Abstract
Two subsets of murine splenic dendritic cells, derived from distinct precursors, can be distinguished by surface expression of CD8α homodimers. The functions of the two subsets remain controversial, although it has been suggested that the lymphoid-derived (CD8α+) subset induces tolerance, whereas the myeloid-derived (CD8α−) subset has been shown to prime naive T cells and to generate memory responses. To study their capacity to prime or tolerize naive CD4+ T cells in vivo, purified CD8α+ or CD8α− dendritic cells were injected subcutaneously into normal mice. In contrast to CD8α− dendritic cells, the CD8α+ fraction failed to traffic to the draining lymph node and did not generate responses to intravenous peptide. However, after in vitro pulsing with peptide, strong in vivo T cell responses to purified CD8α+ dendritic cells could be detected. Such responses may have been initiated via transfer of peptide–major histocompatibility complex complexes to migratory host CD8α− dendritic cells after injection. These data suggest that correlation of T helper cell type 1 (Th1) and Th2 priming with injection of CD8α+ and CD8α− dendritic cells, respectively, may not result from direct T cell activation by lymphoid versus myeloid dendritic cells, but rather from indirect modification of the response to immunogenic CD8α− dendritic cells by CD8α+ dendritic cells.
Keywords: dendritic cell, T cell, antigen presentation, tolerance
Appropriate induction of tolerance and immunity (memory) is a crucial function of the normal immune system. The discovery of a novel subset of dendritic cells (DCs) derived from a common lymphoid precursor (1) and located in the thymic medulla and the T cell zones of secondary lymphoid tissue (2), together with preliminary in vitro functional data (3, 4), has led to the suggestion that such lymphoid DCs (LDCs) may be responsible for generating tolerance in naive T cell populations (5, 6). We have recently proposed an evolutionary model of self/non-self discrimination (7) in which LDCs are postulated to induce deletional tolerance to self-antigens by means of preferential internalization and presentation of self-antigen via receptors recognizing the characteristic chemical structures generated by self–biosynthetic enzymes. In contrast, myeloid DCs (MDCs) are already known to express a number of “pattern recognition receptors” (8) that recognize the biosynthetic footprints of foreign organisms, and to stimulate naive T cells in such a way as to generate T cell memory (9–11).
As an experimental test of whether LDCs and MDCs induce distinct in vivo responses in naive T cells, splenic DCs were fractionated on the basis of CD8α expression, which has been defined as a marker capable of distinguishing splenic LDCs and MDCs in the mouse (2). A novel approach (6) was used in an attempt to ensure that only the injected DCs were capable of presenting the test antigen, a peptide of moth cytochrome c which is known to bind to IE but not IA molecules. By injecting IE+ DCs into a host that expressed IE only in the thymus, peptide presentation was restricted to the adoptively transferred APCs, to which the host T cells were nonetheless tolerant as a result of negative selection to IE in the thymus. Adoptive transfer of a cohort of purified moth cytochrome c (MCC)-specific naive T cells provided a sensitive detection system for presentation of MCC peptide in vivo.
Surprisingly, we found no evidence that CD8α+ DCs migrated into the draining lymph nodes (DLNs) after subcutaneous injection. Nonetheless, peptide-pulsed, sorted CD8α+ DCs were able to stimulate a significant T cell response. As expected, donor-derived CD8α− DCs were found in the DLNs and also stimulated T cell division. These data suggest an alternative interpretation of recent experiments in which subcutaneous injection of antigen-pulsed LDCs was shown to induce Th1 priming, whereas MDCs biased the response towards Th2 unless IL-12 was coinjected (12, 13).
Materials and Methods
Experimental Animals.
Transgenic (Tg) mouse lines were bred and housed under specific pathogen–free conditions at the Centenary Institute Animal Facility. Approval for all animal experimentation was obtained from the Institutional Ethics Committee at the University of Sydney. 107-1 and 36-2 lines of IEαd Tg mice (14, 15) were originally the gift of D. Lo (Scripps Research Institute, La Jolla, CA). The -D line TCR Tg line expressing the 5C.C7 receptor, which recognizes the COOH-terminal epitope of MCC in the context of IEαkβk, IEαkβb, or IEαdβb (16, 17), was maintained on a C57BL/6 background and crossed with 107-1 to provide double Tg offspring for use in experiments. In some experiments, donor DCs were derived from (107-1 × B6.SJLPtprca)F1 mice, to introduce the Ly5.1 allele used to track cells in vivo.
T Cell Purification, Labeling, and Injection.
Pooled inguinal, axillary, subscapular, cervical, and paraaortic LNs of naive (TCR × 107-1) mice served as the source of MCC-specific T cells. Purified T cells were prepared from single cell suspensions and labeled with 5-carboxy fluorescein succinimidyl ester (CFSE) as described previously (18). 107 T cells were injected into the lateral tail vein of unirradiated mice 2 d before injection of DCs.
DC Purification, Labeling, and Injection.
A modification of the protocol of Vremec and Shortman (19) was used to purify splenic DCs. Digestion with collagenase/EDTA and density centrifugation (ρ = 1.077) was followed by a two-step positive magnetic bead selection, replacing the negative selection/FACS® sorting steps in the original protocol. Thus, CD11c positive selection was performed using mAb N418 (20), anti–hamster FITC (Caltag), and anti-FITC Multisort microbeads (Miltenyi Biotec) followed by passage over a MACS® column (Miltenyi Biotec). The beads were removed by enzyme digestion, and a further positive selection for expression of CD8α was performed using anti-CD8α– coupled microbeads (Miltenyi Biotech) and a further MACS® column passage. Because this protocol failed to achieve >70% purity of CD8α+ cells, FACS® sorting for CD11c+CD8α positive and negative populations was substituted for the final CD8α bead selection in some experiments.
For peptide-pulsing, DCs were incubated in tissue culture medium (TCM; reference 18) containing 1 μM MCC87–103 peptide (Chiron Mimotopes) for 2 h at 37°C, then washed twice before injection. DCs (1–7 × 105) were injected subcutaneously into one hind footpad of each recipient mouse. Alternatively, recipients were immunized with 20 μg MCC87–103 peptide by injection into the lateral tail vein 12 h after DC administration.
In some experiments, DCs were labeled with 5-chloromethylfluorescein diacetate (CMFDA; Molecular Probes) before injection. Labeling was performed by resuspending cells at 107 cells/ml in TCM, incubating with 2 μm CMFDA for 15 min at 37°C, then washing and incubating in fresh TCM for an additional 30 min at 37°C. Cells were then washed twice before injection.
Flow Cytometry.
Five color antibody staining was performed as described previously for analysis of CFSE-labeled T cells (18). For detection of CMFDA-labeled DCs in DLNs, individual popliteal LNs were digested in collagenase/EDTA, as for spleen. A combination of anti-Ly5.1 (biotinylated A20.1 [provided by E.A. Boyse, Memorial Sloan-Kettering Cancer Center, New York] plus allophycocyanin-conjugated streptavidin), anti-CD11c (N418 supernatant plus anti–hamster Texas red [Caltag]), and CMFDA fluorescence was used to distinguish donor-derived DCs. Expression of CD8α was detected with PE-conjugated anti-CD8α (Caltag).
7-channel data acquisition (0.5–1 × 106 events per sample) was performed on a FACStarPLUS® flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Treestar).
Results and Discussion
Functional Responses of Antigen-specific T Cells to Subcutaneously Injected DCs.
The experimental protocol, designed to limit antigen presentation to the DC subset of interest, made use of a pair of MHC Tg lines in which IEαd was expressed on the H-2b background, allowing it to pair with endogenous IEβb to form a functional IE molecule capable of presenting MCC87–103 peptide to naive Tg T cells expressing the 5C.C7 TCR. Mice from the 36-2 line (14, 15), which expresses IEαd only in the thymus, were used as hosts of adoptively transferred purified responder T cells and of subcutaneously injected DCs. Mice from the 107-1 line (14, 15), in which IEαd is expressed with a wild-type distribution, served both as direct DC donors in the functional experiments and as parents of (107-1 × B6.SJLPtprca)F1 donors of Ly5.1+ DCs and (TCR Tg × 107-1)F1 donors of T cells.
To test the capacity of subcutaneously injected DCs to migrate to the DLNs and act as APCs presenting intravenously administered peptide to naive T cells, CD8α− and CD8α+ DCs were purified from the spleens of 107-1 Tg donors by two-step positive magnetic bead selection, first for expression of CD11c and then for CD8α. This yielded two fractions, a CD8α− fraction containing <1% CD8α+ contaminants, and a “CD8α+” fraction containing 50% CD8α− cells and 50% CD8α− cells. An aliquot of each purified fraction was pulsed with peptide in vitro. The DCs were then injected into hind footpads of unirradiated 36-2 Tg mice reconstituted with CFSE-labeled T cells 2 d before. 12 h after DC injection, MCC87–103 peptide was administered intravenously to the animals that had received unpulsed DCs, and antigen-specific T cell responses were determined 3 d later in the popliteal LNs. The marked increase in effectiveness of in vitro versus in vivo peptide loading was apparent from comparison of responses in the two groups (Fig. 1, A vs. B). We have previously noted a difference of similar magnitude between in vitro and in vivo peptide loading of naive B cells (6). The size of the response to intravenous peptide correlated with the number of CD8α− DCs in the inoculum, being barely detectable in the group that received the “CD8α+” DCs consisting of a mixture of CD8α− and CD8α+ DCs, and highest in the animals receiving a high dose of pure CD8α− DCs (Fig. 1 A). The response to both fractions of peptide-pulsed DCs was high. Analysis of the CFSE division profiles indicated that the number of cells recruited into division was again proportional to the number of CD8α− cells in the inoculum (Fig. 1 B, bottom panels). As expected, all the T cell responses were localized to the DLNs, as indicated by the lack of response in the contralateral popliteal node.
Figure 1.
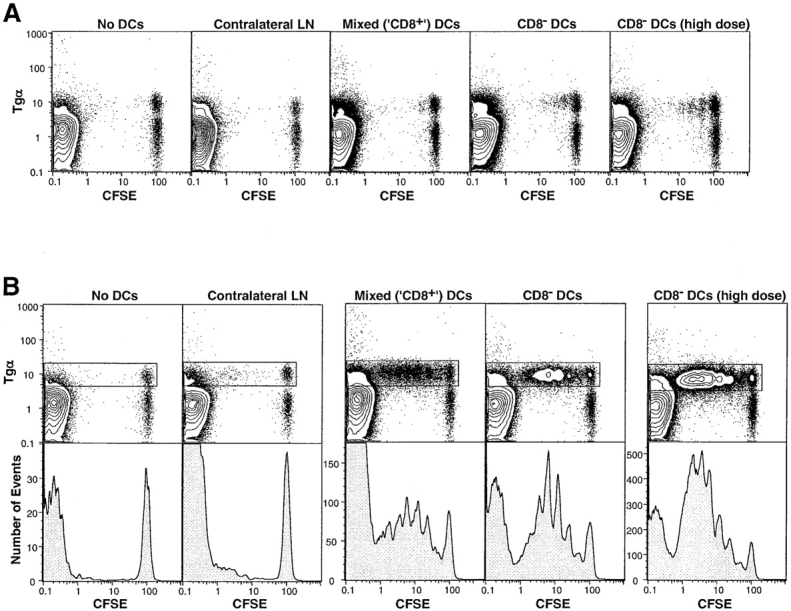
Local response of CFSE-labeled T cells to (A) intravenous peptide or (B) in vitro–pulsed peptide, after subcutaneous inoculation of DCs purifed using MACS® beads. The total numbers of CD11c+ DCs in the inocula were 6 × 105 “CD8+” (3.0 × 105 CD8+ plus 3.0 × 105 CD8−), 6.5 × 105 CD8− (6.2 × 105 CD8− plus 0.3 × 105 CD8+), and 12.0 × 105 CD8− (high dose; 11.4 × 105 CD8− plus 0.6 × 105 CD8+). Popliteal LN cells were gated for CD4+ propidium iodide (PI)− cells. The data are presented as 10% probability plots plus outliers. The histograms (B, bottom panels) show CFSE division peaks within the CD4+PI−Tgα+ T cell gate (boxed). Note the different histogram scales, indicating significantly different responses in the different groups.
Because of difficulties in achieving sufficient DC purity in the experiment described above, CD11c+ DCs obtained by positive bead selection were sorted on the basis of CD8α expression, yielding a CD8α− fraction with <0.01% contaminating CD8α+ cells, and a CD8α+ fraction contaminated with 1.0% CD8α− cells. After peptide pulsing, the cells were injected into the hind footpads of 36-2 mice. Once again, the response to CD8α− cells was marginally higher, as indicated by stimulation of one more T cell division than was seen in the response to CD8α+ cells (Fig. 2 A). As expected, expression of the activation markers CD69 and CD44 was increased before the first cell division (Fig. 2 B), and the pattern of CD69 downregulation was consistent with previous data derived from direct peptide administration to TCR Tg mice (Smith, A.L., and B. Fazekas de St. Groth, manuscript in preparation).
Figure 2.
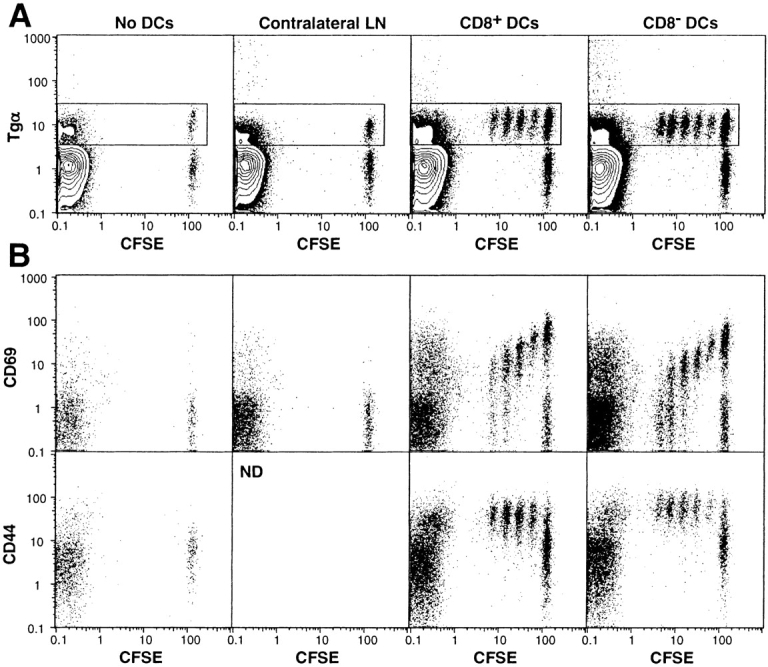
Local response of CFSE-labeled T cells to in vitro–pulsed peptide after subcutaneous inoculation of sorted DCs. The total numbers of CD11c+ DCs in the inocula were 2.5 × 105 CD8+ (2.5 × 105 CD8+ plus 0.03 × 105 CD8−) and 3.0 × 105 CD8− (no detectable CD8+). (A) Popliteal LN cells were gated for CD4+PI− cells. The data are presented as 10% probability plots plus outliers. (B) Dot plots of CFSE versus CD69 or CD44 expression within the CD4+PI−Tgα+ T cell gate (boxed in A).
Detection of Subcutaneously Injected DCs in the DLNs.
Tracking CMFDA-labeled DCs purified using the two methods described above indicated a very striking result, namely that the donor DCs isolated from the DLNs 22 h after inoculation were uniformly CD8α− (Fig. 3). The absence of donor-derived CD8α+ DCs in the DLNs could have resulted either from downregulation of CD8α by the inoculated CD8α+ DCs, or from failure of the CD8α+ DCs to migrate to the DLNs. The correlation between the number of CD8α− DCs in the inoculum and in the DLNs indicated that the latter was the more likely explanation. However, the disparity between the substantial T cell response to peptide-pulsed, sorted CD8α+ DCs (Fig. 2) and the tiny number of donor-derived DCs isolated from the DLNs (Fig. 3 B) also suggested that antigen may have been transferred from donor to host cells after injection. Since simple peptide transfer from donor to host cells was excluded by the experimental protocol (Fig. 1), any antigen transfer from donor cells must have involved transfer of both peptide and MHC, presumably as a complex formed during in vitro pulsing. This type of peptide/MHC transfer between DCs has recently been documented in vitro (21), and may account for previous reports in which thymic DCs presented endogenous self-antigen–MHC complexes synthesized by epithelial cells (22). It could also account for the phenomenon of cross-presentation of MHC class I molecules in the DLNs (23–25), a mechanism that is essential to ensure that viruses do not escape immune surveillance merely by failing to infect the APCs required for priming cytotoxic T cells.
Figure 3.
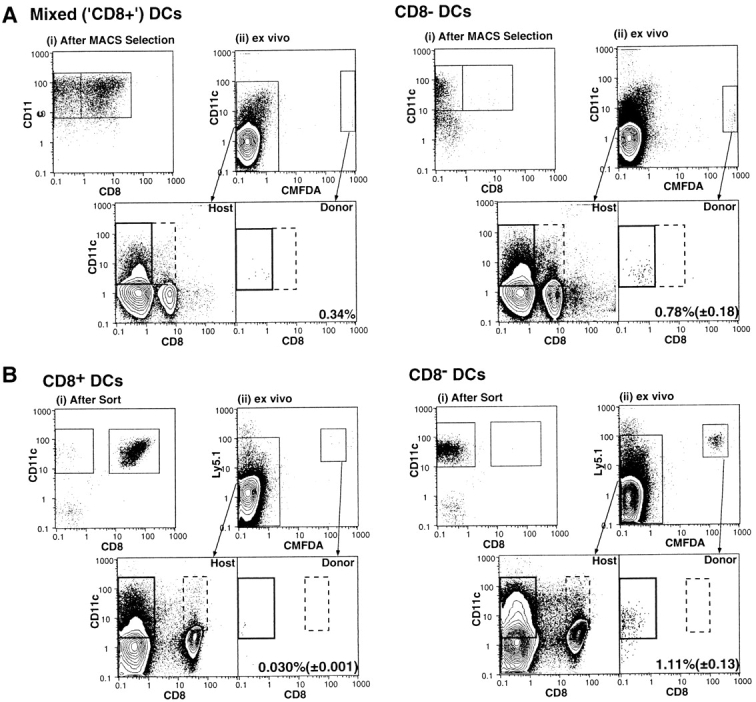
Detection of CMFDA-labeled DCs in the DLNs 22 h after subcutaneous inoculation. DCs were first detected in the DLNs 17 h after inoculation, and were undetectable 2 d later (not shown). The total numbers of CD11c+ DCs in the inocula were as follows: (A) 8.8 × 105 “CD8+” purifed using MACS® beads (4.1 × 105 CD8+ plus 4.7 × 105 CD8−) and 9.7 × 105 CD8− (9.7 × 105 CD8− plus 0.025 × 105 CD8+); and (B) 1.0 × 105 CD8+ (1.0 × 105 CD8+ plus 0.025 × 105 CD8−), of which a total of 0.059 ± 0.0013% were recovered from the DLNs, and 6.0 × 105 CD8− (no detectable CD8+), of which 0.31 ± 0.036% were recovered from the DLNs. Transfer of 2.5 × 105 CD8+ (2.5 × 105 CD8+ plus 0.05 × 105 CD8−, not shown) allowed recovery of 0.072% from the DLNs. The figures in the lower right of each panel show the number (± SEM) of donor-derived DCs as a percentage of total host DCs in the DLNs.
Functional Capabilities of LDCs In Vivo.
These data suggest an alternative interpretation of recent experiments characterizing the response of naive T cells to antigen-pulsed purified DC subpopulations (12, 13). These experiments have been interpreted to indicate that lymphoid DCs, purified on the basis either of CD8α expression (12) or of failure to express CD11b at high levels (13), migrate to DLNs after subcutaneous administration and generate preferential priming of a Th1 phenotype by means of their high levels of IL-12 production. In contrast, MDCs (CD8α−, CD11bhi) were suggested to prime for a Th2 response. However, in light of our finding that no CD8α+ DCs of donor phenotype could be found in the DLNs after subcutaneous injection, we suggest that priming for both Th1 and Th2 memory results from direct stimulation of naive T cells by MDCs that have migrated to the DLNs, with the differential outcomes conditional upon the cytokine environment and/or the amount of available antigen, which will differ depending on the constituents of the original inoculum. This interpretation is supported by the data of Maldonado et al. (12), demonstrating that injection of parenteral IL-12 stimulates T cell production of IFN-γ rather than IL-4 in response to subcutaneous injection of MDCs. Thus, when LDCs are injected subcutaneously, it is possible that IL-12 production at the site of injection may influence the ability of MDCs to induce Th1 development in the DLNs, without the LDCs playing any direct role in antigen presentation to naive T cells in the node. Shift in the Th1/Th2 balance of the response to soluble subcutaneous antigen after administration of Flt3 ligand (which preferentially expands the LDC population in vivo) versus GM-CSF (which increases only MDC numbers) (13), is also consistent with this interpretation. In addition, if peptide–MHC is transferred from LDCs to MDCs (either donor- or host-derived) before their migration to the DLNs, the concentration of antigen to which T cells are exposed could change dramatically, which has previously been demonstrated to influence the Th1/Th2 balance after in vitro priming (26).
These experiments do not directly address the physiological role of LDCs resident in the T cell zones of secondary lymphoid tissue. However, they are consistent with a model in which LDCs are generated in situ from precursors within the thymus (1) and secondary lymphoid tissue (27) and comprise a sedentary population which, in contrast to MDCs, relies on mechanisms other than migration from the periphery to capture antigen for presentation to T cells within the T cell zone (7). It has been suggested that LDCs induce deletional tolerance (3, 5, 6, 7). Our unpublished data, derived from comparison of the deletional response of naive peripheral CD4+ T cells to either intravenous peptide or a transgenic neoself-antigen, suggest that induction of deletional tolerance is accompanied by very early production of Th1 (IL-2, IL-3, IFN-γ) but not Th2 cytokines (IL-4 or IL-10), consistent with the high level of IL-12 production by stimulated LDCs (28). Whether Th1 immunogenic (memory) responses to foreign antigen are induced by direct presentation to T cells by a combination of LDCs and MDCs (29), or whether LDCs play only an indirect role in induction of memory responses, namely as cytokine producers but not APCs, remains to be elucidated.
Acknowledgments
The authors wish to thank Karen Knight and her staff for providing expert animal husbandry, and Kate Scott for screening the transgenic mice. A.L. Smith was supported by an Australian Postgraduate Award. B. Fazekas de St. Groth is a Wellcome Trust Senior Research Fellow.
This work was supported by the National Health and Medical Research Council, the Wellcome Trust, and the Medical Foundation of the University of Sydney.
References
- 1.Ardavin C, Wu L, Li C-L, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 2.Wu L, Li C-L, Shortman K. Thymic dendritic cell precursors: relationship to the lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Süss G, Shortman K. A subclass of dendritic cells kills CD4+T cells via Fas/Fas ligand–induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkel KD, Kronin V, Krummel MF, Shortman K. The nature of the signals regulating CD8 T cell proliferative responses to CD8α+ or CD8α−dendritic cells. Eur J Immunol. 1997;27:3350–3359. doi: 10.1002/eji.1830271234. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 6.Fazekas de St. Groth B, Cook MC, Smith AL, Wikstrom ME, Basten A. Role of dendritic cells in induction of tolerance and immunity in vivo. . Adv Exp Med Biol. 1997;417:255–263. doi: 10.1007/978-1-4757-9966-8_42. [DOI] [PubMed] [Google Scholar]
- 7.Fazekas de St. Groth B. The evolution of self tolerance: a new cell is required to meet the challenge of self- reactivity. Immunol Today. 1998;19:448–454. doi: 10.1016/s0167-5699(98)01328-0. [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Knight SC, Mertin J, Stackpoole A, Clark J. Induction of immune responses in vivowith small numbers of veiled (dendritic) cells. Proc Natl Acad Sci USA. 1983;80:6032–6035. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boog CJP, Kast WM, Timmers HTM, Boes J, de Waal LP, Melief CJM. Abolition of specific immune response defect by immunization with dendritic cells. Nature. 1985;318:59–62. doi: 10.1038/318059a0. [DOI] [PubMed] [Google Scholar]
- 11.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maldonado-López R, de Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α−subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulendran, B., J.L. Smith, G. Caspary, K. Brasel, D. Pettit, E. Maraskovsky, and C.R. Maliszewski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. In press. [DOI] [PMC free article] [PubMed]
- 14.Widera G, Burkly LC, Pinkert CA, Böttger EC, Cowing C, Palmiter RD, Brinster RL, Flavell RA. Transgenic mice selectively lacking MHC class II (I-E) antigen expression on B cells: an in vivo approach to investigate Ia gene function. Cell. 1987;51:175–187. doi: 10.1016/0092-8674(87)90145-0. [DOI] [PubMed] [Google Scholar]
- 15.Marrack P, Lo D, Brinster R, Palmiter R, Burkly L, Flavell RH, Kappler J. The effect of thymus environment on T cell development and tolerance. Cell. 1988;53:627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- 16.Fazekas de St. Groth, B., P.A. Patten, W.Y. Ho, E.P. Rock, and M.M. Davis. 1992. An analysis of T cell receptor-ligand interaction using a transgenic antigen model for T cell tolerance and T cell receptor mutagenesis. In Molecular Mechanisms of Immunological Self-Recognition. F.W. Alt and H.J. Vogels, editors. Academic Press, San Diego. 123–127.
- 17.Girgis L, Davis MM, Fazekas de St. Groth B. The avidity spectrum of T cell receptor interactions accounts for T cell anergy in a double transgenic model. J Exp Med. 1999;189:265–277. doi: 10.1084/jem.189.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook MC, Basten A, Fazekas de St. Groth B. Influence of B cell receptor ligation and T cell receptor affinity on T-B collaboration in vitro. . Eur J Immunol. 1999;28:4037–4049. doi: 10.1002/(SICI)1521-4141(199812)28:12<4037::AID-IMMU4037>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs. Cross-correlation of surface markers, changes with incubation and differences among thymus, spleen and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 20.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight SC, Iqball S, Roberts MS, Macatonia S, Bedford PA. Transfer of antigen between dendritic cells in the stimulation of primary T cell proliferation. Eur J Immunol. 1998;28:1636–1644. doi: 10.1002/(SICI)1521-4141(199805)28:05<1636::AID-IMMU1636>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Humbert C, Rudensky AY, Kyewski B. Presentation and intercellular transfer of self antigen within the thymic microenvironment: expression of the Eα peptide-I-Abcomplex by isolated thymic stromal cells. Int Immunol. 1994;6:1949–1958. doi: 10.1093/intimm/6.12.1949. [DOI] [PubMed] [Google Scholar]
- 23.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurts C, Heath WR, Carbone F, Allison JP, Miller JFAP. Constitutive class I–restricted exogenous presentation of self-antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–87. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 26.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+T helper cell phenotype development in a T cell receptor-αβ– transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulendran B, Lingappa J, Kennedy M, Smith J, Teepe M, Rudensky A, Maliszewski C, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- 29.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]


