD4− population. Vβ11 expression in the CD8+CD4− cells is shown in the histogram below.
Table II.
F5/RAG-1− /−/bad Mice Have Decreased Total Numbers of Thymocytes as well as Decreased Numbers of Mature CD8+CD4−Thymocytes
| Total thymo- cyte number | CD4−CD8hi (mature thymocytes) | |||
|---|---|---|---|---|
| F5/RAG-1−/−/Control | 19.8 × 107 ± 4.4 | 2.4 × 107 ± 0.6 | ||
| F5/bad/RAG-1−/− | 5.4 × 107 ± 2.5 | 0.20 × 107 ± 0.04 | ||
| (n = 4) |
Akt Kinase Activity Regulates Bad Function in Primary T Cells.
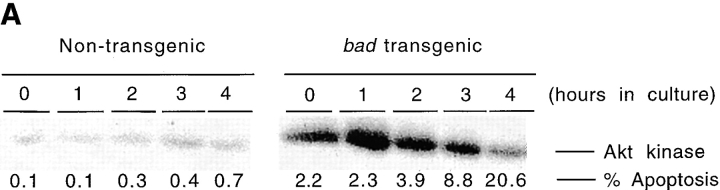
Recent studies in an IL-3–dependent cell line and in primary neurons have shown that Akt kinase has a direct role in the phosphorylation of Bad and hence regulation of the action of Bad (22, 23). The data suggest that Bad is downstream of the PI-3-K/Akt pathway and is a central player in the interaction between signal transduction and the cell death machinery. Therefore, we examined whether Akt kinase activity could regulate Bad-induced apoptosis in primary T cells. Thymocytes from bad transgenic mice and nontransgenic mice were cultured with either wortmannin, an irreversible inhibitor, or LY294002, a competitive inhibitor of PI-3-K (40, 41). Inhibition of PI-3-K blocks Akt kinase activity (42) and thus should block the phosphorylation of Bad (23). Therefore, if Akt regulates Bad-induced apoptosis in T cells, the addition of LY294002 and wortmannin to bad transgenic thymocytes should inhibit Bad phosphorylation. This will block the dissociation of Bad from Bcl-xL or Bcl-2 and hence have a proapoptotic action which should be reflected in increased apoptosis of the thymocytes. To evaluate the effect of PI-3-K inhibitors on Bad-induced apoptosis, thymocytes from bad transgenic and nontransgenic mice were cultured with LY294002 (20 μM) or wortmannin (2 μM) and the levels of apoptosis measured against time. The experiments were carried out using thymocytes from both line 1 and line 3 of bad transgenic mice. Wortmannin significantly accelerated apoptosis in bad transgenic thymocytes from both lines while having no effect on nontransgenic thymocytes (Fig. 7 A). Similarly, LY294002 accelerated Bad-induced apoptosis while having no effect on nontransgenic thymocytes (Fig. 7 B). The lack of effect of PI-3-K inhibitors on apoptozing nontransgenic thymocytes during the time course studied is most likely due to the low levels of Bad present in normal thymocytes (Figs. 1 and 2 C).
Figure 7.
Inhibition of Akt accelerates bad-induced apoptosis. (A) Total thymocytes from bad transgenic mice and control littermates of both transgenic lines were cultured in vitro in the presence of 2 μM wortmannin (irreversible inhibitor of PI-3-K) and the percentage of apoptotic cells at each time point was determined as before. (B) Similar studies were carried out with 20 μM LY294002 (competitive inhibitor of PI-3-K).
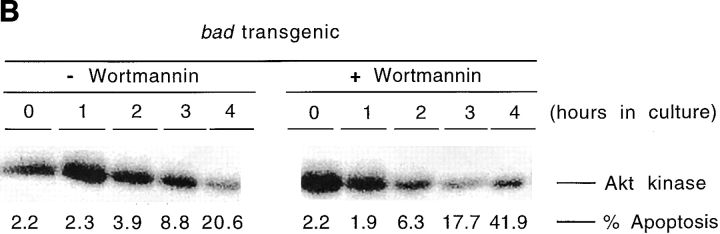
To confirm that the effect of the PI-3-K inhibitors was on Akt activation, we directly measured the Akt kinase activity in thymocytes from bad transgenic mice in the presence or absence of the inhibitors. Akt kinase activity was assayed by in vitro kinase assay after Akt immunoprecipitation using histone H2B as substrate (23). Akt kinase activity was measured in thymocytes from bad transgenic and nontransgenic mice after in vitro culture. The constitutive level of Akt kinase activity (0 h) is much higher in bad transgenic mice than in nontransgenic mice (Fig. 8 A). This high level of Akt kinase activity falls as the level of apoptosis increases with time, whereas the level of Akt kinase activity in the wild type remains negligible. In the presence of wortmannin the rate of decrease of Akt activity is greater in the bad transgenic thymocytes than without wortmannin (Fig. 8 B). For example, as determined by densitometry, the level of Akt kinase activity is almost halved in bad transgenic thymocytes after 2–3 h in culture in the presence of wortmannin. Thus, the effect of PI-3-K inhibitors in accelerating Bad-induced apoptosis appears to be mediated via the reduction of Akt kinase activity. However, we were surprised to discover that bad transgenic thymocytes have a significantly elevated level of constitutive Akt kinase activity. This suggests that, although Bad is biochemically downstream of Akt, elevated levels of Bad can influence the level of Akt activity, possibly by a paracrine/autocrine feedback mechanism.
Figure 8.
Akt kinase activity in bad thymocytes is constitutively higher and is inhibited by wortmannin. Total cell lysates were prepared from thymocytes of bad transgenic mice and control littermates which had been cultured for up to 4 h. Akt was immunoprecipitated and an in vitro kinase assay was carried out using histone H2B as substrate. (A) The constitutive (0 h) level of kinase activity and the activity versus time of the nontransgenic control is shown in the left panel, and that of the bad transgenic mice in the right panel. (B) Akt kinase activity of bad transgenic thymocytes is shown in the absence (left) or presence (right) of 2 μM wortmannin over 4 h.
Bad Overexpression Enhances Cell Cycle Progression and Upregulates IL-2 Production after T Cell Activation.
We then analyzed the functional consequences of bad overexpression on the PI-3-K/Akt pathway. The signaling pathway involving PI-3-K/Akt has been shown to have a role in regulating progression through the cell cycle of T cells (43). We measured the DNA content of thymocytes from bad transgenic and nontransgenic littermates by propidium iodide staining. In nontransgenic mice the vast majority (∼90%) of thymocytes are resting in G0/G1 while <10% are actively progressing through the cell cycle and found in S phase (Table III). However, in bad transgenic mice the fraction of thymocytes that are in the S phase of the cell cycle is two- to threefold higher (Table III). The proportion of cells in G0/G1 is consequently reduced in bad transgenic thymuses. Therefore, overexpression of bad is sufficient to increase the number of T cells entering the cell cycle. This is a similar effect to that found in bax transgenic mice (27) and the opposite to that found in bcl-2 transgenic mice, where the number of thymocytes in S phase is reduced (44, 45).
Table III.
bad Transgenic Mice Have Increased Numbers of Thymocytes in S Phase of the Cell Cycle
| Percentage of thymocytes in cycle | ||||
|---|---|---|---|---|
| Percent G0/G1 phase | Percent S phase | |||
| bad | 67.0 ± 6.4 | 26.9 ± 4.7 | ||
| Nontransgenic | 86.9 ± 3.7 | 9.1 ± 1.8 | ||
| (n = 12) | ||||
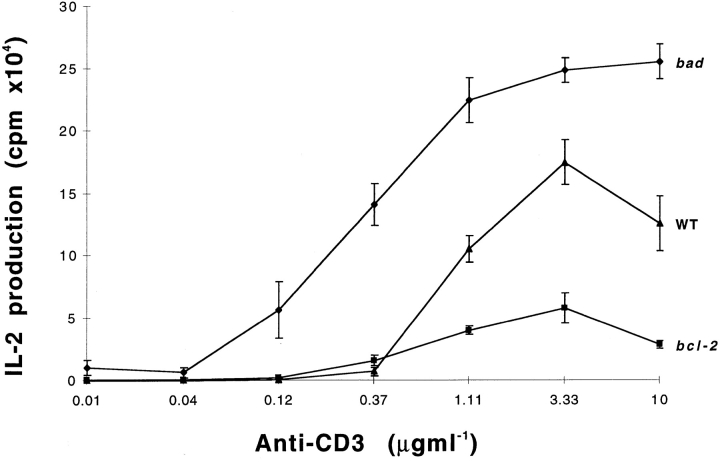
Activation of T cells is associated with their progression through the cell cycle. Activated T cells produced IL-2, which directly promotes cell cycle entry (46). Having observed that bad can promote cell cycle progression, we examined whether bad could affect the production of IL-2 in mature T cells after activation via anti-CD3. A precedent for this comes from studies on T cells from bcl-2 transgenic mice which show that bcl-2 overexpression significantly decreases IL-2 production (44). Therefore, we purified T cells from the lymph nodes of bad transgenic, bcl-2 transgenic, and nontransgenic mice, activated the T cells using serial dilutions of plate bound anti-CD3 antibody, and assayed the production of IL-2. The bcl-2 transgenic T cells produced much less IL-2 than those from nontransgenic mice, as reported previously (44). However, the bad transgenic T cells produced substantially more IL-2 than T cells from nontransgenic mice (Fig. 9). This effect on IL-2 production was not apparent after costimulation of purified bad transgenic T cells with anti-CD28 as well as serially diluted anti-CD3 antibody (data not shown). This may be due to the fact that anti-CD28 strongly induces bcl-xL expression (47), which would then bind the excess Bad present.
Figure 9.
bad transgenic T cells produce higher levels of IL-2 on stimulation with anti-CD3 antibody. T cells purified from the lymph nodes of bad, bcl-2 transgenic mice, and nontransgenic mice were activated with serial dilution of cross-linked anti-CD3 antibody. IL-2 production was determined by CTLL assay and [3H]thymidine incorporation.
The action of Bad on cell cycle progression and cytokine production suggests that a feedback mechanism may exist whereby T cells may try to accommodate the elevated levels of Bad. For example, elevated Bad leads to increased protective cytokine production upon activation which could act in an autocrine manner to enhance survival. This could also enhance Akt kinase activity and enhance cell cycle entry.
Discussion
Bad Acts as a Proapoptotic Molecule in Primary T Cells.
The Bcl-2 family member, Bad, has emerged as a key regulator of apoptosis from studies on hematopoietic cell lines (41). We have examined the function of Bad in regulating apoptosis of primary T cells both in culture and in vivo. Our initial observation was that the level of Bad expression is greatly upregulated in thymocytes exposed to apoptotic stimuli such as γ-radiation or dexamethasone. To determine whether elevated Bad was an effector mechanism in T cell apoptosis and how Bad might function in this context, we generated mice overexpressing a bad transgene in their T cells. Overexpression of bad is sufficient to accelerate apoptosis in primary thymocytes from bad transgenic mice maintained in culture, confirming its proapoptotic nature. The excess Bad may well act by binding the available endogenous Bcl-2 and Bcl-xL, thus preventing their function and accelerating apoptosis.
Thymocytes from bad transgenic mice also exhibit significantly increased levels of apoptosis after treatment with low dose γ-radiation, dexamethasone, and anti-CD95 antibody. These data indicate that Bad is capable of acting in multiple apoptotic pathways. Bcl-2 has been shown to protect thymocytes against γ-radiation and dexamethasone (9, 10) and Bax has the opposite effect, accelerating apoptosis in response to these stimuli (32). Surprisingly, our data also indicate that Bad is capable of influencing CD95-mediated apoptosis, which occurs via a distinct pathway from DNA damage and glucocorticoid-induced apoptosis (6). This effect of Bad is contrary to the action of some of the other members of the Bcl-2 family. Bcl-2 has been shown to have no effect on CD95-induced apoptosis in the lymphoid cells of bcl-2 transgenic mice (6) and Bax is unable to accelerate CD95-mediated apoptosis in thymocytes transgenic for bax (27). We propose that the effect of Bad on CD95-induced apoptosis is a result of its preferred interaction with Bcl-xL (14). Bcl-xL has been shown to inhibit apoptosis in the CD95 pathway (33, 34). We suggest that overexpressing Bad leads to sequestration of all the endogenous Bcl-xL, thus removing its protective function. In addition, excess unbound Bad may also actively accelerate apoptosis in response to anti-CD95. This effect of Bad could be related to the structural properties of Bad which distinguish it from other Bcl-2 family members. It lacks the COOH-terminal signal anchor sequence found in other Bcl-2–related molecules apart from Bid (14, 18), suggesting that Bad may not exist as an integral membrane protein. The homology of its BH1 and BH2 domains to other Bcl-2 family members is limited (14). Although Bad may preferentially bind Bcl-xL (14), using thymocytes from mice carrying both bad and bcl-2 transgenes, we have shown that Bcl-2 can, at least partially, rescue the proapoptotic action of Bad. This is at variance with the previously reported lack of effect of Bad on Bcl-2 in a transfected IL-3–dependent cell line (14). However, the rescue is only partial, which may be due to stoichiometry or a reflection of the weak interaction between Bad and Bcl-2.
The Action of Bad on T Cell Development.
Bad transgenic mice have a significantly smaller thymus compared to nontransgenic littermates. The bad transgenic mice have only 15–20% of the thymocytes present in the control mice. This effect correlates with the increased apoptosis found in bad transgenic thymocytes. We also found reduced numbers of mature SP thymocytes which suggests that overexpressing bad interferes with the process of T cell selection. It is also significant to note that the effect of Bad is only found on maturation of T cells bearing α/β TCRs rather than those bearing γ/δ TCRs. This is consistent with known differences between α/β and γ/δ T cells, including differences in their developmental pathway in the thymus, the absence of positive selection in γ/δ T cell development, and the mechanisms of antigen recognition by γ/δ T cells (48, 49).
We sought to clarify the action of Bad on T cell development using MHC class I restricted F5 TCR transgenic mice on a RAG-1−/− background to produce mice whose T cells express a unique TCR. Expression of bad in F5/ RAG-1−/− mice reduced the size of the thymus almost fourfold (Table II). The number of CD4−CD8hi T cells produced is reduced 10-fold and the upregulation of TCR (Vβ11) expression in the CD4loCD8hi population normally associated with maturation is also reduced. Therefore, Bad can act directly on the processes involved in T cell selection. The impairment of both T cell maturation and TCR upregulation suggest that Bad can disrupt positive selection or enhance negative selection. Further analysis is required to determine if Bad acts in one or both of these ways. It should be noted that the mature T cells found in the spleen of bad transgenic mice, although diminished in numbers, do exhibit comparable levels of TCR expression to nontransgenic controls. This indicates that the thymocytes that do mature and enter the periphery are normal and that the effect of Bad is mainly on the developmental and selection processes which occur in the thymus.
Biochemical Regulation of Bad and Consequences for T Cell Function.
Akt kinase can directly regulate the action of Bad through phosphorylation of serine 136 (22). Akt activity itself is regulated via PI-3-K (24). Phosphorylation of Bad results in loss of its proapoptotic function and leads to binding to 14-3-3 (19, 21). Our experiments confirm that Akt kinase activity is directly involved in the regulation of Bad in primary T cells. By using the specific PI-3-K inhibitors wortmannin and LY294002, we demonstrate that inhibition of Akt kinase activity leads to an increase in the level of apoptosis of bad transgenic thymocytes in culture. The most likely explanation for this effect is that inhibition of Akt kinase leads to increased levels of unphosphorylated Bad which is free to exert its proapoptotic effect either by binding Bcl-xL/Bcl-2 or through its direct action. In further support of this hypothesis, we directly show that wortmannin decreases Akt kinase activity in bad transgenic thymocytes, concomitant with the increase in thymocyte apoptosis. Hence the Akt–Bad regulation pathway acts in primary T cells, although as Akt only phosphorylates one of the two phosphorylated serines on Bad (22) it is likely that Akt-independent regulation of Bad may also take place in primary T cells. We also observed that the constitutive level of Akt kinase activity in bad thymocytes is significantly higher than that in control littermates. Akt activity has been shown to be upregulated by IL-2 and this effect is PI-3-K mediated (50). Furthermore, wortmannin is able to block this upregulation of Akt activity (40, 41). This raises the possibility that bad overexpression has the downstream effect of modulating, directly or indirectly, IL-2 production which activates Akt by a feedback loop and so enhances cell survival to compensate for elevated level of Bad.
The bad transgene increases the number of thymocytes in S phase of the cell cycle by two- to threefold. This is a similar effect to that shown for another Bcl-2 family member, Bax (27). In the case of Bax, we have shown previously that the effect on cell cycle is a direct one, impinging on the molecules that regulate G1–S transition. The phosphorylation of Bad has been shown to be regulated by growth factors (19, 23, 22) and furthermore, one of the key factors which influences the G1–S transition in T cells is cytokines, such as IL-2 (46). We have demonstrated that IL-2 is produced at much higher levels in activated bad transgenic T cells than in control T cells. Therefore, it is possible that the modulation of IL-2 production, or of other cytokines, increases cell cycle progression of bad transgenic thymocytes. The same modulation may then act in a paracrine or autocrine manner to activate the signaling pathway involving PI-3-K leading to the greatly increased Akt kinase activity found in bad transgenic T cells. Conversely, bcl-2 transgenic T cells have greatly decreased IL-2 production in comparison to control T cells. The suppression of IL-2 production by Bcl-2 in activated T cells (44) has been shown to be due to its ability to block the calcineurin- dependent nuclear translocation of NFAT, a transcription factor which is required for the induction of IL-2 gene expression (51). Therefore, we suggest that the effect of Bad on IL-2 induction is mediated either by antagonizing the suppressive effect of Bcl-2 on NFAT translocation via calcineurin or that Bad itself can enhance NFAT translocation by some novel mechanism. Whichever precise mechanism is the correct one, the net effect is that bad overexpression allows hyperinduction of IL-2 expression after activation.
The evidence of Bad upregulation during apoptosis and the data from bad transgenic mice identify Bad as a potential key regulator of T cell apoptosis. Further experiments will involve the use of T cells from bad transgenic mice to examine the effect of Bad overexpression on the signal transduction pathways implemented after T cell activation. This will determine whether the action of Bad in elevating cytokine production is due to an effect on a discrete point in the signaling pathway, a generalized effect, or if it is limited to the NFAT-calcineurin junction. Other studies will address the manner in which Bad acts on T cell selection, to increase negative selection or reduce positive selection (13).
Acknowledgments
We thank Dr. Stanley Korsmeyer (Washington University, St. Louis, MO) for the kind gift of the murine bad cDNA. We are grateful to Dr. Lesley Smyth for her advice on Western blotting.
Footnotes
This research was funded by the Medical Research Council. G. Gil-Gómez was partially funded by a fellowship from the Spanish Government's Ministerio de Educacion y Cultura and by European Commission contract CHRX-CT94-0584. O. Williams was the recipient of a Leukemia Research Fund fellowship.
Abbreviations used in this paper: BH, Bcl-2 homology; HA, hemagglutinin; LCR, locus control region; PI-3-K, phosphatidylinositide-3-kinase; RAG-1, recombination activation gene 1.
References
- 1.von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 2.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 4.Strasser A, Huang DC, Vaux DL. The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochem Biophys Acta. 1997;1333:F151–F178. doi: 10.1016/s0304-419x(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 5.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 6.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO (Eur Mol Biol Organ) J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cory S. Regulation of lymphocyte survival by the bcl-2 gene family. Ann Rev Immunol. 1995;13:513–543. doi: 10.1146/annurev.iy.13.040195.002501. [DOI] [PubMed] [Google Scholar]
- 8.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 9.Sentman CL, Shutter JR, Hockenbery D, Kanagama O, Korsmeyer SJ. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 10.Strasser A, Harris AW, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 11.Strasser A, Harris AW, von Boehmer H, Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci USA. 1994;91:1376–1380. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao W, Teh SJ, Melhado I, Jirik F, Korsmeyer SJ, Teh HS. The T cell repertoire of CD4-8+thymocytes is altered by overexpression of the BCL-2 protooncogene in the thymus. J Exp Med. 1994;179:145–153. doi: 10.1084/jem.179.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams O, Norton T, Halligey M, Kioussis D, Brady HJM. The action of Bax and bcl-2 in T cell selection. J Exp Med. 1998;188:1125–1133. doi: 10.1084/jem.188.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 15.Kitada S, Krajewska M, Zhang X, Scudiero D, Zapata JM, Wang HG, Shabaik A, Tudor G, Krajewski S, Myers TG, et al. Expression and location of pro-apoptotic Bcl-2 family protein BAD in normal human tissues and tumor cell lines. Am J Pathol. 1998;152:51–61. [PMC free article] [PubMed] [Google Scholar]
- 16.Ottilie S, Diaz JL, Horne W, Chang J, Wang Y, Wilson G, Chang S, Weeks S, Fritz LC, Oltersdorf T. Dimerization properties of human BAD. Identification of a BH-3 domain and analysis of its binding to mutant BCL-2 and BCL-XL proteins. J Biol Chem. 1997;272:30866–30872. doi: 10.1074/jbc.272.49.30866. [DOI] [PubMed] [Google Scholar]
- 17.Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem. 1997;272:24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- 18.Kelekar A, Chang BS, Harlan JE, Fesik SW, Thompson CB. Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-XL. Mol Cell Biol. 1997;17:7040–7046. doi: 10.1128/mcb.17.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 21.Hsu SY, Kaipia A, Zhu L, Hsueh AJ. Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)– induced apoptosis in mammalian cells by 14-3-3 isoforms and P11. Mol Endocrinol. 1997;11:1858–1867. doi: 10.1210/mend.11.12.0023. [DOI] [PubMed] [Google Scholar]
- 22.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 23.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3–induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 24.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell–specific expression in transgenic mice. J Immunol Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- 26.Nicoletti I, Miglioratti G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 27.Brady HJM, Gil-Gomez G, Kirberg J, Berns AJM. Baxα perturbs T cell development and affects cell cycle entry of T cells. EMBO (Eur Mol Biol Organ) J. 1996;15:6991–7001. [PMC free article] [PubMed] [Google Scholar]
- 28.Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 29.Tomonari K, Lovering E. T-cell receptor specific monoclonal antibodies against a Vb11-positive mouse T-cell clone. Immunogenetics. 1988;28:445–451. doi: 10.1007/BF00355377. [DOI] [PubMed] [Google Scholar]
- 30.Lang G, Wotton D, Owen MJ, Sewell WA, Brown MH, Mason DY, Crumpton MJ, Kioussis D. The structure of the human CD2 gene and its expression in transgenic mice. EMBO (Eur Mol Biol Organ) J. 1988;7:1675–1682. doi: 10.1002/j.1460-2075.1988.tb02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greaves DR, Wilson FD, Lang G, Kioussis D. Human CD2 3′-flanking sequences confer high level, T cell– specific, position independent gene expression in transgenic mice. Cell. 1989;56:979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- 32.Brady HJM, Salomons GS, Bobeldijk RC, Berns AJM. T cells from baxα transgenic mice show accelerated apoptosis in response to stimuli but do not show restored DNA damage–induced cell death in the absence of p53. EMBO (Eur Mol Biol Organ) J. 1996;15:1221–1230. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Li L, Choe J, Krajewski S, Reed JC, Thompson C, Choi YS. Up-regulation of Bcl-xL expression protects CD40-activated human B cells from Fas-mediated apoptosis. Cell Immunol. 1996;173:149–154. doi: 10.1006/cimm.1996.0260. [DOI] [PubMed] [Google Scholar]
- 34.Medema JP, Scaffidi C, Krammer PH, Peter ME. Bcl-xL acts downstream of caspase-8 activation by the CD95 death-inducing signaling complex. J Biol Chem. 1998;273:3388–3393. doi: 10.1074/jbc.273.6.3388. [DOI] [PubMed] [Google Scholar]
- 35.Cohen JJ, Duke RC, Fadsk VA, Sellins KS. Apoptosis and programmed cell death in immunity. Ann Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 36.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 37.Mamalaki C, Norton T, Tanaka Y, Townsend AR, Chandler P, Simpson E, Kioussis D. Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T cell receptor transgenic mice. Proc Natl Acad Sci USA. 1992;89:11342–11346. doi: 10.1073/pnas.89.23.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spanopoulou E, Roman CAJ, Corcoran LM, Schlissel MS, Silver DP, Nemazee D, Nussenzwieg MC, Shinton SA, Hardy RR, Baltimore D. Functional immunoglobulin transgenes guide ordered B-cell differentiation in RAG-1 deficient mice. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 39.Townsend AR, McMichael AJ, Carter NP, Huddleston JA, Brownlee GG. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39:13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- 40.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 41.Franke TF, Cantley LC. Apoptosis. A Bad kinase makes good. Nature. 1997;390:116–117. doi: 10.1038/36442. [DOI] [PubMed] [Google Scholar]
- 42.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 43.Brennan P, Babbage JW, Burgering BMT, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3–kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 44.Linette GP, Li Y, Roth K, Korsmeyer SJ. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proc Natl Acad Sci USA. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Reilly LA, Huang DC, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO (Eur Mol Biol Organ) J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 46.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2–mediated elimination of the p27Kip1 cyclin–dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 47.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–89. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 48.Schweighoffer E, Fowlkes BJ. Positive selection is not required for thymic maturation of transgenic γ/δ T cells. J Exp Med. 1996;183:2033–2041. doi: 10.1084/jem.183.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang J, Coles M, Cado D, Raulet DH. The development fate of T cells is critically influenced by TCR γδ expression. Immunity. 1998;8:427–438. doi: 10.1016/s1074-7613(00)80548-8. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]