Abstract
Antigen injection into animals causes antigen-specific T cells to become activated and, rapidly thereafter, die. This antigen-induced death is inhibited by inflammation. To find out how inflammation has this effect, various cytokines were tested for their ability to interfere with the rapid death of activated T cells. T cells were activated in vivo, isolated, and cultured with the test reagents. Two groups of cytokines were active, members of the interleukin 2 family and the interferons (IFNs) α and β. This activity of IFN-α/β has not been described previously. It was due to direct effects of the IFNs on the T cells and was not mediated by induction of a second cytokine such as interleukin 15. IFN-γ did not slow the death of activated T cells, and therefore the activity of IFN-α/β was not mediated only by activation of Stat 1, a protein that is affected by both classes of IFN. IFN-α/β did not raise the levels of Bcl-2 or Bcl-XL in T cells. Therefore, their activity was distinct from that of members of the interleukin 2 family or CD28 engagement. Since IFN-α/β are very efficiently generated in response to viral and bacterial infections, these molecules may be among the signals that the immune system uses to prevent activated T cell death during infections.
Keywords: interferon γ, apoptosis, interferon type I, cell survival, T cell
Injection of antigen or superantigen into animals activates specific T cells and allows them to go through several rounds of division. However, these activated cells rapidly go on to die (1–4). Activation-induced death is caused by several processes in vivo. These probably include engagement of Fas and TNF receptors on the surfaces of activated T cells by their ligands, lack of essential growth factors such as IL-2 or -4 (5–8), and also other unknown phenomena.
Whatever the causes of activated T cell death, clearly this phenomenon is not compatible with a productive immune response since because of it animals frequently contain fewer antigen-specific T cells several days after exposure to the antigen than they did before antigen was given. However, it is now known that activation-induced death is inhibited in animals if they are given antigen and an agent that stimulates inflammation. For example, we have shown that superantigen-specific T cells survive better if superantigen-immunized animals are also exposed to bacterial LPS, TNF-α, or vaccinia virus (9, 10, and Mitchell, T., J. Kappler, and P. Marrack, manuscript submitted for publication). Others have shown that antigen-specific T cells can be rescued by similar agents (4, 11).
In attempts to find out what makes activated T cells die or live in animals, we have developed an in vitro system in which the behavior of the T cells in tissue culture reflects their fate in vivo. In this system T cells are activated in animals with antigen or superantigen in the presence or absence of an inflammatory agent. 2 d later the T cells are purified and placed in culture. T cells that have been activated in animals by exposure to antigens and inflammatory agents die slowly in culture. T cells activated in animals by antigen alone and which therefore are destined to die in animals also die rapidly in culture. We and others have previously shown that members of the IL-2 family of cytokines prevent this rapid death via a pathway that probably involves Bcl-2 induction (12–14). In an attempt to find other factors that may interfere with activated T cell death, we screened a large number of other cytokines. Of these only one family, the type I IFNs (IFN-α/β) were effective. Experiments with cells from IFN-α/βR–deficient mice showed that, in vitro, the IFN-α/β acted directly on the T cells and not, as has been described in vivo (15), via intermediary induction of IL-15. The IFN-α/β did not act by raising levels of Bcl-2 or Bcl-XL in the activated cells. We conclude that IFN-α/β can act as survival factors for activated T cells, and that they act via an intracellular pathway that is not shared by IFN-γ and its receptor and is not the same as that induced by the IL-2 family or CD28 engagement (12–14, 16–24).
Materials and Methods
Mice.
C57BL/10SgSnJ (B10) mice were purchased from The Jackson Laboratory. 129/SvEv animals came from Taconic Farms and IFN-α/βR–deficient mice (25) were bred from pairs of mice provided by Drs. R. Schreiber (Washington University, St. Louis, MO) and M. Aguet (Institute of Experimental Immunology, Zurich, Switzerland). All animals were kept under specific pathogen-free conditions in the Biological Resource Center at the National Jewish Medical and Research Center.
Mouse Manipulations.
T cells were activated in mice by injection of the superantigen staphylococcal enterotoxin B (SEB; Toxin Technology).1 Mice were given 150 μg SEB intravenously in balanced salt solution.
Cell Purification and Staining.
T cells were purified on nylon wool columns (26). Intact cells were stained as previously described (27) in balanced salt solution, 2% FBS, 0.1% sodium azide (staining buffer) with biotinylated anti-Vβ3, 6, 8.1–3, or 17. The biotinylated antibodies were detected with streptavidin coupled to PE or cychrome (Cy). Cells were also stained with FITC or Cy-coupled anti–mouse CD4 or anti–mouse CD8 and PE-coupled antibody to the MHC class I protein, Kb.
Cells were analyzed for expression of Bcl-2 as previously described (17). In brief, intact cells were stained as described above with anti-Vβ and/or anti-CD4 and/or anti-CD8 antibodies. The cells were then permeabilized in staining buffer containing 0.03% saponin and hamster anti–mouse Bcl-2. The mixture was incubated at room temperature for 30 min. The cells were then washed twice with staining buffer, 0.03% saponin before addition of FITC-coupled mouse anti–hamster immunoglobulin antibodies. Controls included cells stained without addition of anti–Bcl-2 or stained with anti–human Bcl-2.
Intracellular Bcl-X was analyzed by a method similar to that used for Bcl-2. Intact cells were stained as described above, washed, permeabilized with 0.1% saponin, and incubated with rabbit anti–mouse Bcl-X antibodies (Transduction Labs.) at room temperature for 30 min. The cells were washed again and incubated for an additional 30 min at room temperature with FITC– coupled goat anti–rabbit immunoglobulin (Fisher Scientific Co.) before final washing and analysis.
In some experiments T cells from IFN-α/βR–deficient mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Inc.) (28) by incubation for 15 min at room temperature in balanced salt solution concentrations of 107 cells/ml and 0.1 μg/ml CFSE.
In most of the experiments in this paper, dead cells were distinguished from live by their characteristics on the forward versus side scatter bit maps from the cytofluorographs. This method of distinguishing live from dead lymphocytes was in agreement with analyses in which dead cells were identified by their possession of <2 N amounts of DNA (see Fig. 1), by their ability to take up propidium iodide or to bind annexin V (Teague, K., D. Hildeman, and T. Mitchell, unpublished results).
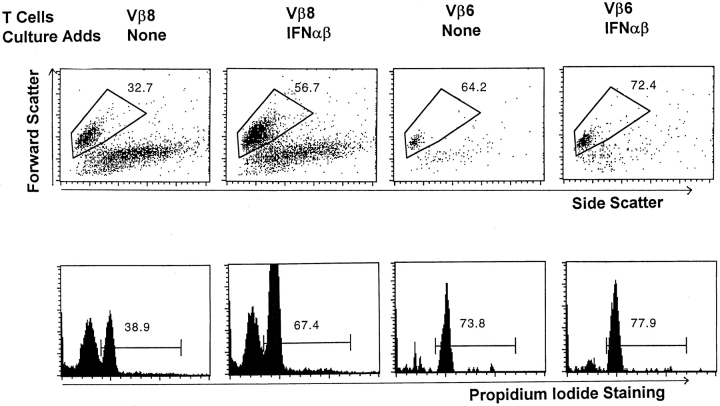
Figure 1.
IFN-α/β prevent the death of activated T cells. T cells were isolated from the lymph nodes of mice given 150 μg SEB 2 d previously. The cells were cultured for 24 h in the absence or presence of 3,333 U/ml IFN-α/β. The cells were then stained with FL-anti-CD4, biotinylated anti-Vβ8x, or anti-Vβ6 followed by PE-labeled streptavidin and Cy-labeled anti-CD8. These cells were then identified as live or dead based on their light scatter properties (top). Separate wells of cells cultured under identical conditions were stained with FL-anti-Vβ6 or anti-Vβ8. These cells were then permeabilized with saponin and incubated with propidium iodide to measure their DNA content. Light scatter gates were set for the permeabilized cells and propidium iodide staining was assessed as shown (bottom). Results shown are typical of duplicate cultures. Values shown on the figure are the percentage of the indicated cells that were alive as defined by the method used.
Except where otherwise mentioned, all reagents were prepared in our laboratory or purchased from PharMingen. Staining was read on FACScan® or FACScalibur® instruments (Becton Dickinson) and analyzed using Cell Quest software (Becton Dickinson).
Cell Culture and Reagents.
Purified T cells were cultured as previously described (9, 14) in the presence or absence of cytokines. Mouse IL-2 and human IL-15 were purchased from R&D Systems. A mixture of mouse IFN-α/β was bought from Sigma Chemical Co. Recombinant mouse IFN-α and -β were obtained from CalBiochem. The IFN preparations all contained <10 ng LPS/105 U IFN.
Analysis of Cell Division.
Lymphocyte proliferation in culture was assayed by uptake of [3H]TdR. In brief, cells were cultured at a starting concentration of 3 × 105 cells/150 μl in the wells of 96-well tissue culture plates. 2–3 d later, 1 μCi [3H]TdR was added to each well and the cells were incubated for an additional 6 h before harvest using a 1450 Microbeta Plus liquid scintillation counter (Wallac). Cell division was also analyzed after permeabilization of the cells in 0.3% saponin and addition of 5 μg/ml propidium iodide. The percentages of cells in G1 and G2/S, and with <2 N amounts of DNA were then identified cytofluorographically.
Results
IFN-α/β Improve the Survival of Activated T Cells.
T cells that have been activated in animals die rapidly in vivo or in culture unless their activation occurred in the presence of an inflammatory agent such as LPS or an adjuvant (1–4, 9, 10). To find out how inflammation has this effect, we screened a large number of agents for their ability to prevent the death of T cells activated in vivo and then cultured in vitro (Table I). Many of the agents had no effect in this assay. Members of two cytokine families, the IL-2 family and the type I IFN (IFN-α/β) family, were active. Several groups including ourselves have previously described the effects of IL-2 family members (12–14, 29, 30); however, the ability of IFN-α/β to prevent activated T cell death has not been reported previously.
Table I.
Agents Tested for Their Effects on the Survival of Activated T Cells In Vitro
| Agents that have no effect | Agents that increase survival | |||
|---|---|---|---|---|
| IL-1β | IL-8 | IL-2 | ||
| IL-6 | RANTES | IFN-α | ||
| IL-12 | MIP-1α | IL-4 | ||
| IFN-γ | MIP-1β | IFN-β | ||
| TNF-α | MIP-2 | IL-7 | ||
| TNF-β | VEGF | IL-15 | ||
| IL-3 | PEGF | |||
| IL-5 | TGF-β | |||
| IL-10 | CD28 engagement | |||
| IL-9 | LPS | |||
| IL-13 | Platelet-activating factor | |||
| Thrombopoietin | ||||
Agents were tested at concentrations recommended by the manufacturers or obtained from the literature.
An example of an IFN-α/β experiment is shown in Fig. 1. T cells were activated in vivo by injection of SEB, a superantigen which reacts with T cells bearing members of the Vβ8 family in their TCRs, into mice. 2 d later lymph node T cells were purified and cultured in the presence or absence of IFN-α/β. 1 d later, the percentages of live Vβ8+ or control Vβ6+ T cells were measured using either live/ dead gates of the forward versus side scatter bit maps of the cells or DNA content, as assessed by staining of permeabilized cells with propidium iodide.
The results of both types of assays for dead cells were similar, except that in every case fewer of the cells were alive after culture as defined by forward versus side scatter than as defined by DNA content (Fig. 1). This difference was probably due to the fact that DNA degradation is a relatively late event in apoptosis (31–33). Because measurement of cell survival by light scatter properties allowed more flexible use of staining reagents and identified dead cells at an earlier stage, this assay was used to define dead cells in the remaining experiments described in this paper. However, in most cases the results were confirmed using DNA content or cell permeability as measures of cell survival.
Fig. 1 shows that <40% of the control Vβ6+ cells died during overnight culture. In contrast, almost 70% of the SEB-activated, Vβ8+ T cells died. Thus, as we have previously described, T cells die more quickly in culture if they have previously been activated in vivo (9). Culture with IFN-α/β slightly increased the survival of the resting, Vβ6+ T cells in overnight culture, but dramatically prevented the death of the activated, Vβ8+ T cells (Fig. 1).
The fact that IFN-α/β have only a very slight effect on the rate of death of resting T cells was also demonstrated in an experiment in which IFN-α/β was titrated. Increasing doses of IFN-α/β had increasing abilities to promote the survival of activated T cells and had very little effect on the survival of resting T cells (Fig. 2). This was not due to absence of IFN-α/β receptors on resting T cells, since, as shown in Fig. 3, culture in IFN-α/β increased the levels of class I MHC on resting T cells. For CD4+ T cells, the effects of IFN-α/β on class I expression were less dramatic if the T cells were resting compared with if they were activated. On the other hand, for CD8+ T cells the dose– response curve was almost exactly the same whether or not the cells were activated.
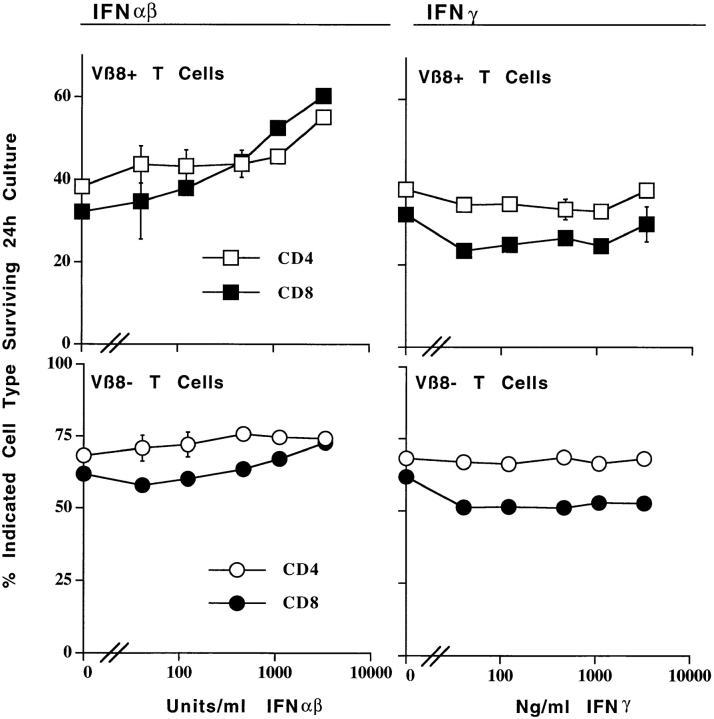
Figure 2.
IFN-α/β prevent the death of activated T cells. IFN-γ does not. T cells were isolated from the lymph nodes of control B10 mice, or from B10 mice that had been injected with 150 μg SEB 2 d previously. The cells were cultured for 20 h in the presence of the indicated concentrations of IFN-α/β or IFN-γ. The percentages of live Vβ8+ or Vβ8− cells in the cultures were determined by flow cytometry as described in the legend to Fig. 1, using light scatter properties as an indicator of survival. Results shown are the mean ±SE of triplicate cultures.
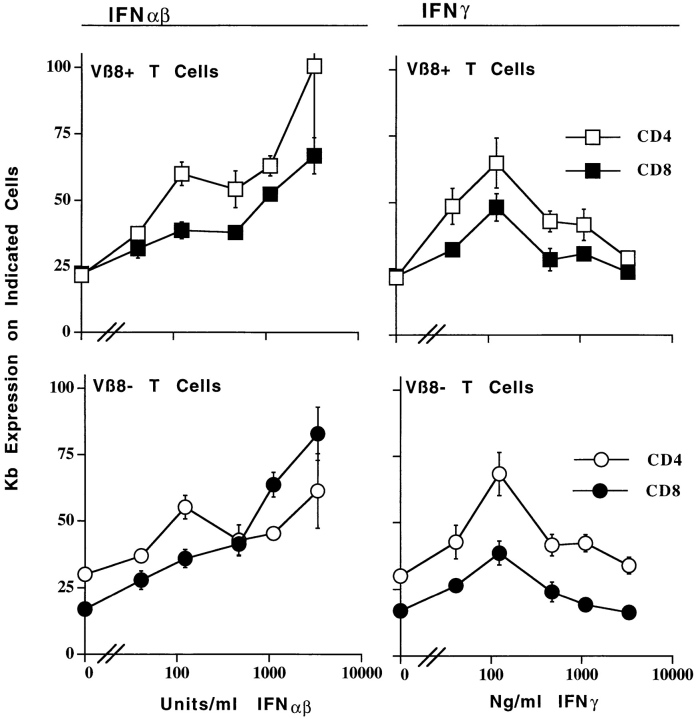
Figure 3.
Activated and resting T cells bear receptors for IFN-α/β and IFN-γ. Mice were primed and T cells were cultured as described in the legend to Fig. 2. 20 h after the start of culture, the cells were isolated and stained for Vβ8, Kb, and CD4 or CD8 as described in Materials and Methods. The results shown are the mean ± SE of triplicate cultures of staining with anti-Kb antibody in arbitrary units based on staining intensities.
IFN-α/β increased the survival of both CD4+ and CD8+ T cells, providing the cells were activated. Thus, as shown in Fig. 2, increasing doses of IFN-α/β kept increasing percentages of CD4 or CD8+ SEB-activated, Vβ8+ T cells alive. However, the effects of IFN-α/β on cell survival were consistently more pronounced for CD8+ T cells than for CD4+ T cells. This was not because CD4+-activated T cells are intrinisically less sensitive to IFN-α/β than are CD8+-activated cells since, as shown in Fig. 3, culture in increasing concentrations of IFN-α/β raised class I MHC expression on both CD4+- and CD8+-activated T cells. In fact, class I was induced to higher levels on activated CD4+ cells than on CD8+ cells.
The extent to which activated T cells died, and therefore to which IFN-α/β rescued them, varied with time in culture and strain of mice. For example, the cultures shown in Fig. 2 were incubated for 20 h and those shown in Fig. 4 were incubated for 24 h, and there was more extensive death in the absence of, and proportional rescue in the presence of, IFN-α/β in the latter experiments than in the former. The mouse strain from which the activated T cells were derived also affected rates of death in vitro. Thus, the activated T cells from 129 mice (Table II) died more slowly than those from C57Bl/10 mice, a difference between 129 and C57Bl/10 T cells that we have consistently observed in many experiments.
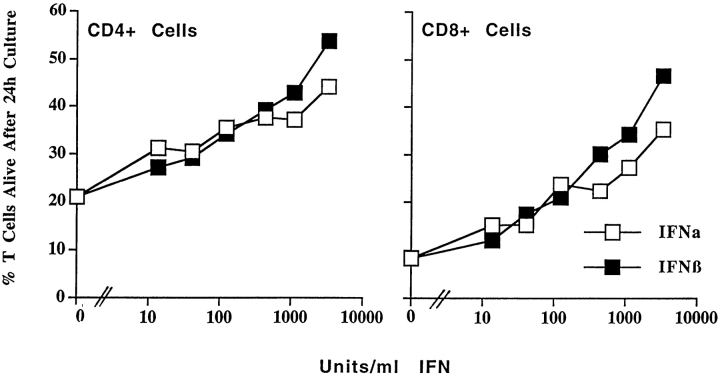
Figure 4.
Both IFN-α and IFN-β affect the survival of activated T cells. T cells were activated and purified as described in the legend to Fig. 1. The cells were cultured for 24 h in the presence of various concentrations of IFN-α or IFN-β and the percentages of live activated (Vβ8+) T cells were determined by flow cytometry. Results shown are the mean ± SE of triplicate cultures.
Table II.
IFN-α/β Act Directly on T Cells
| Additions to culture | Percentage indicated of cells surviving in mixed cultures | |||||||
|---|---|---|---|---|---|---|---|---|
| Wild-type | IFNR-α/β–deficient | |||||||
| CD4 | CD8 | CD4 | CD8 | |||||
| None | 43.6 ± 1.8 | 33.5 ± 0.4 | 45.9 ± 2.4 | 21.5 ± 0.9 | ||||
| IFN-α/β | 49.7 ± 0.6 | 54.4 ± 0.8 | 43.9 ± 0.4 | 22.4 ± 0.6 | ||||
IFN-α/βR–deficient mice and mice of their closely related wild-type strain, 129/SvEv, were injected intravenously with 150 μg SEB. 2 d later T cells were isolated from the lymph nodes of the mice. The cells from the IFN-α/βR–deficient mice were staIned with CFSE as described in Materials and Methods. Mixtures containing equal numbers of cells from each type of mouse were cultured for 28 h in the absence or presence of 3,333 U/ml IFN-α/β and then stained with PE–labeled anti-CD4 or anti-CD8 and biotinylated anti-Vβ8 followed by CySA. Results shown are the mean ± SE of data from three identical cultures.
IFN-β is distinguished from the large family of IFN-α's by sequence differences. However, both IFN-α and IFN-β are thought to act via the same heterodimeric receptor (IFN-α/βR; references 34, 35). Since both IFN-α and IFN-β act on the same receptor, we expected that each type of protein would have the same effect on T cells as the mixture of the two that was used for the experiments shown thus far in this paper. To find out whether this was so, T cells were activated in vivo with SEB, harvested and purified 2 d later, and cultured in various concentrations of IFN-α or IFN-β. As shown in Fig. 4, both kinds of IFN increased the life expectancy of activated T cells in vitro; however, IFN-β was somewhat more active than the IFN-α protein tested. Whether this represents a consistent difference between IFN-β and all the members of the IFN-α family awaits further investigation.
In summary, these experiments show that IFN-α and IFN-β promote the survival of activated T cells in vitro. IFN-α/β has little or no effect on the survival of resting T cells, despite the fact that these cells can respond to IFN-α/β by raising their surface levels of class I strongly on CD8+ T cells and to a lesser extent on CD4+ cells.
IFN-α/β Act Directly on Activated T Cells and Do Not Stimulate T Cell Proliferation.
Tough et al. recently reported that IFN-α/β make CD44hi CD8+ T cells divide in vivo (36). Subsequently this group showed that the IFNs probably do not act directly, but rather indirectly by induction of IL-15, which in turn affects the T cells (15). To find out whether IFN-α/β were acting in a similarly indirect manner in vitro, we set up cultures containing mixtures of SEB-activated T cells from normal and IFN-α/βR–deficient mice. The normal T cells were distinguished from the IFN-α/βR–deficient cells by labeling the latter with CFSE. IFN-α/β increased the survival of the T cells from normal mice but had no effect on T cells lacking IFN-α/βRs (Table II), even though the two types of cells were cultured together. Toxicity due to CFSE labeling did not contribute to this since identical results were obtained in mixed cultures in which the normal T cells were labeled with CFSE and the IFN-α/βR cells were unlabeled. Thus, IFN-α/β were acting directly on T cells in culture. Of course under some circumstances they may also act indirectly via induction of cytokines such as IL-15, but these effects were not manifest in our cultures of purified T cells.
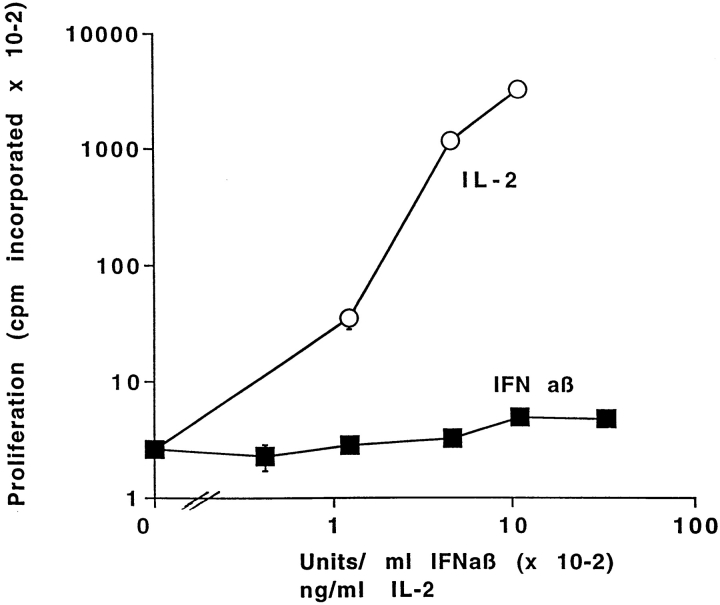
IL-15 stimulates the proliferation of activated T cells but IFN-α/β do not (37–40). To confirm the idea that, in the experiments described in this paper, IFN-α/β were not acting via IL-15, we tested their effects on T cell proliferation. T cells were isolated from mice given SEB 2 d previously and cultured in the presence of different concentrations of IFN-α/β or as controls without added cytokines or with IL-2. The cells cultured alone did not proliferate. They divided vigorously in response to IL-2. Such cells also divided in response to IL-15 (data not shown). The cells did not divide at all in response to IFN-α/β (Fig. 5). The same conclusion was drawn from experiments in which T cells were labeled with CFSE and cultured in the presence or absence of IFN-α/β or IL-15. After 24 h, CFSE staining showed that, of the activated CD8+ T cells that were still alive, 8.9 ± 1.2% had divided if the cells were cultured alone, whereas 5.5 ± 0.6 or 15.5 ± 0.74% had divided if the cells were cultured in IFN-α/β or IL-15, respectively. Likewise, counts of total cell yield showed that there was no difference in the number of T cells per culture after 24 h of incubation in the presence or absence of IFN-α/β. In one experiment, 6.1 ± 0.5 × 105 cells were recovered from cultures incubated for 24 h without IFN-α/β, and 5.4 ± 0.3 × 105 cells were recovered from cultures incubated with 3,333 U/ml IFN-α/β. Hence, IFN-α/β do not cause activated T cells to divide.
Figure 5.
IFN-α/β do not stimulate the proliferation of activated T cells. Lymph node T cells were purified from B10 mice primed 2 d previously with 150 μg/mouse SEB. The cells were cultured for 3 d in the presence of the indicated concentrations of IFN-α/β or IL-2 and then pulsed with [3H]TdR as described in Materials and Methods Results shown are the mean ± geometric SE of triplicate cultures. Similar results were obtained from cells tested after 2 d of culture.
These results showed that IFN-α/β were not acting via induction of IL-15, since they did not stimulate activated T cell division. Also, IFN-α/β did not cause a higher percentage of activated T cells to survive culture because they stimulated T cells to proliferate and hence overgrow dying cells. Rather, IFN-α/β promoted the survival of previously activated T cells.
IFN-γ Has No Effect on the Survival of Activated T Cells.
IFN-α/β share many biological effects with IFN-γ, even though the two types of IFN act via different receptors (33, 34, 40). However, IFN-γ did not prevent the deaths of activated T cells. Thus, as shown in Fig. 2, incubation with various concentrations of IFN-γ had no effect on the survival of CD4+ or CD8+ activated or resting T cells. This was not because these cells did not bear receptors for IFN-γ since, as shown in Fig. 3, incubation with IFN-γ did raise class I MHC levels on these T cells, albeit with a bell-shaped dose–response curve.
It was surprising that IFN-γ did not have the same activity as IFN-α/β. Although the two types of IFNs act through different receptors (34, 35, 41), they share many intracellular signaling pathways and therefore have many similar effects on cells (42, 43). The fact that the two types of IFNs differ in this assay shows that IFN-α/β prevents T cell death via an intracellular pathway that is not triggered by the IFN-γ receptor. Therefore, although Stat1 activation may be required for the effects described here (42), it is not sufficient.
The Protective Effects of IFN-α/β Were Not Mediated by Increased Bcl-2 or Bcl-X Levels.
Other cytokines, including IL-15, that protect activated T cells against death raise the amounts of Bcl-2 in the activated cells (12, 14, 29). If IFN-α/β were acting via IL-15, or by a route similar to that induced by IL-2 family members, then they should also raise Bcl-2 in the T cells. To check this, T cells were isolated from mice primed 2 d previously with SEB and stained for Bcl-2 content. At the time of isolation the activated, Vβ8+ T cells contained about half of the amount of Bcl-2/cell that resting, Vβ6+ T cells did (footnote to Table III). This lowered level of Bcl2 may contribute to the increased rate of death of the activated cells.
Table III.
IFN-α/β Do Not Raise Bcl-2 Levels in Activated T Cells
| Culture conditions | Percentage of Vβ8+ cells alive | Bcl-2 levels in | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vβ8+ cells with | Vβ6+ cells with | |||||||||||
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |||||||
| No Addition | 33.5 ± 0.4 | 11.9 ± 0.3 | 9.1 ± 2.1 | 8.2 ± 1.2 | 13.0 ± 1.6 | 20.6 ± 2.5 | ||||||
| IFN-α/β | 48.0 ± 2.3 | 48.9 ± 1.0 | 7.1 ± 1.3 | 7.9 ± 1.4 | 13.3 ± 1.6 | 24.7 ± 1.3 | ||||||
| IL-2 | 73.3 ± 4.3 | 73.3 ± 4.2 | 18.1 ± 2.1 | 36.5 ± 1.5 | 15.0 ± 1.5 | 39.0 ± 2.7 | ||||||
| IL-15 | 80.7 ± 2.2 | 63.9 ± 2.7 | 23.8 ± 3.7 | 37.8 ± 3.2 | 18.9 ± 2.8 | 49.9 ± 8.5 | ||||||
Lymph node T cells were purified from C57BL/10 mice given SEB 2 d previously. At that time, levels of Bcl-2 were 17.8 ± 0.6 and 17.6 ± 0.9 in Vβ8 cells bearing CD4 and CD8, respectively, and 28.2 ± 0.9 and 38.7 ± 0.9 in Vβ6 cells bearing CD4 and CD8, respectively. Cells were cultured for 24 h alone or with 3,333 U/ml IFN-α/β or 100 ng/ml IL-2 or 100 ng/ml IL-15 and then assayed for intracellular amounts of Bcl-2 as described in Materials and Methods. Results shown are the mean ± SE of staining with anti–Bcl-2 antibody in arbitrary units based on staining intensities of three identical cultures minus the background without primary antibody.
The cells were then cultured in the presence or absence of IFN-α/β or IL-2 or IL-15. As shown in Table III, all three of these cytokines increased the survival rate of the activated T cells. The effects of IL-2 and IL-15 were more profound than those of IFN-α/β.
T cells cultured alone contained less Bcl-2 per cell after culture than before. Activated, Vβ8+ T cells cultured alone continued to contain less Bcl-2 per cell than resting, Vβ6+ T cells did. This was also true for activated Vβ8+ T cells cultured in IFN-α/β, even though these cells had a markedly improved survival rate over that of activated cells cultured alone. The amounts of Bcl-2 in the activated Vβ8+ T cells did not fall so much or were maintained by culture in IL-2 or IL-15.
As we have previously described, the high concentrations of IL-2 or IL-15 used in this experiment also increased the survival rate of the resting T cells (Table III, reference 14, and data not shown). Commensurate with this, the IL-2 family members helped to maintain Bcl-2 levels to a very small extent in CD4+ T cells and much more markedly in the CD8+ T cells.
Bcl-X can also promote the survival of T cells (12, 13, 20, 24). Therefore we tested the effects of culture with IFN-α/β on Bcl-X levels. Vβ8+ T cells were activated in vivo using SEB and isolated 2 d later as described above. At the time they were removed from the animals, the activated, Vβ8+ T cells contained ∼50% more Bcl-X per cell than did the resting T cells (footnote to Table IV). This was probably due to induction of Bcl-X in the activated cells by CD28/B7 engagement (12, 13, 20, 24). After 1 d in culture, the levels of Bcl-X in the activated, Vβ8+ and resting, Vβ6+ T cells were the same. These levels were unaffected by culture in IL-2, raised slightly (especially in the CD8+ T cells) by culture in IL-15, and completely unaffected by culture in IFN-α/β (Table IV). Although these results do not distinguish between the two forms of Bcl-X, Bcl-XL and Bcl-XS, they do suggest that levels of either form are not affected by IFN-α/β.
Table IV.
IFN-α/β Do Not Raise Bcl-X Levels in Activated T Cells
| Culture conditions | Bcl-X levels in | |||||||
|---|---|---|---|---|---|---|---|---|
| Vβ8+ cells bearing | Vβ6+ cells bearing | |||||||
| CD4 | CD8 | CD4 | CD8 | |||||
| No addition | 23.0 ± 2.2 | 29.0 ± 1.7 | 21.6 ± 2.5 | 32.0 ± 5.5 | ||||
| IFN-α/β | 25.2 ± 2.9 | 27.7 ± 4.4 | 22.1 | 24.1 ± 3.7 | ||||
| IL-2 | 25.7 ± 2.8 | 28.3 ± 0.5 | 20.3 ± 2.8 | 25.7 ± 2.3 | ||||
| IL-15 | 30.3 ± 1.0 | 46.2 ± 6.1 | 23.2 ± 4.4 | 46.5 ± 4.0 | ||||
Lymph node T cells were purified from C57BL/10 mice given SEB 2 d previously. At that time Bcl-X levels were 51.3 ± 1.9 and 49.9 ± 1.9 in Vβ8+ cells bearing CD4 and CD8, respectively, and 35.5 ± 0.9 and 37.9 ± 1.3 in Vβ6+ cells bearing CD4 and CD8, respectively. Cells were cultured for 24 h alone or with 3,333 U/ml IFN-α/β or 100 ng/ml IL-2 or IL-15 and then assayed for intracellular amounts of Bcl-X as described in Materials and Methods. Results shown are the mean ± SE of three identical cultures in arbitrary units of Bcl-X based on staining intensities minus the background without primary antibody. Cell survival rates were as listed in Table III.
These results demonstrate that the rescuing activity of IFN-α/β does not depend upon induction of either Bcl-2 or Bcl-X in activated T cells. Thus, the means whereby IFN-α/β protect activated T cells against death must be different than those used in protection by IL-2 family members or by CD28 engagement.
Discussion
Experiments described elsewhere show that T cells that have been activated in vivo in the absence of adjuvant die rapidly in animals or after isolation and culture (1–4, 9–12). In vitro, a number of cytokines prevent the deaths of these activated cells. Previously, we and others have shown that members of the IL-2 family do this very effectively (12–14, 29). In this paper we show that IFN-α/β are also active.
The IL-2 family and IFN-α/β do not act in the same way. IL-2 and its relatives all have the ability to induce Bcl-2 synthesis in responsive T cells (12–14, 29). It is likely that it is this increase in Bcl-2 which increases the life expectancy of the cells. Members of the IL-2 family also have, to varying degrees, the ability to induce proliferation of the responding T cells. IL-2 and IL-15 are particularly effective in this regard, and IL-4 and IL-7 are less so (14). On the other hand, the IFN-α/β family does not act in any of these ways. IFN-α/β do not increase Bcl-2 levels in T cells. IFN-α/β also do not stimulate T cell division. In fact, IFN-α/β are often thought to be inhibitors rather than stimulators of proliferation (38–40).
Recently, Tough et al. have studied the effects of IFN-α/β on T cells. They showed that induction of IFN-α/β in animals caused a subset of what appear to be activated or memory CD8+ T cells to divide (36). Later experiments showed that this was probably due to the ability of IFN-α/β to induce IL-15 production by cells such as macrophages (15). We do not think that the IFN-α/β is acting in such a way in the experiments described here. Experiments with mixtures of wild-type and IFN-α/βR–deficient cells showed that IFN-α/β were acting directly on the T cells and not indirectly via induction of some other molecule. Also, as discussed above, apart from their common ability to prevent the death of activated T cells, IFN-α/β and IL-15 affect T cells in completely different ways.
The IFN-α/β were not as efficient in preventing the deaths of activated T cells as the IL-2–related cytokines were. This was not simply because the IL-2 family members induced cell division and thus overgrowth of dead cells by dividing T cells. Rather, at optimal concentrations IL-2 like cytokines appeared to prevent activated T cell death almost entirely, whereas the IFN-α/β simply slowed the process.
The IFN-α/β do not act via increased induction of another survival protein, Bcl-X. Therefore, their effects are probably not due to induction of members of the B7 family and increased engagement of CD28 on T cells (19–24).
IFN-γ did not share the ability of IFN-α/β to inhibit the death of activated T cells. This was not because activated T cells lacked receptors for IFN-γ, since IFN-γ increased class I MHC expression on such cells. Thus, the rescuing activity of IFN-α/β must involve a feature of signaling of the IFN-α/βR that is not shared with that of the IFN-γR. There are several candidates for differential factors. Although both receptors activate Stat1, only the IFN-α/βR activates Stat2 and Stat4 and creates the heterodimeric transcription factor, ISGF3 (38, 44–46). In addition, in T cells, the engaged IFN-α/βR is associated with several proteins (p54lck, CD45, and Zap70) known to be important in T cell activation (42). Perhaps one or more of these properties of the IFN-α/βR distinguishes signaling by IFN-α/β from that of IFN-γ to T cells.
The fact that the IFN-α/β prevent activated T cell death has considerable implications. It may contribute to the large numbers of antigen-specific and nonspecific T cells that appear during virus infections (47–50). It may also account for the long suspected association between virus infections and induction of autoimmune diseases (51–53).
Acknowledgments
The authors thank Drs. Schreiber and Aguet for their generous gift of breeding pairs of IFN-α/βR–deficient mice.
This work was supported in part by United States Public Health Service grants AI17134, AI18785, and AI22295.
Footnotes
Abbreviations used in this paper: CFSE, carboxyfluorescein diacetate succinimidyl ester; CY, cychrome; SEB, staphylococcal enterotoxin B.
References
- 1.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 2.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ cells in mice tolerant to Staphylococcus aureusenterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 3.McCormack JE, Callahan JE, Kappler J, Marrack P. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- 4.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 5.Duke RC. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- 6.Debatin KM, Goldmann CK, Bamford R, Waldmann TA, Krammer PH. Monoclonal antibody- mediated apoptosis in adult T-cell leukaemia. Lancet. 1990;335:497–500. doi: 10.1016/0140-6736(90)90735-n. [DOI] [PubMed] [Google Scholar]
- 7.Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 8.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/Apo-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 9.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell tolerance. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 10.Vella AT, Mitchell T, Groth B, Linsley PS, Green JM, Thompson CB, Kappler JW, Marrack P. CD28 engagement and proinflammatory cytokines contribute to T cell expansion and long term survival in vivo. J Immunol. 1997;158:4714–4720. [PubMed] [Google Scholar]
- 11.Chiller JM, Weigle WO. Termination of tolerance to human gamma globulin in mice by antigen and bacterial lipopolysaccharide (endotoxin) J Exp Med. 1973;137:740–750. doi: 10.1084/jem.137.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akbar AN, Bothwick NJ, Wickremasinghe RG, Panayiotidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M. Interleukin-2 receptor common γ chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xl) but not proapoptotic (bax, bcl-xs) gene expression. Eur J Immunol. 1996;26:294–299. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 13.Mueller DL, Seiffert S, Fang W, Behrens TW. Differential regulation of bcl-2 and bcl-x by CD3, CD28 and the IL-2 receptor in cloned CD4+ helper T cells. A model for the long term survival of memory cells. J Immunol. 1996;156:1764–1771. [PubMed] [Google Scholar]
- 14.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–600. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 16.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 17.Veis DJ, Stentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 18.Nakayama K, Nakayama K-I, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2αβ in mice: occurrence of gray hair, polycystic kidney disease and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 20.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XLand Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 stimulation can enhance T cell survival by inducing expression of Bcl-XL . Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 22.Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naïve T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- 23.Radvanzi LG, Shi Y, Vaziri H, Sharma A, Dhala R, Mills GB, Miller RG. CD28 costimulation inhibits TCR-induced apoptosis during a primary immune response. J Immunol. 1996;156:1788–1798. [PubMed] [Google Scholar]
- 24.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636–642. [PubMed] [Google Scholar]
- 25.Muller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet MA. Functional role of type I and type II interferons in antiviral defense. Science. 1994;261:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 26.Julius MH, Simpson E, Herzenberg L. A rapid method for the isolation of functional thymus-derived murine T lymphocytes. Eur J Immunol. 1973;3:645–650. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 27.Scherer MT, Ignatowicz L, Pullen A, Kappler J, Marrack P. The use of Mtvmice to evaluate the effects of endogenous viral superantigens on the T cell repertoire. J Exp Med. 1995;182:1493–1504. doi: 10.1084/jem.182.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko S, Suzuki N, Koizumi H, Yamamoto S, Sakane T. Rescue by cytokines of apoptotic cell death induced by IL-2 deprivation of human antigen-specific T cell clones. Clin Exp Immunol. 1997;109:185–193. doi: 10.1046/j.1365-2249.1997.4191324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maraskovsky E, Teepe M, Morrissey PJ, Braddy S, Miller RE, Lynch DH, Peschon JJ. Impaired survival and proliferation in IL-7 receptor deficient peripheral T cells. J Immunol. 1996;157:5315–5323. [PubMed] [Google Scholar]
- 31.Swat W, Ignatowicz L, Kisielow P. Detection of apoptosis of immature CD4+8+ thymocytes by flow cytometry. J Immunol Methods. 1991;137:79–87. doi: 10.1016/0022-1759(91)90396-w. [DOI] [PubMed] [Google Scholar]
- 32.Wesselborg S, Kabelitz D. Activation-driven death of human T cell clones: time course kinetics of the induction of cell shrinkage, DNA fragmentation, and cell death. Cell Immunol. 1993;148:234–241. doi: 10.1006/cimm.1993.1106. [DOI] [PubMed] [Google Scholar]
- 33.Hotz MA, Gong J, Traganos F, Darzynkiewicz Z. Flow cytometric detection of apoptosis: comparison of the assay of in situ DNA degradation and chromatin changes. Cytometry. 1994;15:237–244. doi: 10.1002/cyto.990150309. [DOI] [PubMed] [Google Scholar]
- 34.Novick D, Cohen B, Rubenstein M. The human α/β interferon receptor: characterization and molecular cloning. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 35.Uze G, Lutfalla G, Gresser I. Genetic transfer of a functional interferon α receptor into mouse cells: cloning and expression of its cDNA. Cell. 1990;60:225–234. doi: 10.1016/0092-8674(90)90738-z. [DOI] [PubMed] [Google Scholar]
- 36.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 37.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Sr inivasan, V. Fung, C. Beers, J. Richardson, M.A. Schoenborn, M. Ahdieh, et al., . Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 38.Fradelizi D, Gresser I. Interferon inhibits the generation of allospecific suppressor T lymphocytes. J Exp Med. 1982;155:1610–1622. doi: 10.1084/jem.155.6.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puri RK, Travis WD, Rosenberg SA. In vivo administration of interferon alpha and interleukin 2 induces proliferation of lymphoid cells in the organs of mice. Cancer Res. 1990;50:5543–5550. [PubMed] [Google Scholar]
- 40.Petricoin EF, III, Ito S, Williams BL, Audet S, Stancato LF, Gamero A, Clouse K, Grimley P, Weiss A, Beeler J, et al. Antiproliferative action of interferon alpha requires components of T cell receptor signalling. Nature. 1997;390:629–632. doi: 10.1038/37648. [DOI] [PubMed] [Google Scholar]
- 41.Aguet M, Dembic Z, Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell. 1988;55:273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 42.Shual K, Ziemiecki A, Wilks AF, Harpur AG, Sadowski HB, Gilman MZ, Darnell JE. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993;366:580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- 43.Ihle JN. The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv Immunol. 1995;60:1–35. doi: 10.1016/s0065-2776(08)60582-9. [DOI] [PubMed] [Google Scholar]
- 44.Leung S, Qureshi SA, Kerr IM, Darnell JE, Stark GR. Role of Stat2 in the alpha interferon signaling pathway. Mol Cell Biol. 1995;15:1312–1317. doi: 10.1128/mcb.15.3.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho SS, Bacon CM, Sudarshan C, Rees RC, Finbloom D, Pine R, O'Shea JJ. Activation of STAT4 by IL-12 and IFN-alpha: evidence for the involvement of ligand-induced tyrosine and serine phosphorylation. J Immunol. 1996;157:4781–4790. [PubMed] [Google Scholar]
- 46.Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany ZD, Andrea A, Livingstone DM. Cooperation of Stat2 and p300/CBP in signalling induced by interferon alpha. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 47.Yang H, Welsh RM. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J Immunol. 1986;136:1186–1193. [PubMed] [Google Scholar]
- 48.Tripp RA, Hou S, McMickle A, Houston J, Dougherty PC. Recruitment and proliferation of CD8+ T cells in respiratory viral infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 49.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute viral infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJD, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 51.Dahlquist GG. Viruses and other perinatal exposures as initiating events for beta-cell destruction. Ann Med. 1997;29:413–417. doi: 10.3109/07853899708999371. [DOI] [PubMed] [Google Scholar]
- 52.Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz D, Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1145. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 53.Phillips PE. Viral arthritis. Curr Opin Rheumatol. 1997;9:337–344. doi: 10.1097/00002281-199707000-00011. [DOI] [PubMed] [Google Scholar]