Abstract
Anti-Ro60 autoantibodies are found in a variety of autoimmune disorders including systemic lupus erythematosus (SLE), Sjögren's syndrome, primary biliary cirrhosis, and active hepatitis. They are the most prevalent autoantibodies in normal individuals and in asymptomatic mothers of infants afflicted with neonatal lupus. In the present study, immune responses to recombinant human Ro60 (rhRo60) and recombinant mouse Ro60 (rmRo60) and selected Ro60 peptides in non–SLE-prone mice were investigated. Multiple T and B cell epitopes were identified in Ro60. Immunizations with either xenogeneic or autologous Ro60 induced autoantibodies to a diverse group of autoantigens. In addition to La and Ro52, proteins in the small nuclear ribonucleoprotein (snRNP) particles such as SmA, SmB, SmD, and 70-kD U1-RNP were unexpectedly identified as targeted antigens. In the studies involving synthetic Ro60 peptides, both human and mouse Ro60316–335 peptides, which differ in three amino acids, were found to contain dominant cross-reactive T cell determinants. Immunizations with these peptides induced autoantibodies to Ro60, La, SmD, and 70-kD U1-RNP without autoantibodies to Ro52, SmA, or SmB. With human Ro60316–335 as the immunogen, additional autoantibodies reactive with the Golgi complex were found. In contrast to the immunodominance of both human and mouse Ro60316–335 peptides, the T cell determinant in human Ro60441–465 was dominant, whereas that in the mouse peptide was cryptic. Immunization with human Ro60441–465 induced primarily anti-peptide Abs. Mouse Ro60441–465 failed to induce an antibody response. These results show that both the nature of the immunogen and the immunogenicity of the related endogenous antigen are important in determining the specificities of the autoantibodies generated. They have significant implications for proposed mechanisms on the generation of complex patterns of autoantibodies to a diverse group of autoantigens in SLE patients.
Keywords: systemic lupus erythematosus, determinant spreading, tolerance, autoimmunity, T and B cell epitopes
Systemic lupus erythematosus (SLE)1 is an autoimmune disorder with antinuclear Abs as a prominent feature (1–3). Among the targeted autoantigens in this disease, Ro60 (or Sjögren's syndrome A, SS-A) and La (SS-B), which are physically associated with hYRNAs, constitute a major class of SLE-related autoantigens, and Abs to them have been shown to be associated with specific clinical presentations (2, 4). The presence of Abs to these autoantigens in normal individuals and in asymptomatic mothers of infants affected with neonatal lupus suggests that there is a lack of tolerance to these autoantigens or that the tolerance can be readily broken. In this regard, autoantibodies to Ro60 and La have been induced in non–SLE-prone mice by immunizations with recombinant proteins (5–8).
Recently, this antigenic system has been utilized to show the role of intramolecular and intermolecular epitope spreading in the diversification of the autoimmune response to these antigens (5–10). Because of the availability of mouse as well as human recombinant proteins, La has been used extensively as the immunogen in many of these studies. In particular, emphasis has been made by Reynolds et al. (6) that there is hierarchical self-tolerance to T cell determinants (epitopes) within La and that cryptic or nondominant T cell epitopes are capable of breaking tolerance, leading to a diversified autoantibody response.
Because of the observation that the most prevalent autoantibodies in asymptomatic normal individuals are anti-Ro60 Abs (4), it is of considerable interest to explore the immune response to this autoantigen in normal non–SLE-prone mice. In this investigation, the immune responses to both autologous and rhRo60 in mice were studied. These responses involve multiple T and B epitopes within the immunogens. In addition to the induction of Ab to La and Ro52, these responses unexpectedly generated autoantibodies to multiple proteins in the small nuclear ribonucleoprotein (snRNP) particle. Evidence has been obtained that tolerance to the dominant T cell epitopes in Ro60 can be readily overcome, leading to a diverse autoimmune response.
Materials and Methods
Cloning and Expression of Recombinant Antigens.
The cDNAs encoding human Ro60, 70 kD U1-RNP (gifts from Jack Keene, Duke University, Durham, NC), SmB and SmD (from Joe Craft, Yale University, New Haven, CT), were cloned into the pQE expression vectors (Qiagen Inc., Chatsworth, CA) to generate recombinant fusion proteins with a 6XHis tag. Mouse Ro52 in pQE expression vector was a gift from James McCluskey (Flinders Medical Center, Bedford Park, South Australia). SmA cDNA in the pET expression vector was a gift from Joe Craft. For cloning of mouse Ro60 cDNA, two λgt10 libraries were used: a mouse liver 5′ stretch plus cDNA library from Clontech (Palo Alto, CA) and 33BTE-67, a mouse λ-δ T cell hybridoma library from Rebecca L. O'Brien (National Jewish Medical and Research Center, Denver, CO). They were screened with a 1.8-kb, EcoRI/NotI DNA fragment of human Ro60 under nonstringent conditions. Two independent clones, MuT 10.1 (2-kb insert from T-cell library) and MuL 23.1 (2.3-kb insert from liver cDNA library) were obtained from screening 1.2 × 106 colonies. Their DNA sequences were determined and data were analyzed using Eugene (Molecular Biology Information Resource, Baylor Medical College, Houston, TX) and GCG (Wisconsin Package, Version 8; Genetic Computer Group, Madison, WI) software. MuT 10.1 and MuL 23.1 had an overlap of 1.446 kb. The combined sequence of these two clones was 85% homologous to the human Ro60 sequence. It lacked a 170-bp fragment at the 5′ end. 5′ RACE (11) was used to amplify the missing 170-bp fragment. The entire coding region of mouse Ro60 was generated by PCR using WEHI 7.1 cDNA and cloned into the KpnI and HindIII sites of the pQE expression vector. Mouse La was similarly cloned from the liver cDNA library screened with full-length human La cDNA. The complete cDNA encoding mouse La was cloned into pQE expression vector. Recombinant proteins were expressed in Escherichia coli. Recombinant antigens expressed in pQE vectors were purified under denaturing conditions following manufacturer's instructions. Purified proteins were dialyzed against distilled water, and stored at −70°C until use. Recombinant Sm was purified as described by Fatenejad et al. (12).
Synthetic Peptides.
Overlapping peptides spanning the entire sequence of hRo60 and mRo60 were synthesized on an automated peptide synthesizer, AMS 422 (Gilson Inc., Middleton, WI) using Fmoc Chemistry. Peptides were analyzed and purified by reverse phase HPLC and their masses confirmed by mass spectrometry. Peptides used for immunizations were made in the Biomolecular Research Facility, University of Virginia.
Immunization.
6–8-wk-old female SJL/J and A/J (both from National Cancer Institute, Bethesda, MD) and BALB/cByJ mice (Jackson Laboratory, Bar Harbor, ME) were maintained in the animal facility at the University of Virginia. For in vitro lymph node cell (LNC) proliferative responses, mice were immunized with either 100 μg of recombinant protein or 50 μg of synthetic peptide emulsified in complete Freund's adjuvant (CFA) (Difco Laboratories, Detroit, MI) in one hind footpad and at the base of the tail. For analysis of antibody production, animals were immunized initially as described above. They were subsequently immunized with 50 μg of antigen emulsified in incomplete Freund's adjuvant (Difco Laboratories) intraperitoneally, on days 14 and 28. Controls were immunized with adjuvant alone in a similar way. Tail bleeds from mice were collected at different time points postimmunization, and sera were assayed for specific Abs.
Lymph Node Proliferation Assays.
2 wk after immunization, draining lymph nodes were removed and single-cell suspensions were prepared. LNCs were cultured in 96-well plates at 3 × 105 cells/well in DMEM (BioWhittaker Inc., Walkersville, MD) supplemented with 10% FCS (Hyclone Laboratories, Logan, UT), 2 mM l-glutamine, nonessential amino acids, sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin (GIBCO BRL, Gaithersburg, MD), and 5 × 10−5 M 2-mercaptoethanol, with or without antigens in triplicates. The cells were incubated at 37°C in a humidified, 5% CO2 atmosphere for 4 d. Plates were pulsed with [3H]thymidine (NEN Research Products, Boston, MA), 0.5 μCi/well, during the last 16 h of culture. The cells were harvested onto glass fiber filters (Wallac Oy, Turku, Finland) using a semiautomated cell harvester (Skatron Instruments Inc., Sterling, VA) and radioactivity measured by liquid scintillation counting using a Betaplate counter (LKB Instruments Inc., Piscataway, NJ). The results are expressed, either as stimulation index (SI), determined by dividing the mean triplicate antigen-specific cpm by mean triplicate cpm from wells without antigen, or as mean triplicate ΔCPM (mean triplicate cpm with antigen minus mean triplicate cpm without antigen).
ELISA.
Microtiter plates, Immulon 2 and Immulon 4 (Dynatech Inc., Chantilly, VA) were coated with rRo60 and synthetic peptides, respectively, at a concentration of 2 μg/well, in carbonate-bicarbonate buffer, pH 9.6, overnight at 4°C. The wells were then blocked with PBS containing 3% BSA (200 μl/ well). Sera diluted in PBS containing 0.1% Tween 20 (PBST) and 3% BSA were added to the washed plates. Bound antibodies were detected with peroxidase-conjugated goat anti–mouse IgG (Southern Biotechnology Associates Inc., Birmingham, AL), using the substrate o-phenylenediamine (0.05%) (Sigma Chemical Co., St. Louis, MO), 0.06% hydrogen peroxide in citrate-phosphate buffer, pH 5.0. Reaction was stopped after 15 min by the addition of 50 μl/well of 2.5 N sulfuric acid. Absorbance was read at 490 nm. Volumes of the diluted sera, Abs, and substrates were 100 μl/well, and incubations were for 2 h at room temperature. Plates were washed five times with PBST between steps.
Immunofluorescence.
HeLa cells or NIH/3T3 cells were grown on round coverslips (1 cm diameter). The cells were fixed in methanol for 7 min at −20°C. Sera diluted in PBS containing 1% BSA were added. After 2 h, bound antibodies were detected with rhodamine-coupled goat anti–mouse IgG. In between steps, coverslips were washed three times with PBS.
Antibody Absorption.
Peptides hRo60316–335 and hRo60441–465 were coupled to CNBr activated Sepharose 4B beads (Pharmacia Biotech Inc., Piscataway, NJ) following the manufacturer's instructions. JS7A, a peptide corresponding to amino acids 330–342 of ZP3, which is a protein in the mouse zona pellucida, was also coupled to beads and used as a control in absorption experiments. The beads were incubated overnight with PBS containing 3% BSA at 4°C. Sera were diluted in PBST containing 3% BSA and incubated with the beads for 2 h at room temperature. The absorbed sera were assayed for anti-peptide activity in ELISA and other antibody activity.
Western Blotting.
WEHI 7.1 cell extract was run on a 7.5% SDS-PAGE and transferred overnight onto nitrocellulose paper. After a 5% milk protein blocking step, sera diluted in PBST containing 5% milk protein were incubated with the nitrocellulose paper. Bound antibodies were detected with peroxidase-labeled goat anti–mouse IgG, and blots were developed using enhanced chemiluminescence (Pierce Chemical Co. Rockford, IL). All incubations were for 2 h at room temperature, and blots were washed with PBST three times in between steps.
Slot Blot.
The slot blot apparatus from Millipore Inc. (Bedford, MA) was used. Each slot had a length of 8-mm. Purified recombinant antigens were loaded at a concentration of 5 μg/slot in 8 M urea. The 8 mm strips were cut into three equal parts. After a blocking step with PBS containing 5% milk protein overnight at 4°C, the strips were incubated with diluted sera and the bound Abs were detected in a manner similar to that described in the preceding paragraph.
Immunoprecipitation of mYRNAs Associated with Ro60.
The mYRNAs associated mRo60 were immunoprecipitated as described by Craft and Hardin (13). Briefly, WEHI 7.1 cells were suspended at 2.5 × 105 cells/ml in phosphate-free RPMI 1640 supplemented with 5% dialyzed FCS. The cells were grown for 14 h in the presence of 10 μCi/ml of 32P (NEN Research Products). The 32P-labeled RNA associated with Ro60 were immunoprecipitated with immune and control sera. The precipitated RNA were electrophoresed and revealed by autoradiography.
Results
The Immune Responses to rhRo60 Were Directed to Multiple T and B Determinants.
T and B cell responses to rhRo60 were studied in SJL/J (H-2s), BALB/c (H-2d), and A/J (H-2a). All three strains mounted a strong T cell proliferative response to rhRo60 (Fig. 1 A). Strong antibody responses were also induced. Abs to the immunogen were readily detected 14 d after the initial immunization. By day 30, high antibody titers were generated and reactivity to rhRo60 was detectable at a serum dilution of 106 (Fig. 1 B). Control mice injected with CFA alone did not give specific T and B cell responses. Two other experiments gave similar results.
Figure 1.
Immune response to rhRo60 in SJL/J (circles), A/J (squares), and BALB/c (triangles) mice immunized with rhRo60. T cell responses were studied in LNC proliferative assays (A) and antibody responses were determined by ELISA (B). The LNC proliferative responses are expressed as mean triplicate ΔCPM. In A, results are shown for mice immunized with rhRo60 (open symbols) and with CFA (filled symbols). (B) The ELISA results are expressed as mean duplicate OD490nm and are shown for day 14 (•••••) and day 30 (−) pooled sera. Sera from control mice immunized with CFA, gave OD490nm < 0.1 at a serum dilution of 1:100.
A panel of overlapping peptides spanning the entire sequence of hRo60 was made and used to map T and B epitopes recognized by mice immunized with rhRo60. In preliminary experiments, peptides at a concentration of 20 μM were found to be optimal in recalling T cell responses in vitro. A peptide was considered to contain a T epitope(s) if it gave an SI > 2.0 in at least three independent experiments. Fig. 2 A shows representative results for SJL/J mice. Multiple peptides recalled the proliferative response in varying magnitudes. Peptides hRo60441–465 and hRo60316–335 were the most dominant peptides, followed by peptide hRo60306–325, hRo6026–45, hRo60401–425, and hRo60481–505. Multiple T cell determinants were also mapped in A/J and BALB/c mice, and the results are summarized in Fig. 2 B.
Figure 2.
Delineation of T cell determinants on hRo60 in mice of different haplotypes. In vitro LNC proliferative responses were recalled, with synthetic peptides at 20 μM concentration. Results are expressed as SI, a ratio of the mean triplicate CPM obtained with peptide and the mean triplicate cpm without peptide. (A) Multiple determinants recognized by SJL/J T cells are present in Ro60. Results are shown as mean triplicate SI ± SEM from three independent experiments. Peptides giving an SI > 2 are marked with an asterisk (*). For comparison, the response to rhRo60 at a concentration of 3 μg/ml is shown. (B) A summary of T cell determinants on hRo60, seen by SJL/J, A/J, and BALB/c mice. Amino acid sequences for peptides giving a SI > 2 are shown.
The B cell epitopes on Ro60 were mapped in ELISA, using microtiter plates coated with synthetic peptides. Fig. 3 shows results for sera obtained on days 14 (closed bars) and 30 (open bars) after immunization. Three clusters of reactivities were detected, with most determinants located on the COOH-terminal region of the molecule (hRo60 amino acids 401–538). B cell epitopes were also mapped in the middle portion of the molecule, hRo60 amino acids 240– 315. The sera recognized only two peptides from the NH2-terminal region. While peptide hRo601–25 was recognized by all strains, peptide hRo6061–85 was recognized only in SJL/J and A/J strains. Similarly, peptides hRo60261–285, hRo60456–475, and hRo60466–485 were recognized in SJL/J and A/J but not in BALB/c. Peptide hRo60201–225 appears to be specific to BALB/c and peptide hRo60316–335 for SJL/J.
Figure 3.
Mapping of B cell determinants on hRo60. Sera from, SJL/J, A/J, and BALB/c strains of mice (four/group), immunized with rhRo60, were pooled and reactivity to overlapping synthetic peptides of hRo60 was determined in ELISA. Sera were used at a dilution of 1:500. Results are expressed as mean duplicate OD490nm and are shown for sera obtained on day 14 (▪) and day 30 (□) after immunization. Sera obtained from mice immunized with CFA alone gave a OD490nm < 0.05 at 1:500 dilution.
Both T and B Cell Responses to rmRo60 Were Inducible.
Because of a sequence difference between human and mouse Ro60, the immune response generated by hRo60 immunization could be strongly mediated by these differences between the xenogeneic and autologous forms of this autoantigen. To determine whether responses to autologous Ro60 could be demonstrated, the cDNA encoding mouse Ro60 was cloned and expressed. Our sequence was in agreement with that reported by Wang et al. (14). There is 90% homology at the amino acid level between the mouse and human Ro60, and the amino acid differences scatter throughout the whole sequence. Immunization with purified rmRo60 induced T cell proliferative responses in all strains of mice (Fig. 4). The response in SJL/J mice was much higher than that in BALB/c and A/J. Proliferative responses were not seen in the animals immunized with only CFA, indicating specificity of the response.
Figure 4.
T cell proliferative responses to rmRo60 in mice of different haplotypes: SJL/J (• rmRo60, ○ CFA), A/J (▪ rmRo60, □ CFA), and BALB/c (▾ rmRo60, ▿ CFA). Results are expressed as mean triplicate ΔCPM. Data for SJL/J strain of mice are represented on the left y-axis. Data for A/J and BALB/c mice are plotted on the right y-axis.
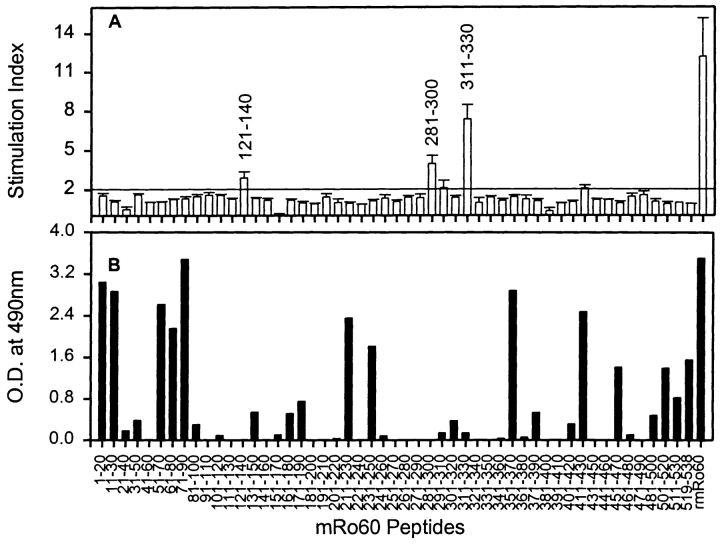
A set of peptides (20 mers with an overlap of 10) spanning the entire sequence of mRo60 was synthesized in order to undertake T cell epitope mapping. Only 3 peptides, mRo60121–140, mRo60281–300, and mRo60311–330, were able to recall the proliferative responses in SJL/J after immunization with rmRo60 (Fig. 5). The most dominant peptide was mRo60311–330. Interestingly, this peptide overlaps the dominant T cell determinant mapped in hRo60, peptide hRo60316–335. These results indicate a lack of tolerance to this region on the Ro60 antigen. The other dominant epitope mapped on hRo60 was in peptide hRo60441–465. However, the mRo60 peptides mRo60441–460 and mRo60451–470 that span this region could not recall the proliferative responses.
Figure 5.
Mapping of T and B cell determinants on mRo60. (A) In vitro T cell proliferative responses were recalled with synthetic peptides of mRo60, at 20 μM concentration. Results are expressed as mean of SI ± SEM from three independent experiments. A mean SI > 2.0 was considered positive. For comparison, the response to rmRo60 at a concentration of 10 μg/ml is included. (B) Reactivity of pooled sera (day 67) at a dilution of 1:500, was checked with synthetic peptides of mRo60 in ELISA. Results are expressed as mean duplicate OD490nm.
The panel of peptides was also used to map B cell determinants on mRo60. As shown in Fig. 5 B, multiple peptides reacted with the pooled sera at day 67 postimmunization. These B cell determinants are present throughout the whole span of mRo60. Immune sera at later time points gave similar reactivity patterns.
Both Xenogeneic rhRo60 and Autologous rmRo60 Induced Intermolecular Determinant Spreading.
Sera from SJL mice immunized with rmRo60 and rhRo60 were positive for antinuclear antibodies (ANA) (Fig. 6, A and B, respectively). Although results are shown for pooled sera, all mice had ANA in their sera. Such antibodies were not detected in mice immunized with only CFA (Fig. 6 C).
Figure 6.

Induction of ANAs following immunization with Ro60 antigens. Sera obtained on day 67, postimmunization, were pooled and used at a dilution of 1:200 to stain methanol-fixed HeLa cells (detected by indirect immunofluorescence). a, b, and c represent sera from mice immunized with rmRo60, rhRo60, and CFA, respectively.
All mice immunized with either rmRo60 or rhRo60 produced high titers of Abs to the immunogens. In both groups of mice, immunoprecipitating Abs to native mRo60 were induced (data not shown). In addition to expected reactivity to La and Ro52 (5–9), Abs to SmA, SmB, SmD, and U1RNP associated 70 kD protein were also observed (Fig. 7). In the control mice immunized with CFA, only weak reactivity to La, SmD, and U1RNP associated 70 kD protein was observed in two of seven mice studied. Surprisingly, recombinant dihydrofolate reductase (DHFR)- 6XHis protein included as a control protein was reactive with some of the immune sera. As presented later, this reactivity was also found in some of the sera from mice immunized with either human or mouse Ro60316–335, which do not have the 6XHis tag. Thus it is concluded that this reactivity was not directed against the 6XHis tag. Similar results were obtained in an additional experiment. These results suggest that intermolecular epitope spreading had occurred in mice immunized with either rhRo60 or rmRo60.
Figure 7.
Intermolecular determinant spreading of antibody responses in SJL/J mice immunized with Ro60 antigens. Reactivities of sera with different ribonucleoproteins are shown in slot blots. Each lane represents a serum sample at a dilution of 1:250.
Intermolecular and Intramolecular Antibody Diversification Is Induced by Peptide hRo60316–335 but Not by Peptide hRo60441–465.
The presence of multiple T and B epitopes on both rhRo60 and rmRo60 makes it difficult to address the phenomenon of spreading and the mechanisms involved due to the complexity of the immune response elicited. For this purpose, we used synthetic peptides representing the dominant T cell epitopes on hRo60 as immunogens.
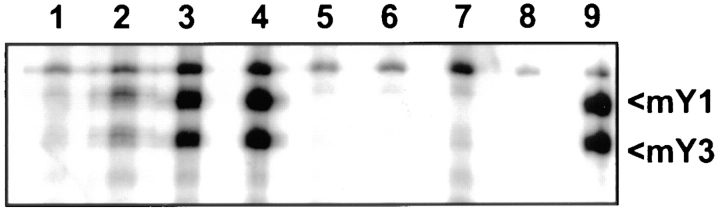
Sera from mice immunized with peptide hRo60316–335 had very high titers of antipeptide Abs. The titers peaked between days 60 and 90. In addition, Abs to nonhomologous peptide hRo60401–425 were detected as early as 24 d after immunization. The titer against hRo60401–425 increased with time up to 60 d postimmunization. With time, the immune sera had increasing amounts of Abs with more diverse specificities. These sera were capable of immunoprecipitating native Ro60 as shown in Fig. 8. Two cytoplasmic RNA moieties, namely mY1 and mY3 associated with mRo60 (15), have been shown to be precipitated by Ro-specific antisera. The appearance of Abs to the nonhomologous peptide and to the native Ro60 determinant(s) represents intramolecular determinant spreading in response to the immunization with the peptide hRo60316–335.
Figure 8.
Immunoprecipitation of mYRNAs associated with mRo60. WEHI 7.1 cells were labeled with 32P, and RNA species were immunoprecipitated and visualized by autoradiography. Lanes 1–4 represent pooled sera on days 24, 37, 60, and 90, respectively, from SJL/J mice immunized with hRo60316–335. Lanes 5–7, represent pooled sera on days 37, 60, and 90, respectively, from mice immunized with CFA. Lane 8 is control, without any serum, and lane 9 is a human anti-Ro60 reference serum from the Center for Disease Control.
Antibody diversification to multiple intracellular determinants was also evident in that the pooled immune sera recognized multiple cellular proteins in WEHI 7.1 cell extract in Western blot analysis (Fig. 9). Of particular interest was the protein recognized at 48 kD (Fig. 9, lanes 2–4), which was also recognized by the CDC anti-La reference serum (Fig. 9, lane 1), suggesting that intermolecular epitope spreading to La might have occurred. This reactivity was not observed in adjuvant immunized mice (Fig. 9, lanes 5–7). These immune sera also reacted with additional protein bands at both high and low molecular weight ranges. Based on this result, the identity of the proteins recognized by these sera can only be speculated on. To identify other autoantigens, the sera were checked for reactivity to purified recombinant ribonucleoproteins in slot blots (Fig. 10). All sera reacted strongly with rmRo60. Interestingly, reactivity to La, SmD, and U1RNP associated 70 kD protein was also detected. In a few mice, reactivity to DHFR was observed. However, no reactivity to Ro52 and SmB, which have a 6XHis tag, was observed. Immunization of mice with a control peptide JS7A did not generate antibodies reactive with these proteins, although very weak reactivity to Ro60 was observed in two of six control mice (Fig. 10).
Figure 9.

Reactivity of pooled immune sera with WEHI 7.1 cell extracts in Western blot. Each lane was loaded with protein equivalent to 2.5 × 106 cells. Pooled sera were used at a dilution of 1:100. Lane 1, human anti-La reference serum from CDC; lanes 2–4, day 37, 60, and 90 sera from mice immunized with peptide hRo60316–335; lanes 5–7, days 37, 60, and 90 sera from mice immunized with CFA.
Figure 10.
Intermolecular determinant spreading of antibody responses in SJL/J mice immunized with hRo60 peptides. Reactivity of sera from mice immunized with peptides hRo60316–335, hRo60441–465, and ZP3 peptide JS7A, with different ribonucleoproteins was tested in slot blots. Each lane represents an individual serum, at a dilution of 1:250.
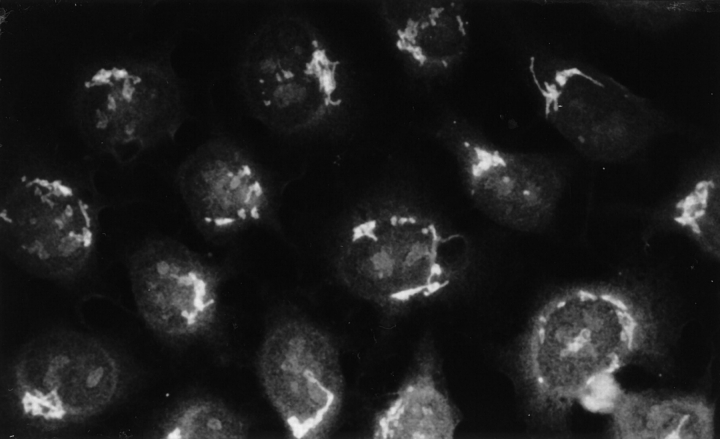
The diversification of the autoreactivity in response to the immunization with hRo60316–335 was further documented in our indirect immunofluorescence study. Anticytoplasmic and antinuclear Abs appeared shortly after immunization. More remarkable was the appearance of a population of Abs that stained the Golgi complex (Fig. 11); the anti-Golgi staining was not seen in HeLa cells treated with brefeldin A, which disrupts the architecture of Golgi apparatus (16). Similar results were obtained when a mouse cell line, NIH/3T3, was used as the substrate. Sera from control mice did not give similar staining.
Figure 11.
Anti-Golgi staining patterns of antibodies generated by hRo60316–335 immunization. HeLa cells grown on coverslips and fixed in methanol were used as substrate. Results are shown for pooled sera (day 60) used at a dilution of 1:200.
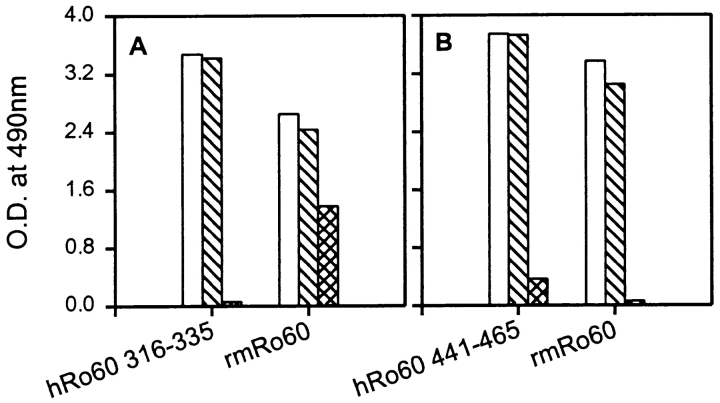
Antibodies reactive to mRo60 epitopes not related to peptide hRo60316–335 were generated in mice immunized with the peptide (Fig. 12). Sera were absorbed with the immunogen to deplete them of antipeptide antibodies. These sera still recognized rmRo60 in ELISA, indicative of intramolecular epitope spreading (Fig. 12 A). Absorption of sera with a control peptide JS7A had little effect on the reactivity of sera, either to the peptide or to rmRo60 (Fig. 12 A). Results presented in Figs. 8–12 were representative of three experiments.
Figure 12.
Intramolecular diversification of antibody responses against mRo60 following immunization with synthetic peptides. Pooled sera from mice immunized either with hRo60316–335 peptide (A), or hRo60441–465 peptide (B) were absorbed with their respective immunogens and peptide JS7A. Reactivity of unabsorbed and absorbed sera with peptides and rmRo60 was determined in ELISA. Results are expressed as mean duplicate OD490nm. Open bars denote unabsorbed pooled sera. Cross bars denote pooled sera, absorbed with peptide JS7A. The hatched bars denote pooled sera absorbed with hRo60316–335 in (A) and with hRo60441–461 in (B).
The immune response against hRo60441–465, which contains a dominant T cell epitope of hRo60, was markedly different than that against peptide hRo60316–335. Immunization with peptide hRo60441–465 generated very high titers (105–106) of antibodies reactive with the peptide in ELISA. Although these antibodies reacted with rmRo60 in ELISA (Fig. 12 B), they did not immunoprecipitate native mRo60 (data not shown). Analysis of antibody reactivities in slot blots (Fig. 10) showed reactivity with rmRo60 in all animals and reactivity to rmRo52 and SmA in only one out of five animals. No reactivity was observed to La, SmB, SmD, U1RNP associated 70 kD protein, and DHFR. Absorption of pooled sera with the immunogen abolished reactivity to the peptide and all reactivity to rmRo60 as shown in Fig. 12 B. Similar results were obtained in two additional experiments involving 10 mice.
Peptide mRo60316–335 Was a Dominant T Cell Epitope, Whereas mRo60441–465 Was a Cryptic T Cell Epitope.
The possibility that the difference observed between the immune responses to the two immunodominant T cell peptides on hRo60 might be due to lack of presentation of the autologous mRo60441–465 was suggestive from data shown in Fig. 5 A. Peptide mRo60311–330 (overlaps hRo60316–335) was able to recall the LNC proliferative response in mice immunized with rmRo60, whereas peptides mRo60441–430 and mRo60451–470 failed to do so. This was further explored. Peptides mRo60316–335 and mRo60441–465 were made and used to recall the LNC proliferative responses in mice immunized with rmRo60. Fig. 13 shows representative results from one of three experiments. While peptide mRo60316–335 could recall the proliferative response, mRo60441–465 could not, indicating that the T cell epitope in the latter peptide is cryptic.
Figure 13.
Recall of in vitro LNC proliferative responses by rmRo60 (open bars) and peptides, mRo60316–335 (cross bars) and mRo60441–465 (hatched bars), in mice immunized with rmRo60. Results are expressed as mean triplicate SI. And an SI > 2.0 was considered positive.
Despite the three amino acid differences between hRo60316–335 and mRo60316–335, the T cell epitopes in these two peptides were cross-reactive. Peptide mRo60316–335 was able to recall proliferative responses in mice immunized with the homologous human peptide (Fig. 14 A), and vice versa (Fig. 14 B). In both cases, the proliferative responses were recalled by the recombinant Ro60 proteins, further confirming the noncryptic nature of the auto T epitope in the peptide mRo60316–335.
Figure 14.
Cross priming between human and mouse peptides. SJL/J mice were immunized with peptides: hRo60316–335 (A), mRo60316–335 (B), hRo60441–465 (C), and mRo60441–465 (D). 2 wk later, proliferative responses were recalled with peptides hRo60316–335 (•), mRo60316–335 (○), hRo60 (▴), mRo60 (▵), hRo60441–465 (▪), and mRo60441–465 (□). Results are expressed as mean triplicate SI. y-axis scales for A, B and C, D are different. Peptide concentrations are in μM and those for Ro60 in μg/ml as shown in the x-axis.
LNC proliferative responses were induced by immunization with both peptides hRo60441–465 and mRo60441–465 (Fig. 14, C and D). However, these responses were significantly lower than those observed for the peptides, hRo60316–335 and mRo60316–335. At best, very weak cross-reactivity was observed between the peptides hRo60441–465 and mRo60441–465. Immunization of mice with the mRo60441–465 peptide induced a weak proliferative response with SI at 2–4. In addition, rmRo60 was not able to recall this response. The weak cross-reactivity between the mouse and human peptides is likely due to the more extensive differences in their amino acid sequences.
Homologous Peptide mRo60316–335 Induced Antibody Diversification.
Immunization of mice with peptide mRo60316–335 resulted in high titers of anti-peptide antibodies, capable of immunoprecipitating native mRo60. Reactivity to La, SmD, and U1RNP associated 70-kD protein and DHFR was observed in slot blots (Fig. 15 A) indicative of intermolecular epitope spreading. The reactivity patterns of the immune sera were similar to those of sera of mice immunized with the human peptide (Figs. 7 and 15 B). With respect to intramolecular spreading, absorption of sera with the immunogen had little effect on the reactivity to Ro60 although almost all reactivity to the peptide was abolished (Fig. 15 C). Absorption with control peptide JS7A had no effect, either on the reactivity with the peptide or with the whole antigen. It is of interest to note that these immune sera did not stain the Golgi complex. Similar results were obtained in an additional experiment.
Figure 15.

Induction of antibody diversification in mice immunized with peptide mRo60316–335. (A) Reactivity of day 30 sera from four mice with different ribonucleoproteins was tested in slot blots. All sera were tested at 1:250 dilution. (B) Reactivity of pooled sera from SJL/J mice immunized with hRo60316–335 peptide (lane 1), JS7A (lane 2), and CFA (lane 3). (C) Pooled sera from mice immunized with mRo60316–335 were absorbed with the immunogen (hatched bars) and control peptide JS7A (cross bars). The reactivities of the unabsorbed (open bars) and the absorbed sera were determined in ELISA with either mRo60316–335 (left) or mRo60 (right) as the target antigen. The sera absorbed with the immunogen did not react with the immunogen mRo60316–335 while the reactivity with mRo60 remained. Results are expressed as mean duplicate OD490nm.
Discussion
Ro60 (SS-A) was initially described by Clark et al. (17) as Ro and by Alspaugh and Tan (18) as SS-A. Later it was documented that Ro and SS-A were identical (19). Ro (SS-A) is a 60-kD protein that is thought to be associated with La through their binding to a common set of hYRNAs (20). Autoantibodies to Ro52 are commonly found in patients with anti-Ro60 and/or anti-La autoantibodies. The absence of physical association between Ro60 and Ro52 has been shown (21). The presence of autoantibodies to Ro60 has been associated with SLE, Sjögren's syndrome, neonatal lupus, primary biliary cirrhosis, and chronic active hepatitis. Antibodies against Ro60 have been found in a substantial number of normal individuals, many of whom have been identified after their children were diagnosed with neonatal lupus (4). In addition, a considerable number of patients with only a mild inflammatory arthritis also have anti-Ro60 Ab. Thus the understanding of the autoimmune response to Ro60 has significant implications in our understanding of autoimmunity and the pathogenesis of SLE.
In this study, T and B cell responses to multiple determinants within Ro60 have been induced by immunization with either human or autologous recombinant Ro60 in multiple non–lupus-prone strains of mice (Figs. 2, 3, and 5). It is of interest that antinuclear Abs are generated in the immunized mice (Fig. 6). In addition, Abs to La, Ro52, and peptides associated with the snRNP particles are also generated (Fig. 7). The generation of Abs to La and Ro52 is anticipated because of the clinical impression that responses to these two antigens are often linked to the anti-Ro60 response. In addition, other investigators have reported that Abs to these three antigens are detected in the sera from mice immunized with any one of these three autoantigens. However, the detection of Abs to Sm peptides and 70-kD U1-RNP is unexpected. Thus, the autoantibody responses to immunizations with both xenogeneic and autologous Ro60 target a much broader range of autoantigens. The involvement of Sm and U1-RNP peptides as targets in the anti-Ro60 response would provide impetus to reexamine some of the current impressions and hypotheses regarding the genesis of SLE-related autoantibodies. Specifically, the clinical impression that SLE-related Abs react to linked sets of autoantigens, i.e., Ro60 and La, Sm and U1-RNP (snRNPs), and histones and DNA (22, 23) should be revisited. The “particle hypothesis” (24), which was postulated to explain the occurrence of autoantibodies in linked sets, and which states that chromatin, snRNPs, and Ro/La complexes are available, as intact particles to the immune system, should be scrutinized.
The identification of multiple T cell determinant containing peptides in both human and mouse Ro60 allows us to explore the immunogenicity of dominant and subdominant T cell determinants of this auto antigen. Ro60316–335 has both T and B cell determinants. Although there are three amino acid differences between the xenogeneic and autologous peptides, both peptides have dominant T cell determinants for multiple strains of mice. Both human and mouse peptides induce a T cell proliferative response, and the intact recombinant proteins recall the induced T cell responses (Fig. 14). These peptides have cross-reactive T cell epitopes and they recall T cell responsiveness induced by the recombinant Ro60 proteins (Figs. 2 and 13). By the criteria of Sercarz et al. (25), the T cell epitopes in the xenogeneic and the autologous peptides should be considered immunodominant. In addition, these peptides induce a diverse autoantibody response to multiple autoantigens. The targets for the diversification of the autoantibody responses include La, SmD, 70-kD U1-RNP, and other unidentified intracellular constituents (Figs. 9–11). Although only the data on SJL/J are presented here, we found similar but not identical data in A/J and BALB/c. Thus, a dominant T cell determinant of an autoantigen need not be tolerogenic. The hierarchical self-tolerance to T cell determinants demonstrated in La (SS-B) (6) may not be generalized to other autoantigens readily.
Although human Ro60441–465 has a dominant T cell epitope for SJL (Fig. 2), the homologous mouse peptide cannot recall the anti-hRo60441–465 T cell response (Fig. 13). In addition, the autologous peptide appears to be cryptic in that it induces a weak T cell response to the immunogen and that the induced response cannot be recalled with the intact mouse Ro60 (Fig. 14). It is of interest to note that human Ro60441–465 induces only very limited autoantibody response to the immunizing peptides with little diversification to other autoantigens. This observation and the diverse autoantibody response induced by either human or mouse Ro60316–335 support the hypothesis that only immune responses to auto–T cell epitopes that can be generated by processing and presentation of the endogenous antigen lead to a diversified autoantibody response (6, 26). This hypothesis suggests a mechanism in which endogenous autoantigens play a significant role in the diversification of the autoantibody responses by relevant self-peptides and provides an explanation for the inability of certain self-peptides of SLE-related autoantigens such as Ro60441–465, certain La (6), and Sm (27) peptides to induce a diverse autoantibody response.
There are differences in the antibody specificities in the immune sera from mice immunized with the recombinant Ro60 proteins and those immunized with Ro60316–335. The immune sera of mice immunized with recombinant Ro60 are reactive with Ro52, SmA, and SmB, whereas those from mice immunized with Ro60316–335 are not reactive. Immune sera from mice immunized either with whole Ro60 protein or the peptide Ro60316–335 have Abs to native Ro60 as demonstrated by immunoprecipitation of the associated mYRNAs. In addition, absorption experiments show that the immune sera from mice immunized with Ro60316–335 have Abs to determinants elsewhere in Ro60. These data are not in congruency with the particle hypothesis as discussed previously. If the particle hypothesis (24) is operative in our system, the differences in the fine specificities of the autoantibodies generated by immunizations with the intact protein and the peptide should not be significantly different. In addition, these data also suggest the presence of an antigenic peptide or peptides within the Ro60 molecule, which can induce autoantibodies reactive with Ro52, SmA, and SmB. Studies with peptides containing dominant T cell epitopes within either human or mouse Ro60 may provide data to support this thesis.
The generation of anti-Golgi Ab in mice immunized with human Ro60316–335 but not in mice immunized with human Ro60, mouse Ro60, or mouse Ro60316–335 requires some comments. The anti-Golgi Ab cannot be absorbed by mouse Ro60 or mouse Ro60316–335. In addition, human Ro60 cannot inhibit the staining. However, the human peptide blocks the staining completely. Further experiments to be presented elsewhere (28) indicate that there is a cross-reactive B cell epitope shared between the Golgi complex and the human peptide. These data indicate that the immunogen determines the specificity of the Ab generated even in the immune responses involving autoantigens.
From the above discussion, the nature of the immunogen is an important factor in determining the specificities of the autoimmune response in our system. This importance is further underscored by our early experience using synthetic peptides as the immunogen. During the early stage of our investigation, synthetic peptides were used as the immunogen without HPLC purification after they were made. These crude peptides induced Abs to Ro52 consistently, in addition to those autoantigens targeted by the immune response to the purified peptides. It is apparent that a contaminating peptide(s) was responsible for the induction of an antibody response with this specificity. This observation has led us to prepare our immunizing peptides carefully to assure the peptides are >95% in purity. In this regard, multiple antigenic peptides (MAPs) have frequently been used to induce autoantibodies and to study determinant spreading and autoimmunity (9, 29–31). In the construction of these MAPs, the purity of the peptides is not assured. More importantly, it is likely that these peptides may contain multiple T and B cell epitopes other than those specified by the amino acid sequences, the structure of which are not readily apparent. For example, recently the use of MAP of PPPGMRPP, a B cell epitope of SmB/B′ as the immunogen, has led to the conclusion that B cell epitope spreading is not H-2 restricted in mice (30). Differential antigen processing and presentation of the immunogen MAP by two strains of mice sharing a similar H-2 region may result in T cell responses to different epitopes which may induce autoantibodies with differing specificities, providing an appearance of non-H2 restriction. Thus, experimental data involving MAP as the immunogen in the study of autoimmunity should be interpreted with caution.
In the present study, evidence has been presented that autoantibody diversification occurs through a process termed determinant spreading (32, reviewed in 10, 33–35). Both intramolecular and intermolecular epitope spreading occurs. Careful absorption experiments to be reported elsewhere (28) indicate that similar conformational epitopes are present in many of the SLE-related autoantigens. With peptide Ro60316–335 as the immunogen, considerable amounts of anti-Ro60 autoantibodies cannot be readily absorbed by the immunogen, indicating that intramolecular determinant spreading occurs by generating specific Ab to Ro60 determinants not present in the immunogen. In contrast, the immunogen can absorb almost completely the Ab to La, SmD, and 70 kD U1-RNP. The latter result indicates that intermolecular determinant spreading may be due to the generation of distinct populations of autoantibodies to the conformational epitopes shared in varying degrees among these autoantigens. Thus, there appears to be a qualitative difference between intramolecular determinant spreading and intermolecular spreading in our system. In addition, molecular mimicry may play an important role in the diversification of an autoimmune response in SLE.
Acknowledgments
We wish to thank Hideko Yamaguchi, Min Chu, and Zoya Mednikova for their technical assistance and Lena Garrison for manuscript preparation.
This work was supported by National Institutes of Health grants RO1 AR-42027, RO1 AR-42465, RO1 DE-12544, RO1 AI-43248, NO1 AI-45199, P50 AR-45222, K11 AR-01906, and P30 CA-44579.
Abbreviations used in this paper
- ANA
antinuclear antibody
- DHFR
dihydrofolate reductase
- LNC
lymph node cell
- MAP
multiple antigenic peptides
- rhRo60
recombinant human Ro60
- rmRo60
recombinant mouse Ro60
- SI
stimulation index
- SLE
systemic lupus erythematosus
- SS-A
Sjögren's syndrome-A
- SS-B
Sjögren's syndrome-B
- snRNP
small nuclear ribonucleoproteins
References
- 1.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus (SLE) Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune disease and probes for cell biopsy. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 3.Reichlin, M., and J.B. Harley. 1997. Antinuclear antibodies: an overview. In Dubois' Lupus Erythematosus. D.J. Wallace and B.H. Hahn, editors. Williams and Wilkins, Baltimore, MD. 397–405.
- 4.Harley JB, Scofield RH, Reichlin M. Anti-Ro in Sjögren's syndrome and systemic lupus erythematosus. Rheum Dis Clin North Am. 1992;18:337–358. [PubMed] [Google Scholar]
- 5.Topfer F, Gordon T, McCluskey J. Intra- and intermolecular spreading of autoimmunity involving the nuclear self-antigens LA (SS-B) and Ro (SS-A) Proc Natl Acad Sci USA. 1995;92:875–879. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds P, Gordon TP, Purcell AW, Jackson DC, McCluskey J. Hierarchical self-tolerance to T cell determinants within the ubiquitous nuclear self-antigen La (SS-B) permits induction of systemic autoimmunity in normal mice. J Exp Med. 1996;184:1857–1870. doi: 10.1084/jem.184.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keech CL, Gordon TP, McCluskey J. The immune response to 52-kDa Ro and 60-kDa Ro is linked in experimental autoimmunity. J Immunol. 1996;157:3694–3699. [PubMed] [Google Scholar]
- 8.Tseng EE, Chan EKL, Miranda E, Gross M, Di Donato F, Buyon JP. The 52-kd protein as a target of intermolecular spreading of the immune response to components of the SS-A/Ro-SS-B/La complex. Arthritis Rheum. 1997;40:936–944. doi: 10.1002/art.1780400523. [DOI] [PubMed] [Google Scholar]
- 9.Scofield RH, Henry WE, Kurien BT, James JA, Harley JB. Immunization with short peptides from the sequence of the systemic lupus erythematosus-associated 60-kDa Ro autoantigen results in anti-Ro ribonucleoprotein autoimmunity. J Immunol. 1996;156:4059–4066. [PubMed] [Google Scholar]
- 10.Craft J, Fatenejad S. Self antigens and epitope spreading in systemic autoimmunity. Arthritis Rheum. 1997;40:1374–1382. doi: 10.1002/art.1780400803. [DOI] [PubMed] [Google Scholar]
- 11.Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatenejad S, Mamula MJ, Craft J. Role of intermolecular/intrastructural B and T cell determinants in the diversification of autoantibodies to ribonucleoprotein particles. Proc Natl Acad Sci USA. 1993;90:12010–12014. doi: 10.1073/pnas.90.24.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft, J., and J.A. Hardin. 1992. Immunoprecipitation assays for the detection of soluble nuclear and cytoplasmic nucleoproteins. In Manual of Clinical Laboratory Immunology. N.R. Rose, H. Friedman, and J.L. Fahey, editors. American Society of Microbiology, Washington, DC. 747–754.
- 14.Wang D, Buyon JP, Chan EKL. Cloning and expression of mouse Ro60-kDa ribonucleoprotein SS-A/ Ro. Mol Biol Rep. 1996;23:205–210. doi: 10.1007/BF00351170. [DOI] [PubMed] [Google Scholar]
- 15.Prujin GJM, Wingens PAETM, Peters SLM, Thijssen JPH, Venrooji WJV. Ro RNP associated Y RNAs are highly conserved among mammals. Biochim Biophys Acta. 1993;1216:395–401. doi: 10.1016/0167-4781(93)90006-y. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara T, Okada K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- 17.Clark G, Reichlin M, Tomasi TB. Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythematosus. J Immunol. 1969;102:117–120. [PubMed] [Google Scholar]
- 18.Alspaugh MA, Tan EM. Antibodies to cellular antigens in Sjogren's syndrome. J Clin Invest. 1975;55:1067–1073. doi: 10.1172/JCI108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alspaugh M, Maddison PJ. Resolution of the identity of certain antigen-antibody systems in systemic lupus erythematosus and Sjogren's syndrome. Arthritis Rheum. 1979;22:796–798. doi: 10.1002/art.1780220719. [DOI] [PubMed] [Google Scholar]
- 20.Boire G, Craft J. Human Ro ribonucleoprotein particles: characterization of native structure and stable association with the La polypeptide. J Clin Invest. 1990;85:1182–1190. doi: 10.1172/JCI114551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelekar A, Saitta MR, Keene JD. Molecular composition of Ro small ribonucleoprotein complexes in human cells. J Clin Invest. 1994;93:1637–1644. doi: 10.1172/JCI117145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northway JD, Tan EM. Differentiation of antinuclear antibodies giving speckled staining patterns in immunofluorescence. Clin Immunol Immunopathol. 1972;1:140–154. [Google Scholar]
- 23.Mattioli M, Reichlin M. Physical association of two nuclear antigens and mutual occurrence of their antibodies: the relationship of the Sm and RNAprotein (Mo) system in SLE sera. J Immunol. 1973;110:1318–1324. [PubMed] [Google Scholar]
- 24.Hardin JA. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986;29:457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- 25.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Ann Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 26.Lou YH, Mcelveen MF, Garza KM, Tung KSK. Rapid induction of autoantibodies by endogenous ovarian antigens and activated T cells: implication in autoimmune disease pathogenesis and B cell tolerance. J Immunol. 1996;156:3535–3540. [PubMed] [Google Scholar]
- 27.Bockenstedt LK, Gee RJ, Mamula MJ. Self-peptides in the initiation of lupus autoimmunity. J Immunol. 1995;154:3516–3524. [PubMed] [Google Scholar]
- 28.Fu, S.M., U.S. Deshmukh, J.E. Lewis, and F. Gaskin. 1998. Diversification of autoantibody response to ribonucleoproteins: role of crossreactive determinants in epitope spreading. The Immunologist. Suppl. 1:75.
- 29.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm BB′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–461. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James JA, Harley JB. A model of peptide-induced lupus autoimmune B cell epitope spreading is strain specific and is not H-2 restricted in mice. J Immunol. 1998;160:502–508. [PubMed] [Google Scholar]
- 31.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14:203–208. doi: 10.1016/0167-5699(93)90163-F. [DOI] [PubMed] [Google Scholar]
- 34.Vanderlugt CJ, Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8:831–836. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung KSK, Lou YH, Garza KM, Teuscher C. Autoimmune ovarian disease: mechanism of disease induction and prevention. Curr Opin Immunol. 1997;9:839–845. doi: 10.1016/s0952-7915(97)80187-2. [DOI] [PubMed] [Google Scholar]