Abstract
T lymphocyte recognition of infected cells is mediated by T cell receptors (TCRs) interacting with their ligands, self–major histocompatibility complex (MHC) molecules complexed with pathogen-derived peptides. Serial TCR interactions with potentially small numbers of MHC/ peptide complexes on infected cells transmit signals that result in T lymphocyte expansion and activation of effector functions. The impact of TCR affinity for MHC/peptide complexes on the rate or extent of in vivo T cell expansion is not known. Here we show that in vivo expansion of complex T cell populations after bacterial infection is accompanied by an increase in their overall affinity for antigen. T cell populations that have undergone additional rounds of in vivo expansion express a narrower range of TCRs, have increased sensitivity for antigen in cytotoxic T lymphocyte assays, and bind MHC/peptide complexes with greater affinity. The selective expansion of higher affinity T cells provides an in vivo mechanism for optimizing the early detection of infected cells.
Keywords: cytotoxic T lymphocytes, Listeria monocytogenes, effector/memory/recall T cells, peptide sensitivity, affinity
Receptor–ligand interactions are fundamental to biological systems and the affinity of these interactions can be an important regulatory parameter. The binding of antibodies to antigen during immune responses to infectious pathogens represents a receptor–ligand interaction in which affinities evolve (1, 2). B lymphocytes are activated and expand in germinal centers of lymph nodes and the spleen upon antigen challenge (3). Over time, antigen-specific B cell populations enhance the affinity of the antibodies they produce by somatically mutating their rearranged immunoglobulin variable (V) genes (4–6). Despite similarities in the strategy for establishing diversity and the general structure of antibodies and TCRs, it is unknown whether TCR–MHC/peptide affinity evolves during antigen stimulation. Since TCRs are coupled to signaling pathways that influence T cell activation, proliferation, and effector functions, evolving TCR affinities could have dramatic effects on T cell phenotypes during immune responses.
The importance of TCR affinity for MHC/peptide complexes has been clearly demonstrated at the level of thymic T cell development (7, 8) and during the activation of mature T cells (9–12). Changes in the affinity of TCR interactions with MHC/peptide complexes can enhance or antagonize T cell activation, or selectively activate different effector functions. However, the role of TCR affinity on T cell responses during infection is unclear. The finding that many antigen-specific T cell responses during infection are highly diverse (13–17) suggests that the affinities of the responding T cells for antigen may not be uniform. Several recent studies have demonstrated evolution of the TCR repertoire during in vivo T cell expansion (18–20). Although it has been postulated that epitope-specific T cell populations undergo affinity maturation during in vivo antigen challenge, the complexity of T cell responses has made it difficult to directly demonstrate this.
We have used murine infection with Listeria monocytogenes as a model system to study MHC class I–restricted T cell responses. Infections of mice with a sublethal dose of L. monocytogenes are rapidly cleared and induce long lasting immunity, which is mediated, in part, by MHC class I–restricted CTLs (21, 22). The peptide specificity of L. monocytogenes–specific CTLs has been determined and the kinetics of the T cell responses to the different epitopes was measured using MHC class I tetramers (23–26). The immunodominant Listeria epitope listeriolysin O (LLO)91–99 1 elicits T cell populations with a diverse TCR Vβ repertoire after primary infection, which is maintained by the memory T cell population (20). Reinfection with L. monocytogenes results in dramatic expansion of memory T cells specific for LLO91–99 and focusing of the TCR repertoire.
In this paper, we demonstrate that in vivo expansion of LLO91–99–specific T cell populations after L. monocytogenes reinfection results in an increase in their affinity for antigen. We used tetrameric MHC/peptide complexes to directly identify complex, LLO91–99–specific T cell populations during primary and recall infection with L. monocytogenes, and we applied and extended a recently described approach (27) using MHC tetramers to estimate the relative affinity of their TCR–MHC/peptide interactions. Using these novel assays, we show that bacterial infection drives the selective expansion of higher affinity T cells, thereby increasing the overall affinity of complex, antigen-specific T cell populations. The functional implications of selective T cell expansion and in vivo T cell affinity maturation for specific immunity to pathogens are discussed.
Materials and Methods
Mice, Bacteria, and Cell Lines
BALB/c mice were obtained from The Jackson Laboratory. For primary infection, 2,000 L. monocytogenes 10403s (obtained from Daniel Portnoy, University of California, Berkeley, CA) were injected into the tail vein of 8–10-wk-old mice. Reimmunization was performed by intravenous injection of 100,000 L. monocytogenes 10403s 5 wk after primary infection. The P815 mastocytoma cell line (H2d) was obtained from the American Type Culture Center.
Generation and Purification of H2-Kd/LLO91–99 Tetramers
Tetrameric H2-Kd/LLO91–99 complexes were generated as recently described (26). After multimerization of refolded and biotinylated MHC/peptide complexes with PE-conjugated streptavidin (SA-PE; Molecular Probes), tetrameric H2-Kd/LLO91–99/ SA-PE complexes were purified by gel filtration over a Superdex 200 HR column (Pharmacia). This final gel filtration step is necessary for tetramer binding or dissociation studies, in order to eliminate the substantial proportion of lower multimeric forms after incubation with SA-PE. Streptavidin conjugates were used because reagents, such as deglycosylated avidin products, form larger aggregates. Purified tetramers were stored at 2–5 mg/ml at 4°C in PBS (pH 8.0) containing 0.02% sodium azide, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 0.5 mM EDTA. To increase the reproducibility and comparability of the presented studies, all experiments were performed using the same batch of H2-Kd/ LLO91–99 tetramer reagent. The reagents were frequently tested on LLO91–99–specific T cell lines to document the maintenance of staining capacity and signal intensity.
Short-Term In Vitro Peptide Stimulation
T cell lines were established by in vitro peptide stimulation as recently described (17, 20). Spleens were removed 7 d after primary infection or 5 d after reinfection with L. monocytogenes, and splenocytes were harvested by dissociation through a wire mesh and lysis of erythrocytes with ammonium chloride, and subsequently resuspended in RP10+, which consists of RPMI 1640 (GIBCO BRL) supplemented with 10% FCS, l-glutamine, Hepes (pH 7.5), β-ME, penicillin (100 U/ml), streptomycin (100 μg/ml), and gentamicin (50 μg/ml). 4 × 107 responder splenocytes were incubated in the presence of 3 × 107 irradiated, syngeneic spleen cells that were peptide-pulsed for 1 h at 37°C with 10−9 M LLO91–99 peptide (17). Cell lines were cultured in 10 ml RP10+ medium supplemented with 5% rat Con A supernatant and were analyzed after 5 d of incubation. Short-term in vitro expansion of LLO91–99–specific T cell lines from peripheral blood was performed as recently described (20).
Enrichment for CD8+ T Cells
Splenocytes were enriched for CD8+ T cells by depletion, using magnetically activated cell sorting (MACS; Miltenyi, Germany) with anti-CD4 (GK1.4), anti–MHC class II (TIB120), anti–MAC-1 (TIB128) mAbs, and anti–rat IgG microbeads (Miltenyi). The purity of CD8+ T cells was between 70 and 85%, as determined by flow cytometry.
CTL Assays
Standard Cr-release assays using 51Cr-labeled P815 target cells were performed as previously described (17). For peptide titrations using T cell lines, the percentage of specific lysis was determined in duplicates over a range of different peptide concentrations at a constant E/T ratio of ∼10:1. For direct ex vivo CTL assays, 6 × 106 CD8+-enriched T cells from a mouse 7 d after primary infection with L. monocytogenes were added per well containing 104 51Cr-labeled P815. This procedure allowed testing of primary, LLO91–99–specific effector T cells at five to six different peptide concentrations, with maximum percentage of specific lysis >30% after 7 h of incubation. To compensate for the ∼10-fold higher frequency of LLO91–99–specific T cells at the peak of a recall response, 10-fold fewer (6 × 105) CD8+-enriched, recall T cells were added per CTL assay well.
Staining and Analysis of CD8+ T Cells
Conventional Procedure.
Phenotypic characterization of LLO91–99– specific T cells using PE-conjugated, tetrameric MHC/peptide complexes together with staining for other surface molecules using directly conjugated mAbs was performed as described previously (20, 26). In brief, after blocking with unconjugated streptavidin (0.5 mg/ml; Molecular Probes) and Fc-block (PharMingen), ∼5 × 105 cells were incubated in FACS staining buffer (SB; PBS, pH 7.45, 0.5% BSA, and 0.02% sodium azide) for 1 h on ice in the presence of saturating concentrations of H2-Kd/LLO91–99 tetramers (0.25–0.5 mg/ml) and other mAbs. Subsequently, cells were washed three times in SB and then fixed in 1% paraformaldehyde/PBS (pH 7.45). Flow cytometry was performed using a FACScalibur® and data were further analyzed with CELLQuest software (Becton Dickinson). The following mAbs were used (all obtained from PharMingen): Cy-Chrome–conjugated anti-CD8α (clone 53-6.7), FITC-conjugated anti-CD8β (clone 53-5.8), FITC-conjugated anti–TCR-α/β (clone H57-597), FITC-conjugated anti-TCR Vβ segments (TCR Vβ2, 3, 4, 5.1/2, 6, 7, 8.1/2, 8.1-3(pan), 9, 10, 11, 12, 13, and 14), and FITC-conjugated anti-CD62L (clone MEL-14).
Tetramer-binding Assays.
Approximately 5 × 105 T cells from in vitro cultured T cell lines or CD8-enriched splenocytes were blocked with unconjugated streptavidin and Fc block as described above, and were subsequently incubated in SB for 0.5 h on ice in the presence of a range of subsaturating concentrations of H2-Kd/ LLO91–99 tetramers and saturating concentrations of Cy-Chrome– conjugated anti-CD8α mAb. The tetramer concentrations tested ranged from 0.4 mg/ml (1:10 dilution of stock solution) to 25 μg/ml (1:160). After incubation, cells were washed three times in SB, fixed in 1% paraformaldehyde, and analyzed by flow cytometry as described above.
Tetramer Dissociation Assays.
Approximately 2 × 106 T cells from T cell lines or CD8-enriched splenocytes were blocked with unconjugated streptavidin and Fc block as described above, and subsequently incubated in SB for 1 h on ice in the presence of saturating concentrations of H2-Kd/LLO91–99 tetramers (0.5 mg/ml) and Cy-Chrome-conjugated anti-CD8α mAb. After incubation, cells were washed three times in SB, and subsequently resuspended in 1 ml SB and transferred to a 15°C waterbath. At the time points 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, and 7 h of incubation at 15°C, 100 μl samples were taken and directly transferred to a FACS® tube containing 100 μl 2% paraformaldehyde/PBS (pH 7.45). Flow cytometry was performed as described above.
Results
Repertoire Focusing of LLO91–99–specific T Cell Populations Occurs during In Vivo T Cell Expansion.
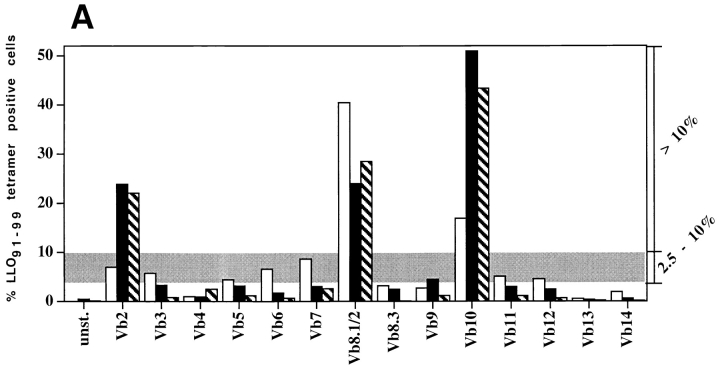
In previous studies we have shown that T cell populations specific for the immunodominant Listeria epitope LLO91–99 are characterized by highly diverse TCR Vβ repertoires at the peak of the primary response to infection, and that this broad TCR repertoire is maintained by memory T cells. However, after reinfection the TCR repertoire of LLO91–99–specific recall T cells becomes more restricted (20). We infected BALB/c mice with L. monocytogenes and stained CD8+ T cells with H2-Kd/LLO91–99 tetramers and a panel of TCR Vβ-specific mAbs during the peak primary response, the peak recall response, and 35 d after the peak recall response, which represents a secondary memory T cell population (Fig. 1). The repertoire of T cells entering the secondary memory pool is nearly indistinguishable from the repertoire established during the peak recall expansion (Fig. 1 A). As a measure of TCR diversity, we calculated the ratio of TCR Vβ staining T cells of intermediate (2.5–10%) to high (>10%) frequency for primary, recall, and secondary memory LLO91–99–specific T cell populations (Fig. 1 B). This analysis demonstrates the loss of TCR diversity after T cell expansion in response to recall infection, and suggests that in vivo T cell expansion is a selective process, favoring the replication of a subset of T cells within a diverse population.
Figure 1.
TCR repertoire focusing occurs during T cell expansion. Primary T cells were obtained from the peripheral blood of BALB/c mice 7 d after sublethal infection and stained with LLO91–99 tetramers and a panel of TCR Vβ-specific antibodies (white bars) as described in Materials and Methods. 35 d after primary infection, the same mice were reinfected with a 50-fold higher dose of L. monocytogenes; peripheral blood lymphocytes were isolated and the TCR Vβ repertoire of LLO91–99–specific T cells was determined 5 d after reinfection (black bars). 35 d after reinfection, CD8+ splenocytes from the same mice were isolated and stained with LLO91–99 tetramers and the panel of TCR Vβ-specific antibodies (hatched bars). (A) Representative TCR Vβ profile of an individual mouse during primary and recall infection with L. monocytogenes. (B) The “degree of diversity” decreases from primary to recall LLO91–99–specific T cells, whereas no further changes occur during the transition from recall effector to secondary memory T cells. The degree of diversity was estimated by the ratio of the number of TCR Vβ segments detected in the range of 2.5–10% within an epitope-specific T cell population (gray area in A) divided by the number of TCR Vβ segments detected at >10%. Results from three different mice and standard deviations are shown.
Is Selective In Vivo Expansion of T Cell Populations Attributable to Enhanced Affinity for Antigen?
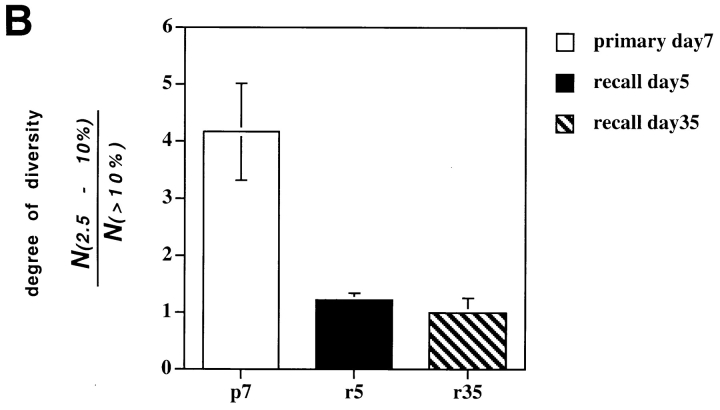
Cytotoxic T cells with higher affinity for a specific MHC/peptide ligand lyse cells in the presence of lower targeting peptide concentrations than CTL with lower affinity (28). If selective in vivo expansion of LLO91–99–specific T cells relates to their affinity, primary and recall CTL should differ in their peptide-sensitivity in cytotoxicity assays. Therefore, we expanded epitope-specific T cells after primary or recall infection and tested them in CTL assays with titrations of the targeting peptide. As shown in Fig. 2 A, T cells derived from the recall response had greater peptide sensitivity than T cells expanded from the primary response. These differences are not attributable to differences in TCR expression levels, since TCR-α/β staining on the two CTL lines was equivalent (Fig. 2 B). These data suggest that recall CTLs have greater affinity for LLO91–99. Because in vitro T cell stimulation might select for nonrepresentative T cell subpopulations, direct ex vivo CTL assays were performed with splenocytes isolated from mice after primary and recall infection. As shown in Fig. 2 C, direct ex vivo CTL assays also demonstrate the higher peptide sensitivity of recall CTLs compared with primary CTLs. We typically find that ex vivo stained CTLs at the peak of the recall response express slightly lower levels of surface TCR (Fig. 2 D) and CD8α (26) than LLO91–99–specific CTLs at the peak of the primary response. Although it might be anticipated that lower TCR expression would decrease peptide sensitivities in CTL assays, any such effect appears to be camouflaged by the overall enhanced peptide sensitivity of recall CTLs.
Figure 2.
Recall LLO91–99–specific T cells are characterized by higher peptide sensitivity than primary effector CTLs. Short-term T cell lines (A and B) and ex vivo T cells (C and D) were assayed in CTL assays (A and C) or stained for TCR expression (B and D) as described in Materials and Methods. (A) T cell lines were generated by short term in vitro peptide stimulation for 5 d from primary day 7 (□) or reinfected day 5 (○) mice, as described in Materials and Methods, and the percentage of specific lysis in the presence of different concentrations of LLO91–99 peptide was determined by standard 51Cr-release assays using P815 (H2d) target cells. (B) TCR-α/β surface expression of the two T cell lines shown in A. (C) Cr-release assays as in A but with cells isolated directly ex vivo from primary and recall infected mice and enriched for CD8+ T cells. Four mice per group, lines show mean values, symbols show data of individual mice. (D) TCR-α/β surface expression of LLO91–99–specific, primary, or recall T cells isolated directly ex vivo.
Binding of Tetrameric H2-Kd/LLO91–99 Complexes Correlates with Relative Affinity.
The two major differences between primary and recall LLO91–99–specific T cell populations are the more focused TCR Vβ repertoire and the greater peptide sensitivity of recall T cells. A recent study showed that the binding kinetics of tetrameric MHC/peptide complexes to T cell hybridomas directly reflect the affinity of TCR binding to MHC/peptide complexes, as determined by BiaCore measurements (27). We therefore tested LLO91–99–specific T cell lines with different peptide-sensitivities for tetramer binding in the presence of a range of tetramer concentrations. As shown in Fig. 3 (using the same T cell lines as in Fig. 2, A and B), T cell lines with higher peptide-sensitivity stain more intensely with subsaturating concentrations of LLO91–99 tetramers than T cell lines with lower peptide sensitivity. The primary T cell line stains with a broader range of intensities than the recall T cell line, suggesting that the affinity of recall T cells is more uniform and, on average, higher than the affinity of primary T cells.
Figure 3.
High affinity T cell lines bind higher levels of H2-Kd/LLO91–99 tetramers than do low affinity T cells. Short-term T cell lines (see Fig. 2, A and B) were tested for their tetramer-binding capacity by incubating in the presence of different, subsaturating concentrations of H2-Kd/LLO91–99 tetramers for 0.5 h on ice. (A) T cell line from the peak of the primary response to L. monocytogenes; (B) T cell line from the peak of the recall response.
Direct Ex Vivo Tetramer-binding Assays Indicate Higher Affinity of Recall T Cell Populations.
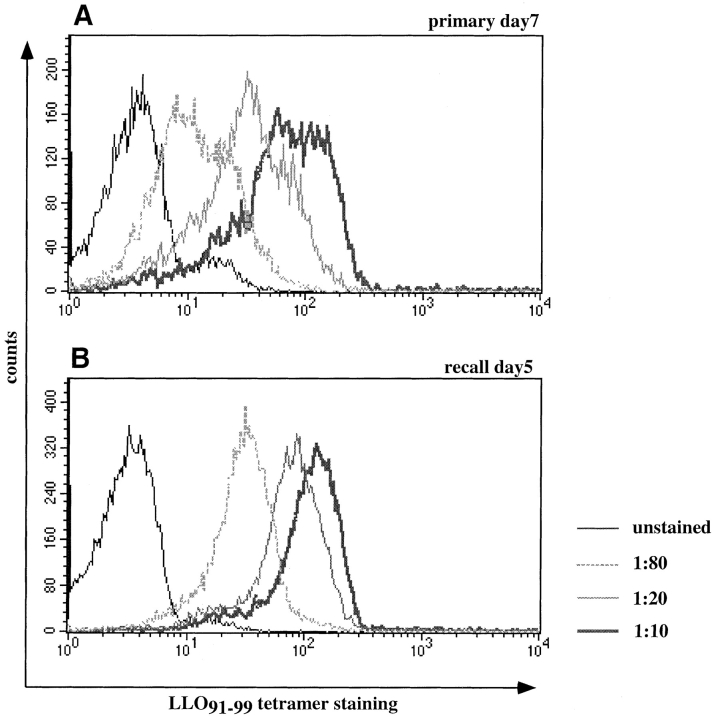
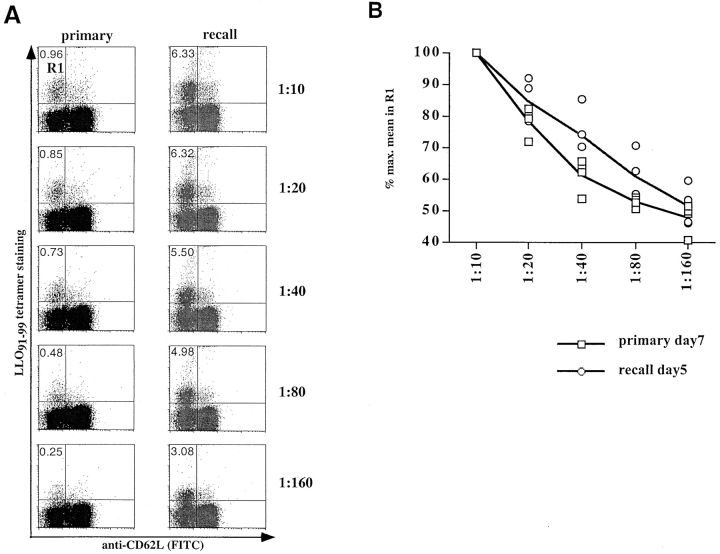
To estimate the in vivo affinities of T cell populations during primary and recall infection, tetramer-binding assays were also performed with directly isolated T cells. The proportion of activated (CD62low) T cells staining with H2-Kd/LLO91–99 tetramers decreases as the tetramers are diluted (Fig. 4). A difference in tetramer staining between primary and recall T cells becomes apparent over the range of these titrations; the percentage of T cells that remain tetramer-positive is higher for recall T cells than for primary T cells (Fig. 4 A). In addition to these quantitative changes, the staining intensity of antigen-specific cells over the range of tetramer concentrations (Fig. 4 B) reveals that recall T cells bind higher levels of tetramers at intermediate concentrations (1:20–1:80) than primary LLO91–99–specific T cells. At lower tetramer concentrations (1:160) the differences in staining intensities between primary and recall LLO91–99–specific T cells are lost.
Figure 4.
Direct ex vivo tetramer-binding assays. CD8-enriched splenocytes from BALB/c mice at the peak of the primary and recall response to L. monocytogenes were tested in tetramer-binding assays. (A) Dot plots of ex vivo binding for CD8+ primary and recall T cells, showing H2-Kd/LLO91–99 tetramer staining on the y axis and anti-CD62L staining on the x axis. The percentages of tetramer-positive cells with an activated phenotype (CD62Llow) are indicated for the different tetramer concentrations (one representative mouse per group). (B) Changes in the staining intensity of tetramer-positive, CD62Llow T cells (gate R1 in A) at different subsaturating tetramer concentrations in direct ex vivo binding assays. Four mice were used per group; the lines represent the mean values for each group, and symbols show intensity of tetramer positive T cells from individual mice. □, peak primary response; ○, peak recall response.
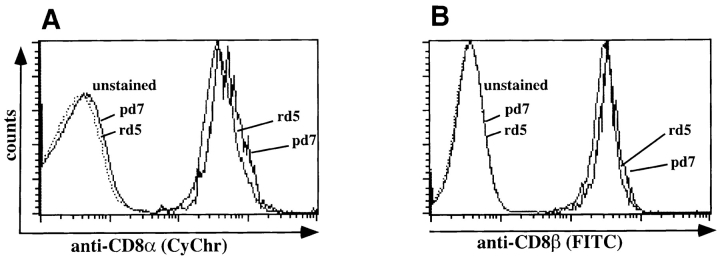
The proportion of cells staining with tetramers and the difference in the intensity of tetramer staining are not attributable to differences in TCR or CD8 surface expression. First, TCR and CD8 expression levels on CTL lines derived from primary and recall infection express identical levels of TCR-α/β and CD8α while differing with respect to tetramer staining at subsaturating concentrations (Fig. 3). Second, with respect to directly isolated antigen-specific T cells, both CD8 and TCR-α/β are more highly expressed during peak primary responses than during peak recall responses (Fig. 2 D and Fig. 5). Because CD8α/β heterodimers can affect the affinity of TCR interactions with MHC/peptide complexes (29, 30), we determined expression levels of CD8α and CD8β on directly ex vivo isolated primary and recall T cells. Staining of LLO91–99–specific T cells revealed that they all express the CD8α/β heterodimer (Fig. 5, A and B). Co-staining with anti-CD8α mAb 53-6.7 does not interfere with tetramer staining (31), whereas anti-CD8β mAb 53-5.8, which can block CD8/ H2-Kd interaction (29), diminishes tetramer-staining intensity of a subpopulation of LLO91–99–specific T cells (data not shown). The percentage of tetramer positive cells whose staining is diminished by anti-CD8β ranges from 10 to 40%, with substantial mouse to mouse variability after both primary and recall infection. Importantly, the degree of CD8 coreceptor dependence among primary and recall T cells is overlapping, ruling out a role for CD8 in determining the differences in tetramer staining. Taken together, these data strongly suggest that TCR affinity for H2-Kd/LLO91–99 complexes evolves and increases as T cells expand during recall responses.
Figure 5.
CD8α/β surface expression of LLO91–99–specific T cell populations. CD8-enriched splenocytes from BALB/c mice at the peak of the primary and recall response to L. monocytogenes were stained for CD8α and CD8β surface expression within the LLO91–99 tetramer–positive T cell population. Cells were first incubated in the presence of anti-CD8 antibodies, followed by staining with NeutrAvidin–PE-conjugated tetramers. Histogram profiles of LLO91–99 tetramer-positive T cells for surface expression of (A) CD8α (Fl-3: CyChr) and (B) CD8β (FL-1: FITC) are shown.
Tetrameric H2-Kd/LLO91–99 Complexes Dissociate from High and Low Affinity T Cell Lines with Different Kinetics.
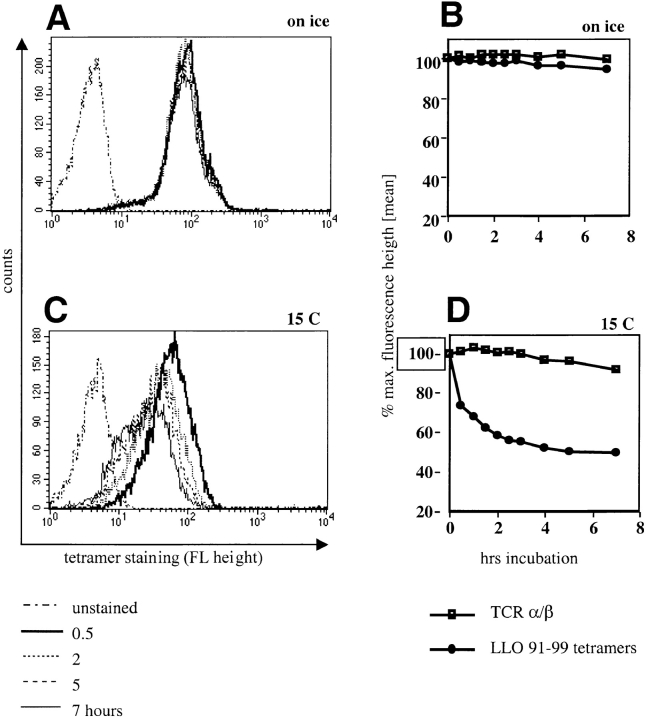
Because the affinity of TCR for MHC/peptide ligands is determined by both on and off rates, we decided to determine whether the dissociation of tetramers from T cell populations could be measured. Maintaining tetramer-stained cells on ice resulted in essentially no loss of staining over 7 h (Fig. 6, A and B). We next determined the amount of tetramer dissociation at 15°C. This temperature was chosen in order to avoid the possibility that MHC class I tetramers might induce TCR internalization, which could confound the interpretation of the results. There is a decrease over time in the staining intensity of tetramer-positive T cells upon incubation at 15°C (Fig. 6, C and D). Staining for TCR after the different incubation intervals did not indicate any changes in TCR surface expression levels, arguing against tetramer-induced TCR internalization. Therefore, the decreased staining intensity is most likely explained by dissociation of tetramers. Tetramer dissociation occurs rapidly during the first hour of incubation at 15°C, perhaps resulting from the dissociation of partially bound complexes. The rapid dissociation phase is followed by a longer phase of slower dissociation.
Figure 6.
Tetramers dissociate from the cell surface of stained cells. An LLO91–99–specific T cell line was stained on ice with saturating concentrations of H2-Kd/LLO91–99 tetramers, and unbound reagent was washed off. Stained cells were either incubated for 7 h either on ice or at 15°C. Samples were taken at increasing time intervals, fixed, and analyzed for the intensity of tetramer staining. Histograms demonstrate tetramer dissociation (decreased staining intensity) on ice (A) and at 15°C (C). The kinetics of tetramer dissociation and TCR-α/β surface expression on ice (B) or at 15°C (D) are plotted. Tetramer staining is expressed as percentage of maximal staining intensity (time point 0 = 100%); TCR-α/β surface expression was determined by staining of samples on ice after the indicated incubation in the dissociation rate assay and is also expressed as percentage of maximal staining intensity (time point 0 = 100%).
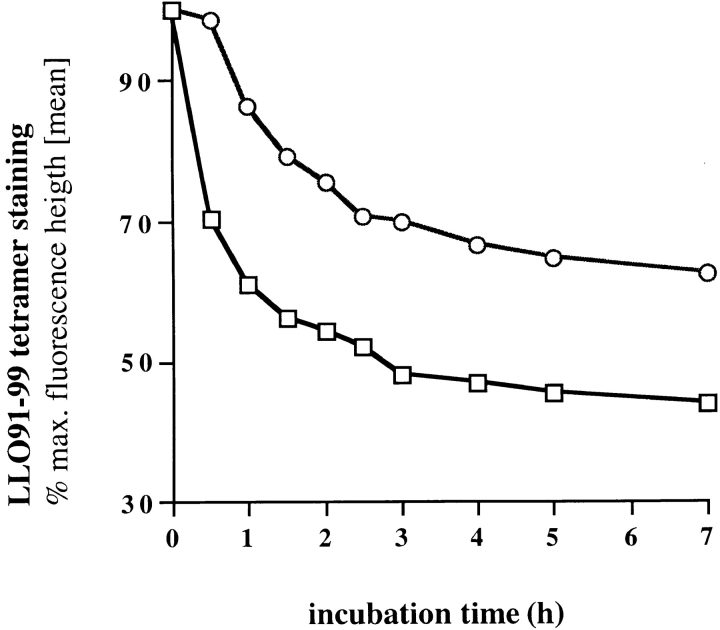
To test if tetramer dissociation rates correlate with the relative affinity of epitope-specific T cells, we tested high and low affinity T cell lines (the same as used in Figs. 2 and 3) for tetramer dissociation (Fig. 7). Tetramers dissociate more slowly from the cell surface of lines with high peptide sensitivity (Fig. 2) and tetramer-binding capacity (Fig. 3) than from low affinity T cell lines. These data indicate that tetramer dissociation rates can be used to contrast the relative affinities of different, antigen-specific T cell populations for their cognate antigen.
Figure 7.
Tetramer dissociation rates of high and low affinity T cell lines. Primary and recall-derived, LLO91–99–specific T cell lines (see Figs. 2 and 3) were tested for tetramer dissociation at 15°C, as described in Fig. 5. □, T cell line from the peak primary response to L. monocytogenes; ○, T cell line from the peak recall response.
Direct Ex Vivo Tetramer Dissociation Assays Confirm Affinity Maturation during Selective Expansion.
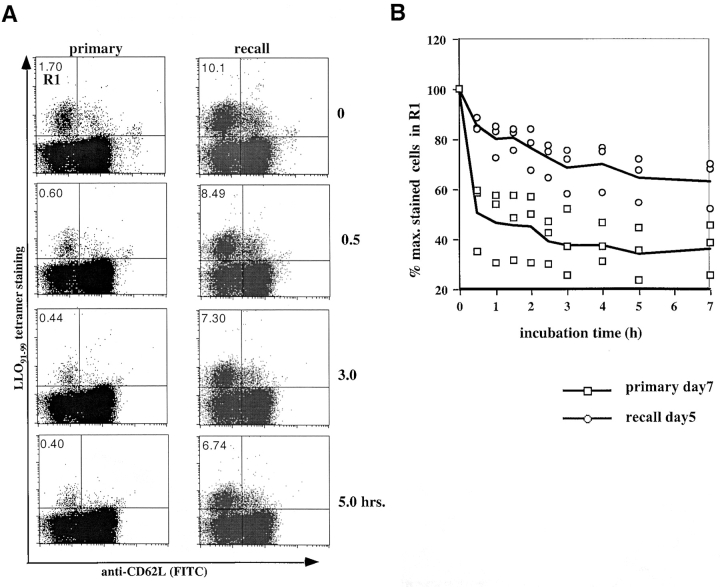
To more directly demonstrate affinity maturation of complex, epitope-specific T cell populations, tetramer dissociation assays were performed on freshly isolated antigen-specific T cells. Splenocytes at the peak of a primary or recall response to L. monocytogenes were enriched for CD8+ T cells and stained with saturating concentrations of tetramers. After washing off unbound tetramers, the temperature was switched to 15°C and tetramer dissociation rates were determined by flow cytometry after increasing time intervals. As shown in Fig. 8 A, more than 50% of H2-Kd/LLO91–99 tetramer-positive, primary T cells with an activated phenotype (CD62Llow) disappear from the R1 quadrant within the first 0.5 h, and only 25% of tetramer-positive T cells remain after 3 h. In contrast, LLO91–99–specific recall T cells are characterized by much lower tetramer dissociation rates; there is minimal loss of surface-bound tetramers during the first 0.5 h, and even at the end of the assay (7 h), >50% of the population remains tetramer positive. This difference between the primary and recall T cell populations is highly reproducible, without any demonstrable overlap in dissociation rates between primary and recall T cells isolated from three mice in each group (Fig. 8 B).
Figure 8.
Direct ex vivo tetramer dissociation rates confirm affinity maturation. Tetramer dissociation assays were performed on CD8- enriched splenocytes isolated from BALB/c mice at the peak of the primary or recall response to L. monocytogenes infection. (A) Dot plots of the ex vivo dissociation assays for CD8+ primary and recall T cells, showing H2-Kd/LLO91–99 tetramer staining on the y axis and anti-CD62L staining on the x axis. Percentages of tetramer-positive cells with an activated phenotype (CD62Llow) for the different time points are indicated (one representative mouse per group). (B) The decline in tetramer staining at 15°C of T cells isolated from primary (□) or recall (○) mice is plotted as the percentage of cells that remain in quadrant R1 after the increasing incubation intervals (T0 is assigned a value of 100%). Three mice were tested for each group; the symbols represent the values obtained for CD8+ T cells isolated from individual mice, with lines indicating the mean of three mice during primary and recall infection.
Discussion
Our studies of the evolution of a complex, antigen-specific T cell population responding to bacterial infection show the following: (a) TCR repertoire focusing occurs during T cell expansion; (b) peptide sensitivity, as measured by direct ex vivo CTL assays, is greater for recall than for primary antigen-specific T cell populations; and (c) the affinity of T cells for antigen, as measured by tetramer association and dissociation rates, increases as memory T cells expand into a recall effector T cell population. These findings are novel, demonstrating for the first time that T cells with higher affinities for their antigen have a selective advantage during in vivo expansion.
Because of the difficulty identifying and characterizing antigen-specific T cell populations, very little is known about their in vivo kinetics and dynamics in response to antigen. Most studies of in vivo T cell responses have focused on experimental systems where T cell populations with very narrow TCR repertoires respond to an immunodominant epitope (18, 32–34). Other studies have used the adoptive transfer of T cells from TCR transgenic mice into antigen-immunized recipient mice to investigate the in vivo activation and expansion of clonal T cell populations (35, 36). However, most primary T cell responses to pathogens are highly complex and characterized by very diverse TCR repertoires. This complexity might enable antigen-specific T cell populations to undergo changes during in vivo T cell responses. Until recently, complex, antigen-specific T cell populations could be studied only indirectly (19, 24). With the introduction of tetramerized MHC/peptide molecules as epitope-specific T cell staining reagents (37), it is now possible to study highly complex, epitope-specific T cell populations directly ex vivo (26, 31, 38).
In this report we show that TCR repertoire focusing of LLO91–99–specific T cells after reinfection with L. monocytogenes results from the selective expansion of high affinity T cells within the epitope-specific population. These findings agree with other studies demonstrating the evolution of antigen-specific T cell populations for CD8+ virus-specific T cell responses (19) and antigen-specific CD4+ T helper cell responses (18). The assays to measure relative TCR–ligand affinities by determining association and dissociation rates of tetrameric MHC/peptide complexes described in this paper should be readily applicable to studies of other model systems. It will be interesting to determine whether affinity maturation is a general feature of in vivo expansion of diverse T cell populations or if there are differences among populations with different antigen specificities. In experimental systems where primary antigen-specific T cell populations are characterized by a restricted TCR repertoire, no changes in the composition of these populations after repeated antigen exposures were found (32, 39). However, it is possible that the limited repertoire of these T cell populations does not provide a sufficient degree of complexity to measure maturation.
What are the mechanisms driving selective expansion of high affinity T cells during the in vivo T cell response to L. monocytogenes infection? It is possible that higher TCR affinity for antigen increases the likelihood that a T cell will become activated and expand upon exposure to antigen. In this setting, antigen concentration and the kinetics of epitope presentation would regulate the extent of affinity maturation; high affinity T cells that recognize low antigen concentrations would be activated earlier and/or for a longer period of time than low affinity T cells. Lower antigen concentrations after reinfection with L. monocytogenes might explain the selective expansion of high affinity T cells during the recall response. However, preliminary experiments analyzing recall responses to different doses of L. monocytogenes demonstrate similar extents of in vivo T cell expansion and TCR repertoire focusing (data not shown), arguing against this hypothesis. Alternatively, T cells within an epitope-specific population may all interact with antigen, but low affinity T cells may receive a qualitatively different signal than high affinity T cells. Studies with altered peptide ligands have shown that differences in the affinity of TCR–ligand engagement can activate different signaling pathways, leading to different effector functions and rates of T cell proliferation (40). The thresholds and requirements for activation of these different signaling pathways are distinct and dependent on the kinetics and affinity of TCR–ligand interactions (41). Qualitatively different signals might turn on different programs for T cell proliferation, with high affinity T cells undergoing more or faster cell divisions than low affinity T cells. It is also possible that qualitatively different signals after TCR–ligand interactions lead to phenotypic differences among activated T cells, such as the surface expression of IL-2 or IL-15 receptors, which influence T cell susceptibility to proliferative and antiapoptotic signals from the inflammatory environment.
What are the functional implications of in vivo T cell affinity maturation for pathogen-specific immunity? It is likely that high affinity T cells respond earlier and faster to infection than low affinity T cells specific for the same antigen. Thus, selectively expanding the high affinity subset of antigen-specific T cells, and consequently increasing their precursor frequency within the memory T cell pool, could increase the quality of immunity. Indeed, adoptive transfer of HIV-specific CD8+ T cells with different affinity for antigen demonstrated that high affinity T cells are 100–1,000-fold more efficient in virus clearance than are low affinity T cells (42). Determining the cellular and molecular basis for the qualitative evolution of epitope-specific T cell populations after in vivo antigen challenge may provide new strategies for vaccination and immunotherapy.
Acknowledgments
We thank P. Cresswell for providing advice, and K. Kerksiek and I.M. Pilip for technical assistance and helpful discussion.
This work was supported by National Institutes of Health grants AI33143 and AI39031. E.G. Pamer is a Pew Scholar in Biomedical Sciences, and D.H. Busch is a research fellow of the Deutsche Forschungsgemeinschaft (DFG) and the Arthritis Foundation.
Footnotes
Abbreviations used in this paper: LLO, listeriolysin O; SA, streptavidin; SB, staining buffer.
References
- 1.Eisen HN, Siskind CW. Variations in affinities of antibodies during the immune response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 2.Jerne NK. A study of avidity based on rabbit skin responses to diphtheria toxin antitoxin mixtures. Acta Path Microbiol Scand. 1951;87(Suppl.):1–183. [PubMed] [Google Scholar]
- 3.Kelsoe G. In situ studies of the germinal center reaction. Adv Immunol. 1995;60:267–288. doi: 10.1016/s0065-2776(08)60587-8. [DOI] [PubMed] [Google Scholar]
- 4.Berek C, Ziegner M. The maturation of the immune response. Immunol Today. 1993;14:400–404. doi: 10.1016/0167-5699(93)90143-9. [DOI] [PubMed] [Google Scholar]
- 5.Nossal GJ. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992;68:1–2. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- 6.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 7.Alam SM, Travers PJ, Wung JL, Nasholds W, Radpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 8.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Tykodi SS, Esser MT, Braciale VL, Braciale TJ. Partial activation of CD8+ T cells by a self-derived peptide. Nature. 1995;378:295–298. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- 10.Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I–restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersh GJ, Allen PM. Essential flexibility in the T cell recognition of antigen. Nature. 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 12.Reis e Sousa C, Levine EH, Germain RN. Partial signaling by CD8+T cells in response to antagonist ligands. J Exp Med. 1996;184:149–157. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex–restricted cytotoxic T lymphocyte clones specific for a Plasmodium bergheinonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174:1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole GA, Hogg TL, Woodland DL. The MHC class I-restricted T cell response to Sendai virus infection in C57BL/6 mice: a single immunodominant epitope elicits an extremely diverse repertoire of T cells. Int Immunol. 1994;6:1767–1775. doi: 10.1093/intimm/6.11.1767. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz MS, Yanagi Y, Oldstone MB. T-cell receptors from virus-specific cytotoxic T lymphocytes recognizing a single immunodominant nine-amino-acid viral epitope show marked diversity. J Virol. 1994;68:353–357. doi: 10.1128/jvi.68.1.352-357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cose SC, Kelly JM, Carbone FR. Characterization of diverse primary herpes simplex virus type 1 gB-specific cytotoxic T-cell response showing a preferential V-beta bias. J Virol. 1995;69:5849–5852. doi: 10.1128/jvi.69.9.5849-5852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch DH, Pamer EG. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation and diversity of responding CTL. J Immunol. 1998;160:4441–4448. [PubMed] [Google Scholar]
- 18.McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann MF, Speiser DE, Ohashi PS. Functional maturation of an antiviral cytotoxic T-cell response. J Virol. 1997;71:5764–5768. doi: 10.1128/jvi.71.8.5764-5768.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busch DH, Pilip I, Pamer EG. Evolution of a complex TCR repertoire during primary and recall bacterial infection. J Exp Med. 1998;188:61–70. doi: 10.1084/jem.188.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann SHE, Rodewald HR, Hug E, Libero GD. Cloned Listeria monocytogenes-specific non-MHC-restricted Lyt-2+ T cells with cytolytic and protective activity. J Immunol. 1988;140:3173–3179. [PubMed] [Google Scholar]
- 22.Pamer, E.G. 1997. Immune response to Listeria monocytogenes. In Host Response to Intracellular Pathogens. S.H.E. Kaufmann, editor. R.G. Landes, Austin, TX. 131–142.
- 23.Pamer EG, Sijts AJ, Villanueva MS, Busch DH, Vijh S. MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses. Immunol Rev. 1997;158:129–136. doi: 10.1111/j.1600-065x.1997.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 24.Vijh S, Pamer EG. Immunodominant and subdominant CTL responses to Listeria monocytogenesinfection. J Immunol. 1997;158:3366–3371. [PubMed] [Google Scholar]
- 25.Busch DH, Bouwer HG, Hinrichs D, Pamer EG. A nonamer peptide derived from Listeria monocytogenes metalloprotease is presented to cytolytic T lymphocytes. Infect Immun. 1997;65:5326–5329. doi: 10.1128/iai.65.12.5326-5329.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 27.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 28.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184:485–492. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T-cell antigen receptor ligand interactions on living cytotoxic T lymphocytes. Nature. 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 30.Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, Teyton L. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 31.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmet R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 32.Walker PR, Wilson A, Bucher P, Maryanski JL. Memory TCR repertoires analyzed long-term reflect those selected during the primary response. Int Immunol. 1996;3:1131–1138. doi: 10.1093/intimm/8.7.1131. [DOI] [PubMed] [Google Scholar]
- 33.Walker PR, Ohteki T, Lopez JA, MacDonald HR, Maryanski JL. Distinct phenotypes of antigen-selected CD8 T cells emerge at different stages of an in vivo immune response. J Immunol. 1995;155:3443–3452. [PubMed] [Google Scholar]
- 34.Maryanski JL, Jongeneel CV, Bucher P, Casanova JL, Walker PR. Single-cell PCR analysis of TCR repertoires selected by antigen in vivo: a high magnitude CD8 response is comprised of very few clones. Immunity. 1996;4:47–56. doi: 10.1016/s1074-7613(00)80297-6. [DOI] [PubMed] [Google Scholar]
- 35.Kearney ER, Walunas TL, Karr RW, Morton PA, Loh DY, Bluestone JA, Jenkins MK. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 36.Zimmerman C, Brduscha-Riem K, Blaser C, Zinkernagel RM, Pircher H. Visualization, characterization, and turnover of CD8+memory T cells in virus-infected hosts. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 38.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 39.Sourdive DJ, Murali-Krishna K, Altman JD, Zajac AJ, Whitmire JK, Pannetier C, Kourilsky P, Evavold B, Sette A, Ahmed R. Conserved T cell receptor repertoire in primary and memory CD8 T cell responses to an acute viral infection. J Exp Med. 1998;188:71–82. doi: 10.1084/jem.188.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Valitutti S, Muller S, Dressing M, Lanzavecchia A. Different responses are elicited by cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and the efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]