Abstract
CD34+ hematopoietic stem cells, which circulate in peripheral blood with very low frequency, exert essential accessory function during lipopolysaccharide (LPS)-induced human T lymphocyte activation, resulting in interferon γ production and proliferation. In contrast, stimulation of T cells by “conventional” recall antigens is not controlled by blood stem cells. These conclusions are based on the observation that depletion of CD34+ blood stem cells results in a loss of LPS-induced T cell stimulation as well as reduced expression of CD80 antigen on monocytes. The addition of CD34-enriched blood stem cells resulted in a recovery of reactivity of T cells and monocytes to LPS. Blood stem cells could be replaced by the hematopoietic stem cell line KG-1a. These findings may be of relevance for high risk patients treated with stem cells or stem cell recruiting compounds and for patients suffering from endotoxin-mediated diseases.
Keywords: antigen presentation; immunity, cellular; immunoreactivity; immunocompetence; lymphocyte cooperation
Lipopolysaccharide (LPS, endotoxin) is the major component of the outer membrane of Gram-negative bacteria. The release of LPS by bacteria is considered to be responsible for systemic reactions of severely infected patients and may cause septic shock associated with high risk for a lethal outcome. LPS exerts its fatal action not directly, but stimulates various types of cells, including monocytes/macrophages (1, 2), endothelial cells (3), or granulocytes (4), to release inflammatory mediators, including IL-1, IL-6, and TNF-α. In addition, murine B lymphocytes were shown to respond to LPS by reacting with differential proliferation and antibody production (5–7). In recent years, it became evident that LPS is also a potent inducer of human as well as murine T lymphocyte proliferation and cytokine production (8–12). We have previously shown that this LPS-induced T cell activation is strongly dependent on direct cell-to-cell contact of responding T lymphocytes with viable accessory monocytes, indicating receptor–ligand interactions (13). Furthermore, this interaction was MHC unrestricted, but strongly dependent on B7 interaction with CD28 (14). Moreover, it could be demonstrated by analysis of the frequency of responding T lymphocytes that LPS is not active as a mitogen, superantigen, or classical antigen, indicating a new mechanism of T cell stimulation (14).
When comparing the stimulatory activity of LPS on PBMCs from adult donors with PBMCs isolated from cord blood, we made the following unexpected finding: adult donors could be grouped, with regard to an LPS-inducible T cell proliferation, into LPS responders and LPS nonresponders (13); however, PBMCs isolated from cord blood always responded to LPS with a proliferation of T lymphocytes. This phenomenon suggested an important role of a cell population present in PBMCs from cord blood samples but rare in PBMCs isolated from adult donors. Only in the presence of larger amounts of this rare cell population is an LPS-induced T cell response observed. As described in this report, this cell type has now been identified as the CD34+ blood hematopoietic stem cell.
Materials and Methods
Isolation of Different Cell Populations.
Human PBMCs were isolated from heparinized blood by discontinuous centrifugation over Ficoll-Hypaque according to the method described by Böyum (15). CD34+ cells were depleted from PBMCs using the CD34 Progenitor Cell Isolation kit (Miltenyi Biotech). Cells were labeled with the hapten-conjugated anti-CD34 mAb QBEND-10, and immunomagnetic beads were coated with hapten-specific goat anti–mouse (GaM)1 antibodies according to instructions given by the manufacturer.
We were unable to control this depletion procedure by flow cytometry, since the number of CD34+ cells was always below the detection limit of this method (in our hands <0.5%). For isolation of CD34+ cells, two subsequent enrichments from PBMCs were performed, resulting in a purity of CD34+ stem cells between 45 and 85%. In some experiments, lymphocytes were separated from monocytes using a JE-6B elutriator (Beckman Instruments). The monocyte fraction collected consisted of >95% monocytes, as determined by FACS® analysis after staining with anti-CD14 (Leu-M3; Becton Dickinson). T cells were isolated from lymphocytes by depletion of CD14+, CD16+, and CD19+ cells using the magnetic activated cell sorting system (MACS®; Miltenyi Biotech) with the following antibodies: CD14 microbeads, CD16 microbeads, and CD19 microbeads (all Miltenyi Biotech). These purified T lymphocytes consisted of >98% CD2+ T lymphocytes (Leu-5b; Becton Dickinson) and <0.01% esterase-positive cells.
Cell Culture.
Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated human serum (HS) and antibiotics in flat-bottomed plates (Nunc) at a final volume of 200 μl. Cells were stimulated with LPS from Salmonella friedenau (1 μg/ml), tetanus toxoid (TT, 1 limit of flocculation [Lf]/ml), purified protein derivatives (PPDs) of Mycobacterium tuberculosis (1 μg/ml), or BCG (4,000 CFU/ml). Cells were cultured for 7 d in a humidified 5% CO2 atmosphere at 37°C. For the last 8 h of stimulation, cells were labeled with [3H]TdR (2 Ci/mmol, 0.2 μCi/culture) and harvested on glass filter mats for measurement of incorporated radioactivity.
Induction of CD80 and Immunofluorescence Staining of Cells.
PBMCs (106/ml) were cultured in 24-well plates (1 ml/well; Nunc) in the presence or absence of LPS (1 μg/ml) in RPMI 1640 supplemented with 10% HS. After 2 d of culture, cells were collected by careful rubbing to yield all adherent monocytes. Indirect immunofluorescent staining of PBMCs was performed in ice-cold PBS (containing 0.1% sodium azide) with anti-CD80– biotin (anti-B7.1, clone BB.1; PharMingen) in concentrations as recommended by the producer. After incubation for 20 min at 4°C, cells were washed by centrifugation on an FCS gradient (200 g for 10 min). Cells were then incubated for another 20 min with streptavidin–Red 670. Unbound streptavidin–Red 670 was again removed by centrifugation over an FCS gradient. Labeled cells were analyzed in a Cytofluorograf (model 50H; Ortho Diagnostic Systems).
IFN-γ Production.
PBMCs (106/ml) were cultured in 24-well plates (1 ml/well; Nunc) in the presence or absence of LPS (1 μg/ml) in RPMI 1640 supplemented with 10% HS. Supernatants were harvested after 24, 48, or 96 h of culture, and IFN-γ production was measured with an ELISA provided by Dr. H. Galatti (Hoffmann-La Roche, Basel, Switzerland).
Culture Conditions for the Induction of Dendritic Cells.
Monocytes (106/ml) were isolated by counter-flow elutriation and cultured in six-well plates in RPMI 1640 plus 10% HS and GM-CSF (100 U/ml), IL-4 (50 U/ml), and IFN-γ (50 U/ml). Weekly, half of the culture medium was replaced by new medium with cytokines.
Results
Depletion of CD34+ Blood Stem Cells Prevents LPS-induced T Cell Proliferation, but Enrichment of CD34+ Blood Stem Cells Restores the Response of LPS Nonresponders.
In previous investigations, we found that only ∼50% of adult blood donors responded to LPS stimulation by a T cell proliferation. However, in all PBMCs isolated from cord blood samples (n > 30), an LPS-induced T cell proliferation could be observed. Thus, we were looking for a very rare accessory cell population which was significantly enriched in cord blood compared with adult blood. CD34+ cells were likely candidates for this cell population, since they are very rare in adult blood (0.03–0.09%) but present in significantly larger amounts in cord blood (0.33–1.98%) (16). Therefore, we depleted CD34+ cells from PBMCs of adult donors using a CD34 isolation kit and the MACS® system. These CD34-depleted PBMCs were either stimulated with LPS or the recall antigen PPD of tuberculin. Furthermore, CD34-enriched cell preparations were added to CD34-depleted PBMCs and then again stimulated with LPS or antigens. Table I shows representative results of one out of seven experiments. Magnetic depletion of CD34+ cells from PBMCs resulted in a clear and almost total loss of the LPS-induced DNA synthesis. The DNA synthesis induced by PPD was not reduced in CD34-depleted cultures, ruling out the possibility that classical APCs were depleted or had lost their accessory capacity during magnetic depletion procedures. The response to LPS was fully restored or even enhanced by addition of 5% CD34-enriched cells to CD34-depleted PBMCs. These findings were supported by the following control experiments: (a) PBMCs were labeled with anti-CD34 mAbs and goat anti–mouse (GaM) microbeads, but not subjected to the MACS® separation columns. This labeling procedure did not affect the LPS-induced T cell proliferation. (b) PBMCs were labeled with isotype-specific antibodies and after binding to GaM microbeads were subjected to MACS® separation columns. These control PBMCs could be stimulated by LPS just as well as untreated PBMCs. (c) CD34-enriched cells by themselves were stimulated with LPS. As shown in Table I, these cells did not respond to LPS (or PPD). This finding excludes the possibility of CD34+ stem cells representing the proliferating cells after stimulation with LPS.
Table I.
Accessory Cell Activity of CD34+ Blood Stem Cells during Stimulation of T Lymphocytes by LPS
| Cell population | DNA synthesis after stimulation with | |||||
|---|---|---|---|---|---|---|
| None | LPS | PPD | ||||
| cpm/culture | ||||||
| PBMCs | 80 ± 10 | 8,150 ± 850 | 12,630 ± 250 | |||
| CD34-depleted PBMCs | 40 ± 20 | 40 ± 20 | 12,060 ± 1,690 | |||
| CD34-depleted PBMCs plus 5% CD34-enriched cells | 470 ± 130 | 8,250 ± 40 | 14,430 ± 340 | |||
| PBMCs, labeled with anti-CD34 and GaM microbeads (control 1) | 100 ± 40 | 8,150 ± 350 | 11,230 ± 120 | |||
| PBMCs after depletion of cells labeled with an isotype-specific mAb (control 2) | 120 ± 50 | 7,980 ± 430 | 12,130 ± 380 | |||
| CD34-enriched cells alone (control 3) | 90 ± 30 | 100 ± 20 | 110 ± 60 | |||
Cells (106/ml) were stimulated with LPS (S. friedenau, 1 μg/ml) or PPD (1 μg/ml) and cultured for 7 d in RPMI 1640 plus 10% HS in a final volume of 200 μl/culture. For the last 8 h of culture, cells were pulsed with [3H]TdR (0.2 μCi/culture), then harvested on glass filter mats, and the radioactivity was measured in a β-counter. The results of one of seven experiments are given. Data are expressed as mean ± SD of three independent cultures.
Next, we investigated whether the addition of CD34-enriched cells to CD34-depleted PBMCs restored an LPS-inducible proliferation of T lymphocytes from LPS nonresponders. As depicted in Table II and demonstrated previously, DNA synthesis to antigens (PPD, donor 1; TT, donor 2; BCG, donor 3) was not influenced by depletion or enrichment of CD34+ cells. On the other hand, neither untreated nor CD34-depleted PBMCs of these LPS nonresponders could be stimulated by LPS. It is noteworthy that addition of CD34-enriched cells to PBMCs of these donors clearly resulted in LPS-induced DNA synthesis. Titration experiments showed that as few as 0.5% CD34+ cells were able to induce significant DNA synthesis after LPS stimulation (data not shown).
Table II.
CD34+ Blood Stem Cells Restore Activation of T Lymphocytes from LPS Nonresponders by LPS
| Cell population | DNA synthesis after stimulation with | |||||
|---|---|---|---|---|---|---|
| None | LPS | Recall antigen | ||||
| cpm/culture | ||||||
| Experiment 1 | ||||||
| PBMCs | 680 ± 260 | 520 ± 90 | 12,080 ± 1,580 | |||
| CD34-depleted PBMCs | 70 ± 10 | 80 ± 10 | 20,370 ± 2,680 | |||
| CD34-depleted PBMCs plus CD34-enriched cells | 590 ± 130 | 8,380 ± 110 | 14,500 ± 90 | |||
| Experiment 2 | ||||||
| PBMCs | 550 ± 290 | 420 ± 10 | 32,170 ± 5,880 | |||
| CD34-depleted PBMCs | 160 ± 100 | 600 ± 170 | 32,640 ± 3,950 | |||
| CD34-depleted PBMCs plus CD34-enriched cells | 160 ± 40 | 2,230 ± 540 | 31,700 ± 2,640 | |||
| Experiment 3 | ||||||
| PBMCs | 140 ± 50 | 250 ± 80 | 2,110 ± 910 | |||
| CD34-depleted PBMCs | 350 ± 40 | 390 ± 60 | 2,490 ± 120 | |||
| CD34-depleted PBMCs plus CD34-enriched cells | 770 ± 240 | 12,010 ± 1,200 | 3,370 ± 1,030 | |||
Cells (106/ml) plus 10% accessory cells were stimulated with LPS (S. friedenau, 1 μg/ml), TT (donor 1, 1 Lf/ml), PPD (donor 2, 1 μg/ml), or BCG (donor 3, 4,000 CFU/ml) and cultured for 7 d in RPMI 1640 plus 10% HS in a final volume of 200 μl/culture. For the last 8 h of culture, cells were pulsed with [3H]TdR (0.2 μCi/culture), then harvested on glass filter mats, and the radioactivity was measured in a β-counter. Data are expressed as mean ± SD of three independent cultures.
CD34+ Blood Stem Cells Are the Responsive Fraction in Elutriated Monocyte Preparations.
In previous experiments, we have shown that the stimulation of human T lymphocytes by LPS depends on the presence of monocytes, isolated by counter-flow elutriation. The demonstrated accessory activity of CD34+ blood stem cells prompted experiments to investigate whether, in fact, CD34+ stem cells are responsible for the accessory activity of these monocyte preparations. The monocyte fractions isolated by counter-flow elutriation were depleted from CD34+ stem cells by the MACS® system. Additionally, CD34+ stem cells were enriched from monocyte fractions by the MACS® system using positive selection. Purified T lymphocytes were isolated from PBMCs by counter-flow elutriation and subsequent purification by magnetic depletion of all non-T cells (CD14+, CD16+, and CD19+ cells). Thereafter, purified T cells were stimulated with either LPS or PPD in the presence of unseparated monocytes, CD34-depleted monocytes, or CD34-enriched cells. Table III shows the representative results of one of three independent experiments. Neither LPS nor the recall antigen PPD was able to induce DNA synthesis in purified T lymphocytes. However, in the presence of 10% unseparated monocytes, DNA synthesis was observed for both stimuli. Depletion of CD34+ cells from the elutriated monocyte fractions clearly resulted in the loss of LPS-induced T cell proliferation, but not of PPD-induced T cell response. Addition of CD34+ cells not only restored the response of purified T lymphocytes to LPS, but resulted in DNA synthesis that was even higher than in the presence of untreated monocytes. As already shown for PBMCs, depletion or enrichment of CD34+ cells from elutriated monocytes had no influence on PPD-induced T cell proliferation.
Table III.
Failure of CD34-depleted Monocytes to Exert Accessory Function during Stimulation of T Lymphocytes by LPS
| Cell population | DNA synthesis after stimulation with | |||||
|---|---|---|---|---|---|---|
| None | LPS | PPD | ||||
| cpm/culture | ||||||
| Purified T lymphocytes | 190 ± 70 | 210 ± 140 | 250 ± 180 | |||
| T plus 10% monocytes | 220 ± 20 | 3,600 ± 320 | 9,370 ± 1,380 | |||
| T plus 10% CD34-depleted cells | 210 ± 20 | 350 ± 50 | 12,300 ± 3,350 | |||
| T plus 10% CD34-enriched cells | 600 ± 290 | 8,520 ± 90 | 8,600 ± 380 | |||
Purified T lymphocytes (106/ml) or T lymphocytes plus 10% of the accessory cell preparations described above were stimulated with LPS (S. friedenau, 1 μg/ml), or PPD (1 μg/ml) and cultured for 7 d in RPMI 1640 plus 10% HS in a final volume of 200 μl/culture. For the last 8 h of culture, cells were pulsed with [3H]TdR (0.2 μCi/culture), then harvested on glass filter mats, and the radioactivity was measured in a β-counter. The results of one of three experiments are given. Data are expressed as mean ± SD of three independent cultures.
CD34+ Blood Stem Cells Are Necessary for the Induction of CD80 on Monocytes after LPS Stimulation.
Previously, we have reported that stimulation of human T lymphocytes by LPS is dependent on the interactions of CD80 (B7.1) on accessory cells and CD28 on T cells (14). We have also shown that CD80 could be induced on monocytes of LPS responders, but not on monocytes of LPS nonresponders after LPS stimulation. To shed light on the role of CD34+ blood stem cells in the expression of CD80, we now compared the CD80 expression on monocytes after LPS stimulation in unseparated PBMCs, CD34-depleted PBMCs, and CD34-depleted PBMCs plus CD34-enriched cells. As a control, DNA synthesis was determined. In Table IV, one representative experiment of three independent experiments is shown. CD80 expression on monocytes after LPS stimulation was clearly dependent on the presence of CD34+ accessory cells. After LPS stimulation of unseparated PBMCs, CD80 was enhanced on monocytes. Depletion of CD34+ cells resulted in a diminished stimulation of DNA synthesis as well as reduced CD80 expression after LPS stimulation. The addition of CD34-enriched cells to CD34-depleted PBMCs not only restored but even surpassed DNA synthesis as well as CD80 expression after LPS stimulation compared with PBMC controls.
Table IV.
CD34+ Blood Stem Cells Are Necessary for LPS-induced Expression of CD80 on Monocytes
| Cell population | CD80 expression | DNA synthesis | ||||||
|---|---|---|---|---|---|---|---|---|
| None | LPS | None | LPS | |||||
| % positive monocytes | cpm/culture | |||||||
| PBMCs | 15.6 | 58.2 | 220 ± 70 | 7,160 ± 340 | ||||
| CD34-depleted PBMCs | 16.2 | 21.6 | 210 ± 110 | 590 ± 230 | ||||
| CD34-depleted PBMCs plus CD34-enriched cells | 13.3 | 80.1 | 340 ± 160 | 11,720 ± 1,080 | ||||
Cells (106/ml) were stimulated with LPS (S. friedenau, 1 μg/ml) and cultured in RPMI 1640 plus 10% HS in a final volume of 200 μl/culture. CD80 expression was measured after 2 d of culture. For determination of DNA synthesis after 7 d of culture, cells were pulsed with [3H]TdR (0.2 μCi/culture) for the last 8 h of culture, then harvested on glass filter mats, and the radioactivity was measured in a β-counter. The results of one of three experiments are given. Data are expressed as mean ± SD of three independent cultures.
CD34+ Blood Stem Cells Are Necessary for the Production of IFN-γ.
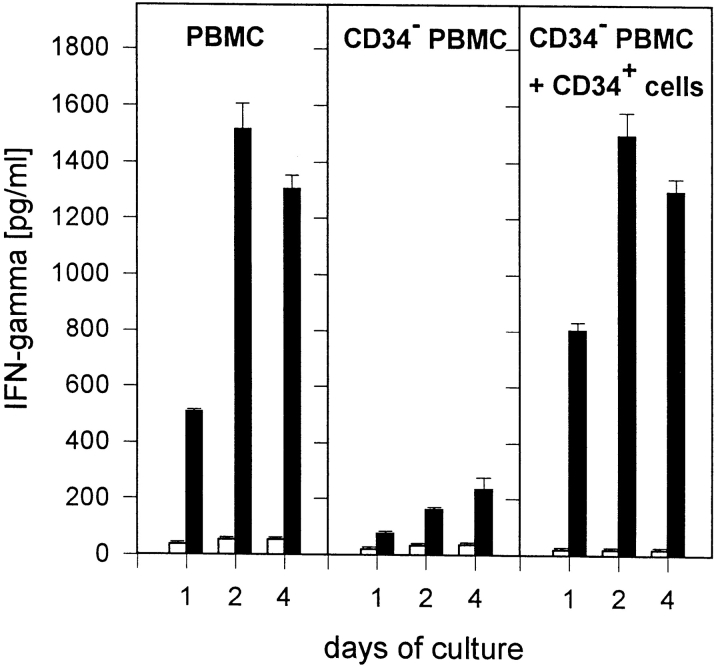
In previous experiments, we have shown that LPS is a potent inducer of IFN-γ production by human T lymphocytes (13). In the experiments presented here, we investigated whether depletion of CD34+ blood stem cells also influences the production of IFN-γ by PBMCs. As shown in Fig. 1, IFN-γ production was clearly reduced in the absence of CD34+ cells over the total culture period of 4 d. On the other hand, IFN-γ production could be fully restored by adding CD34+ cells. It should be noted that IFN-γ could be detected in PBMCs or CD34− PBMCs plus CD34+ cells already after 1–2 h of culture (data not given).
Figure 1.
Accessory cell activity of CD34+ blood stem cells during induction of IFN-γ release in T lymphocytes by LPS. PBMCs (106/ml), CD34-depleted PBMCs (106/ml), or CD34-depleted PBMCs plus 5% CD34+ cells were cultured in 24-well plates (1 ml/well) in RPMI 1640 plus 10% HS. Cells were stimulated with LPS (1 μg/ml; black bars); control cultures remained unstimulated (white bars). After 24, 48, or 96 h of culture, supernatants were harvested and IFN-γ production was measured in an ELISA. Data are expressed as mean ± SD of duplicate cultures.
CD34+ Blood Stem Cells Cannot Be Replaced by Dendritic Cells Derived from Blood Monocytes.
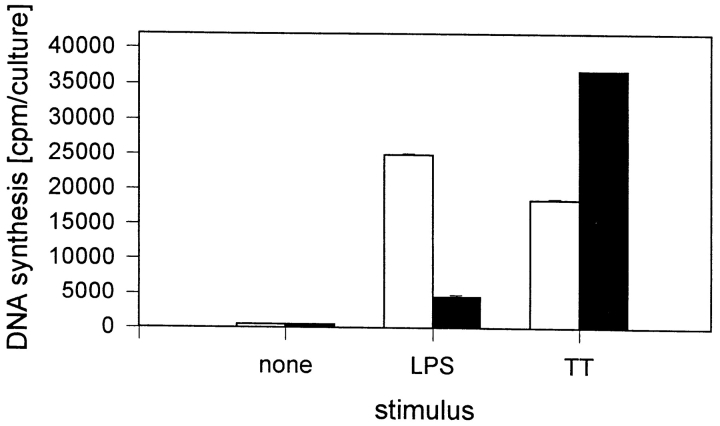
CD34+ blood stem cells are described to be a source for the in vitro generation of dendritic Langerhans cells (17–21). These dendritic Langerhans cells were shown to be highly efficient in processing and presenting soluble antigens to autologous T lymphocytes (20). Thus, we asked whether LPS induces the generation of dendritic cells from CD34+ blood stem cells in the system described. Therefore, it was analyzed whether CD34+ stem cells could be replaced by autologous dendritic cells. To obtain dendritic cells, peripheral blood monocytes were isolated by counter-flow elutriation and cultured for 2 wk in the presence of GM-CSF, IL-4, and IFN-γ. After this culture period, the cells had the typical morphology of dendritic cells and could be phenotypically characterized by the following surface structures: CD14, 4.9 ± 2.5%; HLA-DR, 90 ± 7%; CD80, 47.5 ± 6%; and CD86, 53 ± 16%. The accessory capacity of these dendritic cells was compared with the accessory capacity of freshly isolated monocytes. As shown in Fig. 2, freshly isolated monocytes induce significantly higher DNA synthesis after LPS stimulation than dendritic cells. As expected, dendritic cells function as accessory cells after stimulation with TT. From these experiments, we concluded that it is the CD34+ blood stem cell and not the dendritic cell which provides accessory capacity in LPS-induced T cell proliferation.
Figure 2.
Accessory activity of monocytes and dendritic cells during stimulation of T cells by LPS. Purified T lymphocytes (106/ml) were cultured in the presence of 10% freshly isolated autologous monocytes (white bars) or 10% autologous dendritic cells (black bars) and stimulated with LPS (S. friedenau, 1 μg/culture) in RPMI 1640 plus 10% HS in a final volume of 200 μl/culture. After 7 d of culture, cells were pulsed with [3H]TdR (0.2 μCi/culture), then harvested on glass filter mats, and the radioactivity was measured in a β-counter. The results of one of three experiments are given. Data are expressed as mean ± SD of three independent cultures.
CD34+ Blood Stem Cells Can Be Replaced by KG-1a Cells.
The purity of our CD34-enriched blood stem cell preparations varied between 45 and 85%. This raised the question, whether other cell populations present may contribute to the observed effects in addition to or instead of CD34+ blood stem cells. Therefore, a myeloid stem cell line was used to replace accessory cell preparations used. Indeed, it was found that the hematopoietic stem cell line KG-1a, derived from an acute myelogenous leukemia (22), was able to substitute for CD34+ blood stem cell preparations. Purified T lymphocytes were cultured in the presence of γ-irradiated KG-1a cells and stimulated with LPS. Table V shows representative results of eight experiments. It was found that, indeed, T lymphocytes responded to LPS in the presence of KG-1a cells. Furthermore, controls with irradiated KG-1a cells showed that the resulting DNA synthesis was not due to proliferating KG-1a cells.
Table V.
KG-1a Stem Cell Line Shows Accessory Activity during Stimulation of T Lymphocytes by LPS
| Cell population | DNA synthesis after stimulation with | |||
|---|---|---|---|---|
| None | LPS | |||
| cpm/culture | ||||
| T | 110 ± 10 | 430 ± 90 | ||
| T plus irradiated KG-1a | 320 ± 200 | 25,180 ± 2,700 | ||
| Irradiated KG-1a | 280 ± 130 | 380 ± 90 | ||
Purified T lymphocytes (106/ml) were cultured in the presence of 10% irradiated (5,000 rads) KG-1a cells and stimulated with LPS (S. friedenau, 1 μg/ml) in RPMI 1640 plus 10% HS in a final volume of 200 μl/ culture. After 7 d of culture, cells were pulsed with [3H]TdR (0.2 μCi/ culture), then harvested on glass filter mats, and the radioactivity was measured in a β-counter. The results of one of eight experiments are given. Data are expressed as mean ± SD of three independent cultures.
Discussion
The factors and mechanisms involved in stimulation of human or murine T lymphocytes by LPS are not yet fully understood. All features of T cell stimulation indicate a new, so far unknown mechanism of T cell activation by LPS: LPS is not a T cell mitogen (low frequency of responding cells), a T cell superantigen (living accessory cells and CD80–CD28 interactions are necessary), or a classical T cell antigen (no MHC-restricted interactions between APCs and T lymphocytes) (13, 14). Furthermore, it may be speculated that LPS, as a lipid antigen, might be presented (like lipoarabinomannan [LAM]) by CD1 in an MHC- unrestricted manner. However, monocytes, stem cells, or KG-1a cells do not express CD1; therefore, this mechanism of stimulation can be excluded. CD80 expression and IL-12 have been reported to stimulate proliferation and cytokine production in T lymphocytes (23–25). These data might suggest that LPS stimulates T lymphocytes by this indirect mechanism. However, the low frequency of responding T cells after LPS stimulation (1:1,000; reference 13) excludes this rather unspecific activation mechanism by CD80 and IL-12 alone.
The unique and new characteristic feature of T cell stimulation by LPS is underlined by the results presented in this paper. The necessity of hematopoietic blood stem cells for activation of T cells by LPS represents a new mechanism of T cell activation and introduces stem cells as active, potent cells in inflammatory reactions mediated by T lymphocytes. Peripheral CD34+ blood stem cells are known to be a source for autologous and allogenic stem cell transplantation and have been introduced for the treatment of hematological malignancies (26, 27). Furthermore, these cells have been described to be a source for the in vitro generation of dendritic Langerhans cells by treatment of bone marrow or blood stem cells with GM-CSF and TNF-α (17, 18, 20, 21). These dendritic Langerhans cells were shown to be highly efficient in processing and presenting soluble antigen to autologous T lymphocytes (20). However, an accessory function of freshly isolated CD34+ blood stem cells has not been documented to date. We can only speculate on the mechanisms by which CD34+ blood stem cells exert their accessory functions. We consider it unlikely that LPS induces the generation of dendritic cells from CD34+ blood stem cells in our system. Time–kinetic studies concerning the production of IFN-γ after LPS stimulation as shown in Fig. 1 or the expression of CD80 on monocytes after 2 d of culture (Table IV) argue against this hypothesis. IFN-γ could already be detected after a few hours of stimulation of PBMCs or CD34− PBMCs plus CD34-enriched cells by LPS (Fig. 1, and our unpublished data). However, as demonstrated by others, for the generation of dendritic cells, culture periods of several days are necessary (17–21). Furthermore, classical dendritic cells generated from elutriated monocytes by treatment with IFN-γ, IL-4, and GM-CSF were clearly less efficient than freshly isolated monocyte preparations (Fig. 2).
It has also not been clear whether CD34+ blood stem cells act directly as accessory cells for the induction of T cell proliferation or indirectly by influencing the function of monocytes, which are always present at low numbers in the CD34-enriched blood stem cell preparations. The requirement for CD80–CD28 interaction for T cell stimulation by LPS and the requirement of CD34+ stem cells for CD80 expression on monocytes, together with the results obtained with the KG-1a cell line, argue for the latter hypothesis. This question still requires clarification and is presently under further investigation.
In conclusion, the activation of T lymphocytes by LPS represents a new and unique mechanism of T cell activation differing from that induced by classical mitogens, superantigens, or antigens. The involvement of hematopoietic blood stem cells in this type of activation demonstrated for the first time a new role of these cells in inflammatory and immunological events. The significance of these findings for the medication and prevention of diseases (e.g., septic shock, bone marrow transplantation, stem cell factor treatment) remains to be investigated.
Abbreviations used in this paper
- GaM
goat anti–mouse
- HS
human serum
- PPD
purified protein derivative
- TT
tetanus toxoid
Footnotes
This work was financially supported by the Deutsche Forschungsgemeinschaft (SFB 367, project C5) and the Fond der Chemischen Industrie (to H.D. Flad and E.T. Rietschel).
References
- 1.Cavaillon JM, Haeffner-Cavaillon N. Signals involved in interleukin 1 synthesis and release by lipopolysaccharide-stimulated monocytes/macrophages. Cytokine. 1990;2:313–329. doi: 10.1016/1043-4666(90)90061-w. [DOI] [PubMed] [Google Scholar]
- 2.Cavaillon JM, Fitting C, Haeffner-Cavaillon N, Kirsch SJ, Warren HS. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990;58:2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loppnow H, Libby P. Adult human vascular endothelial cells express the IL-6 gene differently in response to LPS and IL-1. Cell Immunol. 1989;122:493–503. doi: 10.1016/0008-8749(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 4.Schade FU, Burmeister I, Engel R. Increased 13-hydroxyoctadecadienoic acid content in lipopolysaccharide-stimulated macrophages. Biochem Biophys Res Commun. 1987;147:695–700. doi: 10.1016/0006-291x(87)90986-7. [DOI] [PubMed] [Google Scholar]
- 5.Gery I, Krüger J, Spiesel SZ. Stimulation of B-lymphocytes by endotoxin. J Immunol. 1972;108:1088–1091. [PubMed] [Google Scholar]
- 6.Greaves M, Janossy G, Doenhoff M. Selective triggering of human T and B lymphocytes in vitro by polyclonal mitogens. J Exp Med. 1974;140:1–18. doi: 10.1084/jem.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker PJ, Taylor CE, Ekwunife FS. Bacterial polysaccharides, endotoxins, and immunomodulation. Adv Exp Med Biol. 1992;319:31–38. doi: 10.1007/978-1-4615-3434-1_4. [DOI] [PubMed] [Google Scholar]
- 8.Milner ECB, Rudbach JA, VonEschen KB. Cellular responses to bacterial lipopolysaccharide: T cells recognize LPS determinants. Scand J Immunol. 1983;18:21–28. doi: 10.1111/j.1365-3083.1983.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 9.Vogel SN, Hilfiker ML, Caulfield MJ. Endotoxin-induced T lymphocyte proliferation. J Immunol. 1983;130:1774–1779. [PubMed] [Google Scholar]
- 10.Le J, Lin J-X, Henriksen-Destefano D, Vilcek J. Bacterial lipopolysaccharide-induced interferon-γ production: roles of interleukin 1 and interleukin 2. J Immunol. 1986;136:4525–4530. [PubMed] [Google Scholar]
- 11.Blanchard DK, Djeu JY, Klein TW, Friedman H, Steward WE. Interferon-γ induction by lipopolysaccharide: dependence on interleukin 2 and macrophages. J Immunol. 1986;136:963–970. [PubMed] [Google Scholar]
- 12.Nitta T, Imai H, Ogasawara Y, Nakano M. Mitogenicity of bacterial lipopolysaccharide on the T lymphocyte population bearing γδ T cell receptor. J Endotoxin Res. 1994;1:101–107. [Google Scholar]
- 13.Mattern T, Thanhäuser A, Reiling N, Toellner K-M, Duchrow M, Kusumoto S, Rietschel ET, Ernst M, Brade H, Flad H-D, Ulmer AJ. Endotoxin and lipid A stimulate proliferation of human T cells in the presence of autologous monocytes. J Immunol. 1994;153:2996–3004. [PubMed] [Google Scholar]
- 14.Mattern T, Flad H-D, Brade L, Rietschel ET, Ulmer AJ. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J Immunol. 1998;160:3412–3418. [PubMed] [Google Scholar]
- 15.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Lab Clin Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- 16.Sutherland DR, Keating A, Nayar R, Anania S, Stewart AK. Sensitive detection and enumeration of CD34+cells in peripheral and cord blood by flow cytometry. Exp Hematol. 1994;22:1003–1010. [PubMed] [Google Scholar]
- 17.Reid CDL, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+progenitors in human bone marrow. J Immunol. 1992;149:2681–2688. [PubMed] [Google Scholar]
- 18.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 19.Santiago-Schwarz F, Belilos E, Diamond B, Carson SE. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukocyte Biol. 1992;52:274–281. [PubMed] [Google Scholar]
- 20.Caux C, Massacrier C, Dezutter-Dambuyant C, Vanbervliet B, Jacquet C, Schmitt D, Banchereau J. Human dendritic Langerhans cells generated in vitro from CD34+ progenitors can prime naive CD4+ T cells and process soluble antigen. J Immunol. 1995;155:5427–5435. [PubMed] [Google Scholar]
- 21.Bernhard H, Disis ML, Heimfeld S, Hand S, Gralow JR, Cheever MA. Generation of immunostimulatory dendritic cells from human CD34+ hematopoietic progenitor cells of the bone marrow and peripheral blood. Cancer Res. 1995;55:1099–1104. [PubMed] [Google Scholar]
- 22.Hay, R., M. Macy, T.R. Chen, P. McClintock, and Y. Reid. 1988. American Type Culture Collection. Rockville, MD.
- 23.Perussia B, Chan SH, D'Andrea A, Tsuji K, Santoli D, Pospisil M, Young D, Wolf SF, Trinchieri G. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-αβ+, TCR-γδ+T-lymphocytes, and NK cells. J Immunol. 1992;149:3495–3502. [PubMed] [Google Scholar]
- 24.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy EE, Terres G, Macatonia SE, Hsieh C-S, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon γ production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li CK, Yuen PM, Chik KW, Shing MM, Tsang KS, Wong A, Leung TF, Lai C. Allogenic peripheral blood stem cell transplantation in children. Med Pediatr Oncol. 1998;30:147–151. doi: 10.1002/(sici)1096-911x(199803)30:3<147::aid-mpo3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Link H, Arseniev L. CD34 positive blood stem cells for allogeneic progenitor and stem cell transplantation. Leuk Lymphoma. 1997;26:451–465. doi: 10.3109/10428199709050882. [DOI] [PubMed] [Google Scholar]