Abstract
Intracellular signals emanating from cytokine and antigen receptors are integrated during the process of intrathymic development. Still, the relative contributions of cytokine receptor signaling to pre-T cell receptor (TCR) and TCR-mediated differentiation remain undefined. Interleukin (IL)-7 interactions with its cognate receptor complex (IL-7Rα coupled to the common cytokine receptor γ chain, γc) play a dominant role in early thymopoiesis. However, α/β T cell development in IL-7–, IL-7Rα–, and γc-deficient mice is only partially compromised, suggesting that additional pathways can rescue α/β T lineage cells in these mice. We have investigated the potential interdependence of γc- and pre-TCR–dependent pathways during intrathymic α/β T cell differentiation. We demonstrate that γc-dependent cytokines do not appear to be required for normal pre-TCR function, and that the rate-limiting step in α/β T cell development in γc − mice does not involve TCR-β chain rearrangements, but rather results from poor maintenance of early thymocytes. Moreover, mice double mutant for both γc and pre-Tα show vastly reduced thymic cellularity and a complete arrest of thymocyte differentiation at the CD44+CD25+ cell stage. These observations demonstrate that the pre-TCR provides the γc-independent signal which allows α/β T cell development in γc − mice. Thus, a series of overlapping signals derived from cytokine and T cell receptors guide the process of α/β thymocyte development.
Keywords: thymus, interleukin, lymphocyte, development, knockout
The common cytokine receptor γ chain (γc)1 forms a critical functional component of the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15 (for a review, see reference 1). Naturally occurring mutations in γc are responsible for X-linked severe combined immunodeficiency disease (SCIDX1) in humans, characterized by a complete absence of T and NK cells, while B cells are present (for reviews, see references 2 and 3). Targeted deletion of γc in mice also provokes a wide variety of defects in lymphoid development, including a complete absence of NK cells, γ/δ T cells, and gut-associated lymphoid tissue (4–6). Like SCIDX1 patients, γc − mice have some mature peripheral B cells, but these mice also show a remarkable degree of α/β T cell development (4–8). These data demonstrate the important role of γc-dependent signals in lymphopoiesis, but also suggest that fundamental differences exist between the mechanisms that permit α/β T cell development in humans and mice.
Analysis of single cytokine- or cytokine receptor–deficient mice has identified which γc-dependent signals are responsible for some of the observed developmental defects seen in γc − mice. NK cell differentiation is critically linked to the expression of the IL-2Rβ chain (9), thereby implicating IL-2– and/or IL-15–mediated signaling pathways in the development of these cells. Since IL-2 mutant mice develop NK cells (10), this suggests that IL-15 (or another IL-2Rβ–binding ligand) is required for the differentiation of this subset (for a review, see reference 11). In contrast, the defect in γ/δ T cell development in γc − mice appears strictly IL-7 dependent (12). Moreover, since IL-7 was initially identified as an important growth factor for T and B cell precursors (13, 14), this would explain the severely reduced thymic cellularity and defects in bone marrow B cell and intrathymic precursors found in IL-7 and IL-7Rα mutant mice (15–17).
The biological consequences of γc-dependent receptor engagement for α/β T cell development include signals which can potentially promote cell survival, proliferation, and/or differentiation. Experimentally, however, it has been difficult to conclusively define which of these processes are adversely affected in the absence of γc. In theory, the absence of IL-7 signaling in γc − mice could potentially limit thymocyte development by affecting the survival and/ or expansion of intrathymic precursors, or by reducing the efficiency of the recombination process. Evidence for the latter has been suggested by reports that the expression of functionally rearranged TCR-α/β transgenes in γc- or IL-7Rα–deficient mice augmented total thymocyte numbers (18, 19). However, enforced expression of the antiapoptotic factor Bcl-2 could also rescue α/β T cell development in these mice (20–22), supporting a major role for IL-7/γc signaling in promoting a survival program. In all of these cases, thymic reconstitution was not complete, suggesting either that IL-7/γc influences both survival and recombination, or that intrathymic development involves additional mechanisms which are critically dependent on this receptor complex, such as the efficiency of pre-TCR assembly or function.
In this report, we have analyzed the potential interplay between γc-dependent cytokine pathways and signaling through the pre-TCR for α/β T cell development.
Materials and Methods
Animals and Cell Preparation.
γc − mice (5), TCR-α−/− mice (23), pTα−/− mice (24), recombination activating gene (RAG)- 2−/− mice (25), and their control littermates were maintained in a specific pathogen–free animal facility (CNRS/CDTA, Orleans, France). γc −/pTα−/− mice were generated by crossing γc +/− female mice to pTα−/− male mice, and female offspring carrying the γc mutation were identified by PCR (26) and backcrossed to pTα−/− males. γc −/pTα−/− male mice were then identified by PCR. γc −/TCR-α−/− mice were generated in a similar fashion using TCR-α−/− male mice. All mice were on a mixed (129Ola × C57Bl/6) background and were used between 3 and 6 wk of age. Cells isolated from thymus and spleen were prepared as described previously (5).
Antibodies and Immunofluorescence Analysis.
The following mAbs were used as conjugates to fluorescein (FITC), phycoerythrin (PE), Tricolor (TRI), or biotin: CD3 (145-2C11), CD4 (GK1.5), CD8β (35-5.8), TCR-α/β (H57-597), TCR-γ/δ (GL3), HSA (J11d), CD44 (Pgp-1), CD25 (PC-61), IL-7Rα (A7R34), c-kit (2B8), NK-specific (DX5), and B220 (RA3-6B2). Biotinylated mAbs were revealed with either streptavidin-TRI or streptavidin-allophycocyanin (APC; Caltag). Cell suspensions were lysed of erythrocytes and depleted of B cells using sheep anti–mouse Ig–coated magnetic beads (Dynal). Cells were stained in microtiter plates (2 × 106 cells/well in 50 μl), using combinations of directly conjugated mAbs. Simultaneous four-color cell sorting and analysis were performed on a FACSVantage® flow cytometer (Becton Dickinson). Dead cells were excluded by gating based on forward and side scatter characteristics. Sorted populations were routinely 97% pure upon reanalysis.
Cell Cycle Analysis.
In vivo labeling of S phase cells with bromo-deoxyuridine (BrdU; Sigma) was performed by a single intraperitoneal BrdU injection at a dose of 50 mg/kg body wt 15 min before killing. Thymocyte subsets were sorted using a FACSVantage®, and cells incorporating BrdU were identified as described previously (27) using an FITC-coupled anti-BrdU mAb (Becton Dickinson) and a FACScan® flow cytometer.
Intracellular Staining for Bcl-2, pTα, and TCR-β Chains.
Bcl-2 staining was performed as described previously (28). For intracellular staining of pTα or TCR-β chains, surface antigens were stained as above and cells were fixed with 0.1% paraformaldehyde. Cells were subsequently permeabilized in 0.1% saponin before incubation with biotinylated anti–TCR-β (29) or anti-pTα (30) mAbs and finally with streptavidin-APC before analysis on a FACSCalibur® flow cytometer.
TCR-β Rearrangements.
TCR-β V(D)J rearrangements were studied as described (31). In brief, genomic DNA was amplified using a combination of two 5′ primers specific for Vβ6 and Vβ8 together with a 3′ primer hybridizing to the 3′ region of Jβ2.5. PCR products were fractionated on 2% agarose gels, transferred to nylon membranes, and probed by Southern hybridization using a 33P-labeled oligonucleotide specific for the 5′ region of Jβ2.5. Five distinct PCR products ranging in size from 850 to 150 bp are expected depending on the rearrangements of the Vβ segment with either Jβ2.1, Jβ2.2, Jβ2.3, Jβ2.4, or Jβ2.5.
Results
Abnormal Development of Early Thymocyte Progenitors in γc − Mice.
We analyzed intrathymic development in γc − mice to better define the nature of any developmental blocks resulting from the absence of γc. When comparing absolute numbers of CD4−CD8− double negative (DN) cells, CD4+CD8+ double positive (DP) cells, and CD4+CD8− and CD4−CD8+ single positive (SP) mature T cells, the DN and CD8 SP populations were more affected by the absence of γc (Fig. 1 A). While both DP and CD4 SP cells were reduced by 15–20-fold (similar to the overall decrease in thymic cellularity), the DN and CD8 SP subsets were reduced almost 40-fold. The DN compartment contains both early thymocyte precursors (CD3−) as well as mature CD3+ TCR-γ/δ and TCR-α/β cells. While γ/δ T cells do not develop in γc − mice (4, 28), DN α/β T cells are present (32). To specifically evaluate γc − TCR− thymocyte progenitors, we studied CD44 and CD25 expression on CD3−CD4−CD8− (TN) thymocytes by four-color immunofluorescence analysis. Previous studies have demonstrated that these cells differentiate through the following stages: CD44+CD25− → CD44+CD25+ → CD44−CD25+ → CD44−CD25− (33; for a review, see reference 34).
Figure 1.
Early thymocyte development in control and γc − mice. (A) Thymocytes from 3–4-wk-old γc-deficient mice and their littermate controls were stained using CD4-PE and CD8β-FITC, and absolute numbers of DN, DP, CD4 SP, and CD8 SP cells were calculated (mean ± SD from 10 mice of each genotype). (B) Thymocytes were stained with a combination of FITC-conjugated antibodies (CD3, CD4, CD8β, TCR-α/β, TCR-γ/δ, B220, DX5, and Gr-1), CD44-PE, and CD25-biotin followed by streptavidin-TRI. CD44 versus CD25 expression is shown on electronically gated FITC− TN thymocytes. Absolute numbers were calculated (mean ± SD) from six mice of each genotype.
Compared with controls, immature thymocytes from γc-deficient mice showed altered patterns of CD44 and CD25 expression (Fig. 1 B). The most immature CD44+CD25− TN cells, which can also give rise to B cells, NK cells, and thymic dendritic cells (35; for a review, see reference 36), were found in normal frequency, but were clearly reduced in absolute numbers. More striking was the relative accumulation of cells at the CD44+CD25+ stage, which were almost twofold increased in frequency compared with controls (Fig. 1 B). Moreover, absence of γc was associated with a clear defect in the development of cells beyond the CD44+CD25+ stage. Percentages of CD44−CD25+ and CD44−CD25− cells were reduced by a factor of 2 and were markedly reduced (>100-fold) in absolute numbers (Fig. 1 B).
Altered Cell Proliferation in γc − Thymocyte Precursors.
The block in thymocyte maturation seen in γc − thymi could result from abnormal differentiation, reduced cell survival, and/or proliferative defects. The ability of γc − thymocyte precursors to incorporate the analogue BrdU was used as a measure of intrathymic proliferation. The number of cells in S phase of the cell cycle was analyzed after a single injection of BrdU (Fig. 2 A). Only a fraction of the immature thymocytes are labeled under these conditions, and it is clear that T cell precursors in γc − mice show defects in proliferation, as both CD44+CD25− and CD44+CD25+ cells have markedly reduced BrdU incorporation relative to their γc + counterparts (the CD44−CD25+ and CD44−CD25− subsets were not analyzed in γc − thymi due to their extremely small absolute numbers; these subsets were normally labeled in γc + controls [data not shown]). DP cells from γc − mice were similarly affected.
Figure 2.
Altered survival and proliferation of early thymocyte precursors in the absence of γc. (A) Abnormal BrdU incorporation in γc − thymocytes. Mice received a single pulse of BrdU before killing. Indicated thymocyte subsets were sorted, and percentages of cells incorporating BrdU were analyzed. (B) Annexin V staining of γc + or γc − thymocyte subsets. Cells were stained for TN cells (see Fig. 1), CD25, and Annexin V. Propidium iodide–negative CD25− and CD25+ TN cells were electronically gated, and percentages of Annexin V–positive cells were calculated (mean ± SD) from four mice of each genotype. (C) Intracellular Bcl-2 staining of DN thymocyte precursors was performed; γc − thymocytes demonstrated severely reduced Bcl-2 levels (thick lines). Thin lines, isotype control staining.
Decreased BrdU incorporation in γc − thymocyte precursors could result from decreased survival of these cells. γc-dependent cytokines have been shown to promote lymphocyte survival by maintaining levels of antiapoptotic factors such as Bcl-2 and Bcl-XL (37, 38). Therefore, we examined intracellular levels of Bcl-2 in immature thymocytes from γc − mice and their control γc + littermates. γc − precursors had markedly reduced levels of Bcl-2 compared with their γc + counterparts (Fig. 2 B). Moreover, γc − cells showed elevated cell surface staining with Annexin V (Fig. 2 C), indicating commitment to the apoptotic process (39). Taken together, these results are consistent with a critical role for γc cytokines in the survival and expansion of thymocyte precursors, the absence of which could account in part for their reduction in absolute cell numbers.
Pre-TCR Signaling in the Absence of γc.
Having demonstrated a major role for γc in the most immature T cell precursors, we next addressed the impact of the γc mutation on pre-TCR signaling. The activity of the pre-TCR is presumed to begin at the CD44−CD25+ cell stage when functional TCR-β chain rearrangements are achieved (24, 40). The role of γc-dependent cytokines in pre-TCR function has not been previously examined, although it has been suggested that cytokines (like IL-7) may play a role in the expansion of pre-T cells as they differentiate towards the DP stage. This hypothesis gains support from the fact that CD25+ intrathymic precursors express IL-7Rα (41). Moreover, the defect in intrathymic maturation in γc − mice and the marked depletion in CD44−CD25+ and CD44−CD25− cells could be the consequence of abnormal pre-TCR assembly or function.
Although the pre-TCR has its main role in the transit of early α/β T cell precursors to the DP stage (for a review, see reference 42), additional mechanisms independent of the pre-TCR have been described which can permit the generation of DP cells in vivo (43, 44). These include effects mediated by γ/δ TCRs and via α/β TCRs due to early rearrangements at the TCR-α locus. To focus on the pre-TCR–mediated pathway and to exclude these alternative pathways of DP cell generation, we crossed γc − mice (which lack γ/δ T cells) with TCR-α−/− mice (23; to eliminate TCR-α expression). Thymocyte differentiation was examined in these double mutants. Introduction of the TCR-α mutation did not further alter the pattern of differentiation of thymocytes to the DP stage compared with γc − mice or further diminish their total thymic cellularity (Fig. 3 A). These results demonstrate that early TCR-α rearrangements do not play a major role in the differentiation of DP cells in γc − mice.
Figure 3.
Pre-TCR function in the absence of the γc chain. (A) CD4/CD8 profiles of thymocytes from 3–4-wk-old TCR-α–deficient and TCR-α/γc double- deficient mice. Total thymocyte numbers were calculated (mean ± SD) from six mice of each genotype. (B) Intracellular TCR-β chain expression in thymocytes from γc-deficient, pTα-deficient, or wild-type mice. (C) Thymocyte CD4/CD8 profiles from mice bearing a functional TCR Vβ8.2 Tg on γc-deficient or wild-type backgrounds. Absolute thymocyte cell numbers (mean ± SD) were derived from four to six mice of each genotype.
Pre-TCR signaling results in β selection, i.e., the preferential expansion of early thymocytes expressing a single, functionally rearranged TCR-β chain (for a review, see reference 45). β selection was assessed in control, γc −, and pTα−/− thymocytes by intracellular staining of TCR-β expression (Fig. 3 B). As reported previously (44), pTα−/− mice show clear defects in β selection as demonstrated by decreased intracellular levels of TCR-β chains in DP thymocytes compared with control mice. In contrast, γc − DP thymocytes demonstrated intracellular TCR-β levels comparable to controls (Fig. 3 B, and see below).
We also considered that pre-TCR function might be affected due to reduced synthesis of TCR-β chains, thereby limiting the assembly of a pre-TCR complex. Since TCR-β V(D)J rearrangements are detected at the CD44−CD25+ cell stages (40, 46), and previous studies have suggested that IL-7/CD127/γc interactions may be important for RAG expression during the TCR recombination process (for a review, see reference 47), we tested whether defects in TCR-β rearrangements were responsible for the developmental block in γc − thymi. γc − mice were bred with mice bearing a functionally rearranged TCR Vβ8.2 transgene (Tg) (48), and thymocyte development was analyzed. As shown in Fig. 3 C, γc −/TCR-β Tg+ mice demonstrated no change in the distribution or absolute numbers of thymocytes compared with non-Tg γc − littermates. The lack of discernible effect of the TCR-β Tg in γc − mice was not due to an inability to express this TCR, as surface levels of Vβ8.2 were equivalent in γc + and γc − total thymocytes and CD25+ thymocyte precursors (data not shown). These results suggest that defects in the TCR-β chain rearrangement process are not rate limiting in the absence of γc, and are consistent with a role for γc in promoting survival and proliferation of early thymocytes.
γc and Pre-TCR Signaling Pathways Compensate for Each Other in α/β T Cell Development.
To address the relative interdependence of γc and pre-TCR signals, we intercrossed the γc and pTα null strains to generate mice lacking both these molecules. Thymocyte development was analyzed in 3–4-wk-old double mutant mice (γc −/pTα−/−) as well as single mutants for γc or pTα and wild-type mice.
Consistent with previous reports (5, 24), the thymi from either γc or pTα mutant mice demonstrated a reduction in the size (data not shown) and cellularity (20–30-fold; Fig. 4). In contrast, γc −/pTα−/− thymi were severely hypoplastic and showed a drastic reduction (∼4,000-fold) in absolute cell numbers (Fig. 4). Thymocyte cell surface phenotype was further characterized in these mice (Fig. 4, A and B). pTα−/− thymi showed an incomplete block in thymocyte development with an accumulation of cells at the DN stage; however, pTα-deficient thymocytes are capable of further maturation to DP and SP mature cells. In marked contrast, thymi from double mutant γc −/pTα−/− mice contained only immature DN cells (Fig. 4 A). Using CD44 and CD25 markers, γc − thymi demonstrated the characteristic incomplete block at the CD44+CD25+ to CD44−CD25+ transition (Fig. 4 B), whereas the accumulation of cells at the CD44−CD25+ stage in pTα-deficient mice coincides with pre-TCR–mediated cellular expansion and differentiation to the DP stage (for a review, see reference 45). Strikingly, residual thymocytes from γc −/pTα−/− mutant mice showed a developmental block with a complete arrest of differentiation at the CD44+CD25+ stage (Fig. 4 B). To our knowledge, this is the first description of mutant mice harboring this particular intrathymic defect. In terms of absolute cell numbers, γc −/pTα−/− thymi contained ∼4 × 104 TN precursors, all of which were CD44+, and therefore similar to the number of CD44+ TN precursors found in γc − thymi (Fig. 1 B). However, unlike γc − or pTα−/− single mutant mice, the arrest in thymic development in γc −/pTα−/− double mutant thymi was complete, as no mature T cells were found intrathymically or in the peripheral lymphoid organs (data not shown, and see below). These results (a) define a critical period of intrathymic development (the CD44+CD25+ to CD44−CD25+ transition) in which signals delivered by γc and the pre-TCR pathways appear to overlap, and (b) suggest that pre-TCR signals are responsible for rescue of α/β T cell development in γc − mice.
Figure 4.
Intrathymic development in γc/pTα−/− double mutant mice. (A) CD4/CD8 profiles of thymocytes from 3-wk-old mice. (B) CD44/CD25 expression on gated TN thymocytes (see Fig. 1). For these experiments, thymi from four to six γc/pTα−/− double mutant mice were pooled. Absolute thymocyte cell numbers (mean ± SD) were calculated for the indicated mice (n = 6–10 for each genotype).
It would follow from these results that a pre-TCR can form at the CD44+CD25+ stage. Although several studies have reported the rearrangement status of early thymocyte subsets (40, 46, 49), no studies to date have examined pre-TCR protein expression in these cells. Using reagents specific for the TCR-β (29) and a newly developed antibody against the pTα chain (30), we characterized intracellular pre-TCR components in early thymocytes from γc + and γc − mice (Fig. 5). At the CD44+CD25+ stage, thymocytes demonstrate uniform intracellular staining for pTα chains, whereas the level of pTα expression increases slightly as the cells mature to become CD44−CD25+. A small fraction of CD44+CD25+ cells (3.0 ± 1%; n = 4) also stain intracellularly for TCR-β protein; this fraction increases to ∼20% as these cells downregulate CD44 expression (Fig. 5). TCR-β and pTα protein expression on a per cell basis was not qualitatively or quantitatively altered in γc − thymocytes (Fig. 5). These data conclusively demonstrate that a pre-TCR can form during the CD44+CD25+ to CD44−CD25+ transition, a point at which intrathymic precursors express IL-7Rα/γc (41). These results suggest that γc and pre-TCR signals are independent and overlapping for intrathymic development.
Figure 5.
Intracellular expression of pTα and TCR-β chain in CD25+ thymocyte precursors. Thymocytes from γc + or γc − mice were surface stained for TN cells (see Fig. 1), CD44, and CD25, fixed, and permeabilized with saponin before detection of intracellular (IC) TCR-β or pTα chains. Gated CD44+CD25+ and CD44−CD25+ thymocyte subsets are boxed. Negative controls (dotted lines) are staining of thymocytes from RAG-2–deficient (for TCR-β) or pTα-deficient (for pTα) mice.
Characterization of the γc −/pTα− /− Thymic Rudiment.
Due to the severe reduction in thymocyte cell numbers in γc −/pTα−/− mice, a PCR-based strategy (31) was used to identify any TCR-β rearrangements present in these mutant thymi. DNA from control, γc −, or pTα−/− thymi contained abundant TCR-β V(D)J rearrangements, which were diverse with respect to junctional sequences present in the CDR3 region (Fig. 6; and data not shown). In contrast, rearrangements from γc −/pTα−/− mutant thymi were reduced in overall amounts, although samples derived from independent thymi contained multiple and different bands, indicating rearrangements to different Jβ segments (Fig. 6). Sequence analysis of these PCR products revealed unique Vβ CDR3 sequences, suggesting that the observed reduction in rearrangements was related to the paucity of absolute cell numbers and not to a restricted rearrangement potential (data not shown). Finally, TCR-β rearrangements were absent from the spleens of γc −/pTα−/− double mutant mice, demonstrating that the intrathymic block in α/β T cell development was complete and that no mature α/β T cells were produced that could seed the peripheral lymphoid organs (Fig. 6).
Figure 6.
TCR-β rearrangements in thymus and spleen. Genomic DNA from the indicated mice were amplified by PCR using a combination of primers specific for TCR Vβ6 and Vβ8 (sense) and a primer specific for the 3′ region of TCR Jβ2.5 (antisense). Amplification products were detected by blot hybridization using a probe specific for the 5′ region of TCR Jβ2.5.
Discussion
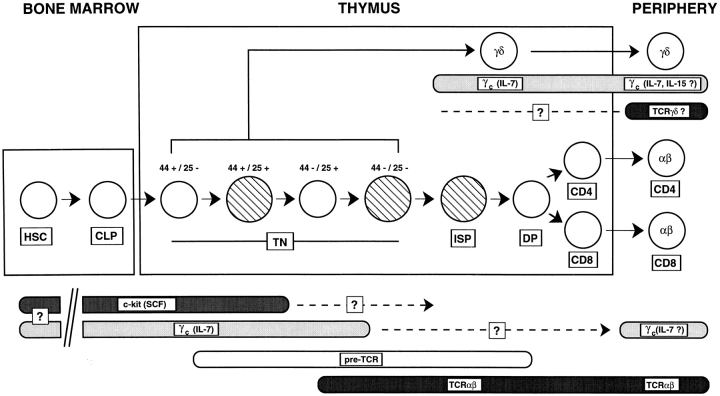
α/β T cells are generated intrathymically through a series of developmental steps involving survival, expansion, and differentiation of immature precursor cells. The γc chain plays a critical role in this process, primarily by relaying signals from stromal cell–derived IL-7 to developing thymocytes. The essential role of IL-7 in early thymocyte differentiation has been difficult to define because this cytokine has been postulated both to act as a trophic factor and to influence the TCR rearrangement process (for a review, see reference 47). Moreover, deficiencies in IL-7/ IL-7Rα/γc abrogate γ/δ T cell development, whereas α/β T cell development is permissive, suggesting either a differential role for the IL-7 receptor complex in the generation of these two T cell subsets or the existence of compensatory pathways that rescue α/β T cells in the absence of IL-7/IL-7Rα/γc. In this report, we identify the pre-TCR as a rescue mechanism for α/β T cell development in γc − mice. This result suggests that a model of intrathymic differentiation involves an overlapping series of signals derived from growth factors and TCRs that guide the maturation process (Fig. 7). This model is consistent with the permissive nature of thymocyte development in c-kit–, IL-7Rα–, γc-, and pTα-deficient mice (4–6, 15–17, 24, 50), and sheds light on the compensatory signaling pathways that exist to insure α/β T cell differentiation in these mutant mice.
Figure 7.
Cytokine and TCR signaling in intrathymic development. Hematopoietic stem cells (HSC) give rise to thymocytes via common lymphoid progenitor cells (CLP). Within the thymus, TN precursor cells can be further subdivided based on expression of CD44 and CD25. Immature SP cells (ISP) are the immediate precursors of DP thymocytes, which after tolerance induction via α/β TCR selection develop into CD4+ or CD8+ SP cells which exit the thymus to seed peripheral lymphoid organs. γ/δ T cells can derive from the various subsets of TN cells. Concerning the molecules required to generate and maintain α/β T cells, growth factor receptors including c-kit and the common γ chain (γc) in concert with the pre-TCR and TCR-α/β appear to provide overlapping signals which guide the developmental process. In the absence of either IL-7Rα, γc, or the pre-TCR, signals provided by adjacent pathway(s) permit a limited degree of α/β thymocyte development. In contrast, when two adjacent signals are absent (for example, in c-kit × γc or γc × pTα double mutants), α/β T cell development is abrogated. Moreover, α/β T cells require continual TCR triggering in the periphery. In contrast, γ/δ T cells appear strictly cytokine dependent, both intrathymically and in the periphery. Hatched circles, cycling cells.
Before the expression of a rearranged TCR chain, immature thymocytes are maintained and proliferate in response to factors provided within the thymic milieu (for a review, see reference 51). Although several cytokines have been shown to act on thymic precursors, stem cell factor (SCF) and IL-7 remain the two dominant factors that can promote their survival and/or expansion (14, 50, 52). The receptors for stem cell factor (c-kit) and for IL-7 (the IL-7Rα/γc complex) are coexpressed on early thymocytes (41, 53, 54), and proliferation of these cells is reduced in the absence of c-kit or IL-7Rα/γc (50, 55; and this report). The permissive nature of thymocyte development in c-kit or IL-7Rα/γc mutants implies redundancy in the pathways that maintain early precursors. The hypothesis that c-kit and IL-7Rα/γc signals could compensate for each other at this stage is strongly supported by the complete abrogation of thymocyte development (before the CD44+CD25− cell stage) in mice deficient in both c-kit and γc (26). Thus, for cells up to the CD44+CD25− stage, c-kit and γc act synergistically to maintain cells before TCR rearrangements. The essential nature of c-kit and γc signals cannot be replaced by other growth factors at this stage of development (26).
At the transition from the CD44+CD25+ stage to the CD44−CD25+ stage, rearrangements of the TCR-β chain begin (40, 46, 49). Since IL-7– and γc-deficient thymocyte precursors are most severely affected at this stage (55; Fig. 1 B), the failure to signal through γc could have an adverse effect on the TCR rearrangement process. Consistent with this hypothesis, previous studies have demonstrated that transgenic expression of a functionally rearranged TCR-α/β (against the male-specific [HY] antigen in association with H-2Db) could partially restore total thymocyte numbers in γc- and IL-7Rα–deficient mice (18, 19). However, these experiments failed to rule out potential effects associated with TCR signaling under conditions of positive selection, since increases in thymic cellularity were only observed in H-2Db female mice. Here we show that the same TCR-β alone has no effect on thymocyte development in γc − mice. This observation strongly suggests that defective TCR-β rearrangements alone cannot account for the abnormal α/β T cell development in the absence of γc, and supports the idea that poor survival and reduced proliferation of the CD44+CD25+ and subsequent thymocyte subsets are the major limiting factors for α/β T cell development in these mice.
The results presented here identify the pre-TCR as an independent and essential signal which acts in concert with γc during α/β thymopoiesis. Although in principle the failure to signal through γc could have an adverse effect on pre-TCR assembly or function, we find that β selection and pre-TCR–mediated expansion appear γc independent. However, the essential role of the pre-TCR in γc − mice is clearly illustrated by the γc/pTα double mutants. In these mice, thymocyte development proceeds only to the CD44+CD25+ stage (thereby delimiting the role of the c-kit pathway), and implies that further development requires either γc or pre-TCR signals. As indicated above, γc can act via IL-7 to maintain early thymocytes at this stage (20–22, 55) and might also serve to drive differentiation to the CD44−CD25+ stage where TCR rearrangements are ongoing (40, 46, 49). How, then, could the pre-TCR rescue γc-deficient cells at such an early stage?
To address this issue, we examined expression of TCR-β and pTα proteins in CD44+CD25+ and CD44−CD25+ thymocytes from γc + and γc − mice. Our results clearly demonstrate that a pre-TCR complex can potentially form in a small subset of CD44+CD25+ cells. The expression of a pre-TCR at this stage could thereby provide a compensatory mechanism in γc − mice to enable α/β T cell development. Moreover, the “window” of pre-TCR expression was similar in γc + and γc − mice. In this respect, γc and pre-TCR signals are independent and overlapping at this stage of intrathymic development (Fig. 7).
The signaling cascades initiated from γc and pre-TCRs appear distinct. γc receptors activate the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway (for a review, see reference 56), whereas the pre-TCR uses immunoreceptor tyrosine-based activation motif (ITAM)-containing CD3 components which couple to the src family and ZAP-70/syk family tyrosine kinases (for reviews, see references 42 and 57). How the pre-TCR mediates proliferation of late thymocyte precursors is unknown, but our results indicate that this process does not require γc-dependent cytokines. Further work will be required to identify the mechanism by which triggering the pre-TCR engages the cell cycle.
Our observations provide insights into the difference between α/β and γ/δ T cell development in IL-7/IL-7Rα/ γc-deficient mice (12, 17, 58). Although the pre-TCR is capable of rescuing TCR-α/β cells in γc − mice, γ/δ T cells lack an equivalent mechanism and therefore must rely on other signals for their final intrathymic differentiation. We have previously shown that transgenic expression of rearranged TCR-γ or TCR-γ/δ chains failed to rescue γ/δ T cell development in γc − mice, suggesting that γc-dependent cytokines provide the dominant signals for γ/δ T cell survival and/or proliferation both intrathymically and in the periphery (28). On the other hand, peripheral maintenance of α/β T cells requires continual TCR stimulation (for reviews, see references 59 and 60) but appears less γc dependent in their development.
Finally, our model suggests that differences in T cell development between human and murine γc deficiency might be related to species-specific differences in the function of c-kit, IL-7/IL-7Rα/γc, or the pre-TCR. Little is known about the specific patterns of expression of these molecules with regard to the various stages of intrathymic development in humans. Moreover, the documented differences between human and mouse pTα cytoplasmic sequences (61) could result in differential signaling properties of the pre-TCR between species. Support for this model will require further studies focusing on these pathways in human thymocyte development.
Acknowledgments
We thank D. Guy-Grand (Paris), M. Malissen, B. Malissen (Marseille), and H.-R. Rodewald (Basel) for stimulating discussions.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Association pour le Recherche sur le Cancer, and the Ligue Nationale Contre le Cancer. The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche.
Footnotes
Abbreviations used in this paper: BrdU, bromo-deoxyuridine; DN, double negative; DP, double positive; γc, common cytokine receptor γ chain; RAG, recombination activating gene; SP, single positive; TN, triple negative; Tg, transgene; TRI, Tricolor.
References
- 1.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Nakamura M, Takeshita T. The common gamma-chain for multiple cytokine receptors. Adv Immunol. 1995;59:225–277. doi: 10.1016/s0065-2776(08)60632-x. [DOI] [PubMed] [Google Scholar]
- 2.Leonard WJ. Dysfunctional cytokine receptor signaling in severe combined immunodeficiency. J Investig Med. 1996;44:304–311. [PubMed] [Google Scholar]
- 3.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor γ chain: its role in multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 4.Cao X, Shores EW, Hu LJ, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 5.Di Santo JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, Moriyama M, Nakamura M, Katsuki M, Takahashi K, et al. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 7.Nakajima H, Shores EW, Noguchi M, Leonard WJ. The common cytokine receptor γ chain plays an essential role in regulating lymphoid homeostasis. J Exp Med. 1997;185:189–195. doi: 10.1084/jem.185.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharara LI, Andersson A, Guy-Grand D, Fischer A, Di Santo JP. Deregulated TCR alpha beta T cell population provokes extramedullary hematopoiesis in mice deficient in the common gamma chain. Eur J Immunol. 1997;27:990–998. doi: 10.1002/eji.1830270428. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor β chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 11.Di Santo JP. Cytokines: shared receptors, distinct functions. Curr Biol. 1997;7:424–427. doi: 10.1016/s0960-9822(06)00208-9. [DOI] [PubMed] [Google Scholar]
- 12.Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7−/−mice. J Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 13.Namen AE, Hjerrild SLK, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S, Cosman D, Goodwin RG. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 14.Murray R, Suda T, Wrighton N, Lee F, Zlotnik A. IL-7 is a growth and maintenance factor for mature and immature thymocyte subsets. Int Immunol. 1989;1:526–531. doi: 10.1093/intimm/1.5.526. [DOI] [PubMed] [Google Scholar]
- 15.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki JI, Ikuta K. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc Natl Acad Sci USA. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Santo JP, Guy-Grand D, Fisher A, Tarakhovsky A. Critical role for the common cytokine receptor γ chain in intrathymic and peripheral T cell selection. J Exp Med. 1996;183:1111–1118. doi: 10.1084/jem.183.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crompton T, Outram SV, Buckland J, Owen MJ. A transgenic T cell receptor restores thymocyte differentiation in interleukin-7 receptor alpha-deficient mice. Eur J Immunol. 1997;27:100–104. doi: 10.1002/eji.1830270115. [DOI] [PubMed] [Google Scholar]
- 20.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 21.Kondo M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 22.Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 23.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ. Lymphoid development in mice congenitally lacking T cell receptor αβ-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 24.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor α gene in the development of αβ but not γδ T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 25.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 26.Rodewald HR, Ogawa M, Haller C, Waskow C, Di Santo JP. Pro-thymocyte expansion by c-kit and the common cytokine receptor gamma chain is essential for repertoire formation. Immunity. 1997;6:265–272. doi: 10.1016/s1074-7613(00)80329-5. [DOI] [PubMed] [Google Scholar]
- 27.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor trangenic mice. Cell. 1991;66:5338–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 28.Malissen M, Pereira P, Gerber DJ, Malissen B, Di Santo JP. The common cytokine receptor γ chain controls survival of γ/δ T cells. J Exp Med. 1997;186:1277–1285. doi: 10.1084/jem.186.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine αβ T cell receptors. J Immunol. 1989;142:2736–2742. [Google Scholar]
- 30.Alfantis I, Azogui O, Feinberg J, Saint-Ruf C, Buer J, von Boehmer H. On the role of the pre-T cell receptor in αβ versus γδ T lineage committment. Immunity. 1998;9:649–655. doi: 10.1016/s1074-7613(00)80662-7. [DOI] [PubMed] [Google Scholar]
- 31.Rodewald HR, Awad K, Moingeon P, D'Adamio L, Rabinowitz D, Shinkai Y, Alt FW, Reinherz EL. FcγRII/III and CD2 expression mark distinct subpopulations of immature CD4−CD8−murine thymocytes: in vivo developmental kinetics and T cell receptor β chain rearrangement status. J Exp Med. 1993;177:1079–1092. doi: 10.1084/jem.177.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lantz O, Sharara LI, Tilloy F, Andersson A, Di Santo JP. Lineage relationships and differentiation of natural killer (NK) T cells: intrathymic selection and interleukin (IL)-4 production in the absence of NKR-P1 and Ly49 molecules. J Exp Med. 1997;185:1395–1401. doi: 10.1084/jem.185.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically distinct subsets of CD3−CD4−CD8−triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 34.Rodewald H-R, Fehling HJ. Molecular and cellular events in early thymocyte development. Adv Immunol. 1998;69:1–112. doi: 10.1016/s0065-2776(08)60606-9. [DOI] [PubMed] [Google Scholar]
- 35.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously within the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 36.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Boise LH, Minn AJ, June CH, Lindsten T, Thompson CB. Growth factors can enhance lymphocyte survival without committing the cell to undergo cell division. Proc Natl Acad Sci USA. 1995;92:5491–5495. doi: 10.1073/pnas.92.12.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akbar AN, Borthwick NJ, Wickremasinghe RG, Panayoitidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol. 1996;26:294–299. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 39.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 40.Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-β gene rearrangement and role of TCR-β expression during CD3−CD4−CD8−thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 41.Laky K, Lefrancois L, von Freeden-Jeffry U, Murray R, Puddington L. The role of IL-7 in thymic and extrathymic development of TCR-γδ cells. J Immunol. 1998;161:707–713. [PubMed] [Google Scholar]
- 42.Fehling HJ, von Boehmer H. Early αβ T cell development in the thymus of normal and genetically altered mice. Curr Opin Immunol. 1997;9:263–275. doi: 10.1016/s0952-7915(97)80146-x. [DOI] [PubMed] [Google Scholar]
- 43.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, Tonegawa S. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 44.Buer J, Aifantis I, Di Santo JP, Fehling HJ, von Boehmer H. Role of different T cell receptors in the development of pre-T cells. J Exp Med. 1997;185:1541–1547. doi: 10.1084/jem.185.9.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Boehmer H, Fehling HJ. Structure and function of the pre-T cell receptor. Annu Rev Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman ES, Passoni L, Crompton T, Leu TM, Schatz DG, Koff A, Owen MJ, Hayday AC. Productive T-cell receptor β-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 47.Candeias S, Muegge K, Durum SK. IL-7 receptor and VDJ recombination: trophic versus mechanistic actions. Immunity. 1997;6:501–508. doi: 10.1016/s1074-7613(00)80338-6. [DOI] [PubMed] [Google Scholar]
- 48.von Boehmer H, Bonneville M, Ishida I, Ryser S, Lincoln G, Smith RT, Kishi H, Scott B, Kisielow P, Tonegawa S. Early expression of a T-cell receptor β-chain transgene suppresses rearrangement of the Vγ4 gene segment. Proc Natl Acad Sci USA. 1988;85:9729–9732. doi: 10.1073/pnas.85.24.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrie HT, Livak F, Burtrum D, Mazel S. T cell receptor gene recombination patterns and mechanisms: cell death, rescue, and T cell production. J Exp Med. 1995;182:121–127. doi: 10.1084/jem.182.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodewald HR, Kretzschmar K, Swat W, Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3:313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 51.Zlotnik A, Moore TA. Cytokine production and requirements during T-cell development. Curr Opin Immunol. 1995;7:206–213. doi: 10.1016/0952-7915(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 52.Morrissey PJ, McKenna H, Widmer MB, Braddy S, Voice R, Charrier K, Williams DE, Watson JD. Steel factor (c-kit ligand) stimulates the in vitro growth of immature CD3−/CD4−/CD8−thymocytes: synergy with IL-7. Cell Immunol. 1994;157:118–131. doi: 10.1006/cimm.1994.1210. [DOI] [PubMed] [Google Scholar]
- 53.Godfrey DI, Zlotnik A, Suda T. Phenotypic and functional characterization of c-kit expression during intrathymic T cell development. J Immunol. 1992;149:2281–2285. [PubMed] [Google Scholar]
- 54.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, Nishikawa S-I. Expression and function of the interleukin 7 receptor in murine thymocytes. Proc Natl Acad Sci USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 56.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 57.Alberola-Ila J, Takaki S, Kerner JD, Perlmutter R. Differential signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- 58.He YW, Malek TR. Interleukin-7 receptor α is essential for the development of γδ+T cells, but not natural killer cells. J Exp Med. 1996;184:289–293. doi: 10.1084/jem.184.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benoist C, Mathis D. Selection for survival? . Science. 1997;276:2000–2001. doi: 10.1126/science.276.5321.2000. [DOI] [PubMed] [Google Scholar]
- 60.Freitas AA, Rocha B. Lymphocyte survival: a red queen hypothesis. Science. 1997;277:1950. doi: 10.1126/science.277.5334.1950. [DOI] [PubMed] [Google Scholar]
- 61.Del Porto P, Bruno L, Mattei MG, von Boehmer H, Saint-Ruf C. Cloning and comparative analysis of the human pre-T-cell receptor α-chain gene. Proc Natl Acad Sci USA. 1995;92:12105–12109. doi: 10.1073/pnas.92.26.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]