Abstract
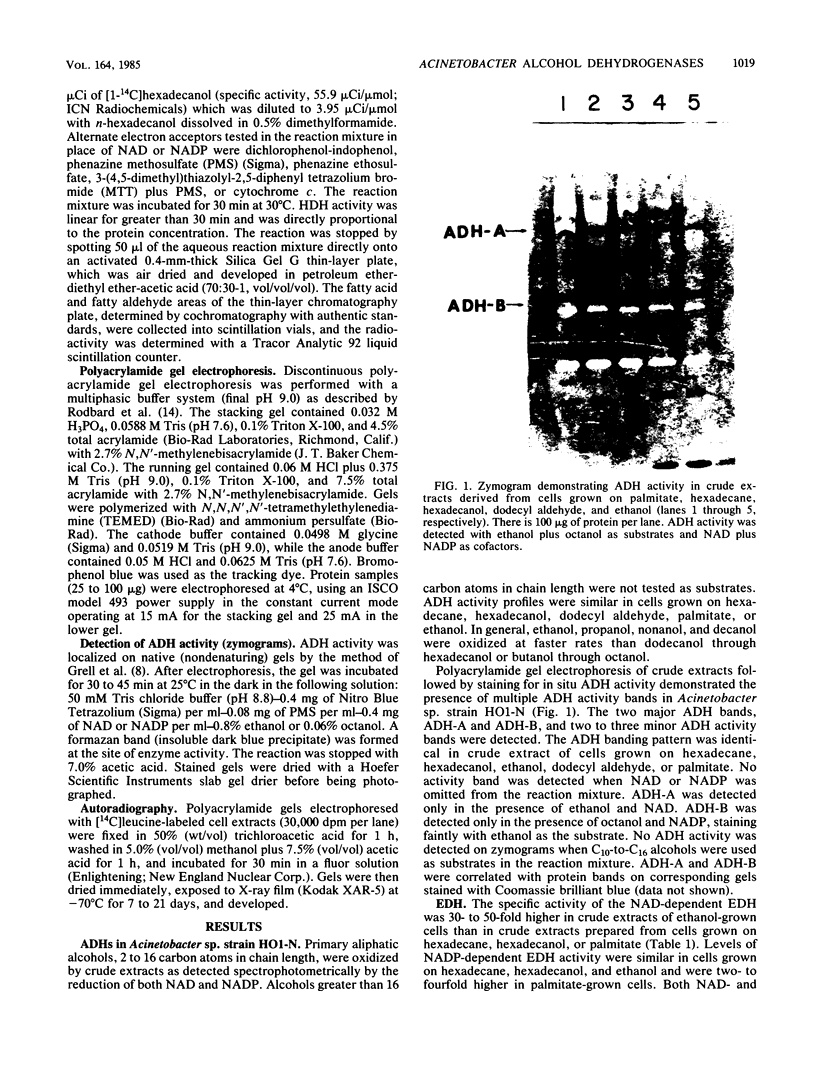
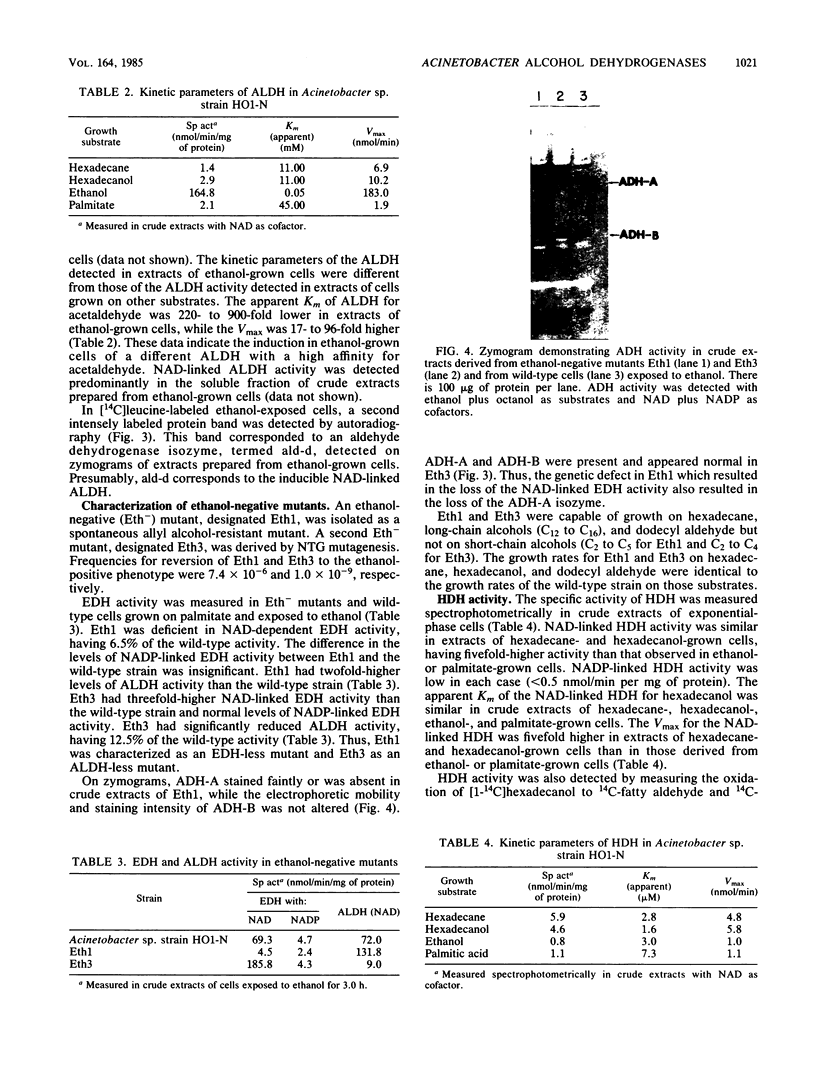
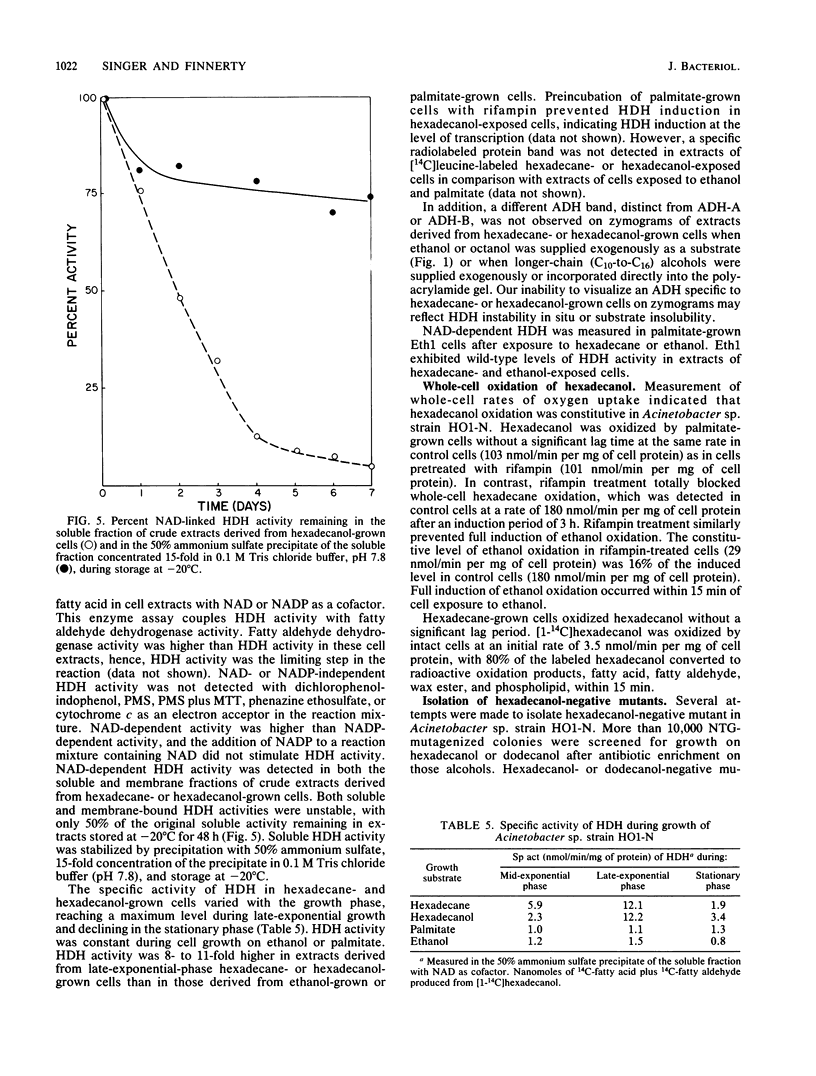
Multiple alcohol dehydrogenases (ADH) were demonstrated in Acinetobacter sp. strain HO1-N. ADH-A and ADH-B were distinguished on the basis of electrophoretic mobility, pyridine nucleotide cofactor requirement, and substrate specificity. ADH-A is a soluble, NAD-linked, inducible ethanol dehydrogenase (EDH) exhibiting an apparent Km for ethanol of 512 microM and a Vmax of 138 nmol/min. An ethanol-negative mutant (Eth1) was isolated which contained 6.5% of wild-type EDH activity and was deficient in ADH-A. Eth1 exhibited normal growth on hexadecane and hexadecanol. A second ethanol-negative mutant (Eth3) was acetaldehyde dehydrogenase (ALDH) deficient, having 12.5% of wild-type ALDH activity. Eth3 had threefold-higher EDH activity than the wild-type strain. ALDH is a soluble, NAD-linked, ethanol-inducible enzyme which exhibited an apparent Km for acetaldehyde of 50 microM and a Vmax of 183 nmol/min. Eth3 exhibited normal growth on hexadecane, hexadecanol, and fatty aldehyde. ADH-B is a soluble, constitutive, NADP-linked ADH which was active with medium-chain-length alcohols. Hexadecanol dehydrogenase (HDH), a soluble and membrane-bound, NAD-linked ADH, was induced 5- to 11-fold by growth on hexadecane or hexadecanol. HDH exhibited apparent Kms for hexadecanol of 1.6 and 2.8 microM in crude extracts derived from hexadecane- and hexadecanol-grown cells, respectively. HDH was distinct from ADH-A and ADH-B, since HDH and ADH-A were not coinduced; Eth1 had wild-type levels of HDH; and HDH requires NAD, while ADH-B requires NADP. NAD- and NADP-independent HDH activity was not detected in the soluble or membrane fraction of extracts derived from hexadecane- or hexadecanol-grown cells. NAD-linked HDH appears to possess a functional role in hexadecane and hexadecanol dissimilation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett C. H., Dodgson K. S., White G. F., Payne W. J. Preliminary observations on alcohol dehydrogenases in Comamonas terrigena that exhibit stereospecificity towards secondary alcohols. Biochem J. 1980 Jun 1;187(3):703–709. doi: 10.1042/bj1870703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardmore-Gray M., Anthony C. The absence of quinoprotein alcohol dehydrogenase in Acinetobacter calcoaceticus. J Gen Microbiol. 1983 Oct;129(10):2979–2983. doi: 10.1099/00221287-129-10-2979. [DOI] [PubMed] [Google Scholar]
- Benson S., Shapiro J. Plasmid-determined alcohol dehydrogenase activity in alkane-utilizing strains of Pseudomonas putida. J Bacteriol. 1976 May;126(2):794–798. doi: 10.1128/jb.126.2.794-798.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Savageau M. A. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl Environ Microbiol. 1977 Feb;33(2):434–444. doi: 10.1128/aem.33.2.434-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. P., Cronan J. E., Jr Acetaldehyde coenzyme A dehydrogenase of Escherichia coli. J Bacteriol. 1980 Oct;144(1):179–184. doi: 10.1128/jb.144.1.179-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D., Cronan J. E., Jr Escherichia coli mutants with altered control of alcohol dehydrogenase and nitrate reductase. J Bacteriol. 1980 Jan;141(1):177–183. doi: 10.1128/jb.141.1.177-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell E. H., Jacobson K. B., Murphy J. B. Alcohol Dehydrogenase in Drosophila melanogaster: Isozymes and Genetic Variants. Science. 1965 Jul 2;149(3679):80–82. doi: 10.1126/science.149.3679.80. [DOI] [PubMed] [Google Scholar]
- Grund A., Shapiro J., Fennewald M., Bacha P., Leahy J., Markbreiter K., Nieder M., Toepfer M. Regulation of alkane oxidation in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):546–556. doi: 10.1128/jb.123.2.546-556.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makula R., Finnerty W. R. Microbial assimilation of hydrocarbons. I. Fatty acids derived from normal alkanes. J Bacteriol. 1968 Jun;95(6):2102–2107. doi: 10.1128/jb.95.6.2102-2107.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. R. Allyl alcohol-induced irreversible inhibition of yeast alcohol dehydrogenase. Biochem Pharmacol. 1974 Aug 15;23(16):2328–2331. doi: 10.1016/0006-2952(74)90563-2. [DOI] [PubMed] [Google Scholar]
- Rando R. R. In situ generation of irreversible enzyme inhibitors. Nat New Biol. 1972 May 10;237(71):53–53. doi: 10.1038/newbio237053a0. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacylamide gel electrophoresis. Anal Biochem. 1971 Mar;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- STEWART J. E., KALLIO R. E., STEVENSON D. P., JONES A. C., SCHISSLER D. O. Bacterial hydrocarbon oxidation. I. Oxidation of n-hexadecane by a gram-negative coccus. J Bacteriol. 1959 Sep;78:441–448. doi: 10.1128/jb.78.3.441-448.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. E., Finnerty W. R. Fatty aldehyde dehydrogenases in Acinetobacter sp. strain HO1-N: role in hexadecanol metabolism. J Bacteriol. 1985 Dec;164(3):1011–1016. doi: 10.1128/jb.164.3.1011-1016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. E., Tyler S. M., Finnerty W. R. Growth of Acinetobacter sp. strain HO1-N on n-hexadecanol: physiological and ultrastructural characteristics. J Bacteriol. 1985 Apr;162(1):162–169. doi: 10.1128/jb.162.1.162-169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchert H., Grunow M., Aurich H. Regulation und einige Eigenschaften einer partikulären, akzeptorabhängigen Alkoholdehydrogenase aus Pseudomonas putida beim Wachstum auf n-Alkanen. Z Allg Mikrobiol. 1978;18(9):675–680. doi: 10.1002/jobm.3630180906. [DOI] [PubMed] [Google Scholar]
- Tauchert H., Grunow M., Harnisch H., Aurich H. Reinigung und einige Eigenschaften der NADP+-abhängigen Alkoholdehydrogenase aus Acinetobacter calcoaceticus. Acta Biol Med Ger. 1976;35(10):1267–1272. [PubMed] [Google Scholar]
- Tauchert H., Roy M., Schöpp W., Aurich H. Phridinnucleotid-unabhängige Oxydation von längerkettigen aliphatischen Alkoholen durch ein Enzym aus Acinetobacter calcoaceticus. Z Allg Mikrobiol. 1975;15(6):457–460. doi: 10.1002/jobm.3630150609. [DOI] [PubMed] [Google Scholar]
- Van der Linden A. C., Huybregtse R. Occurrence of inducible and NAD(P)-independent primary alcohol dehydrogenases in an alkane-oxidizing Pseudomonas. Antonie Van Leeuwenhoek. 1969;35(3):344–360. doi: 10.1007/BF02219154. [DOI] [PubMed] [Google Scholar]
- Wills C., Kratofil P., Londo D., Martin T. Characterization of the two alcohol dehydrogenases of Zymomonas mobilis. Arch Biochem Biophys. 1981 Sep;210(2):775–785. doi: 10.1016/0003-9861(81)90245-9. [DOI] [PubMed] [Google Scholar]






