Abstract
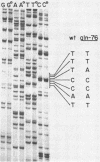
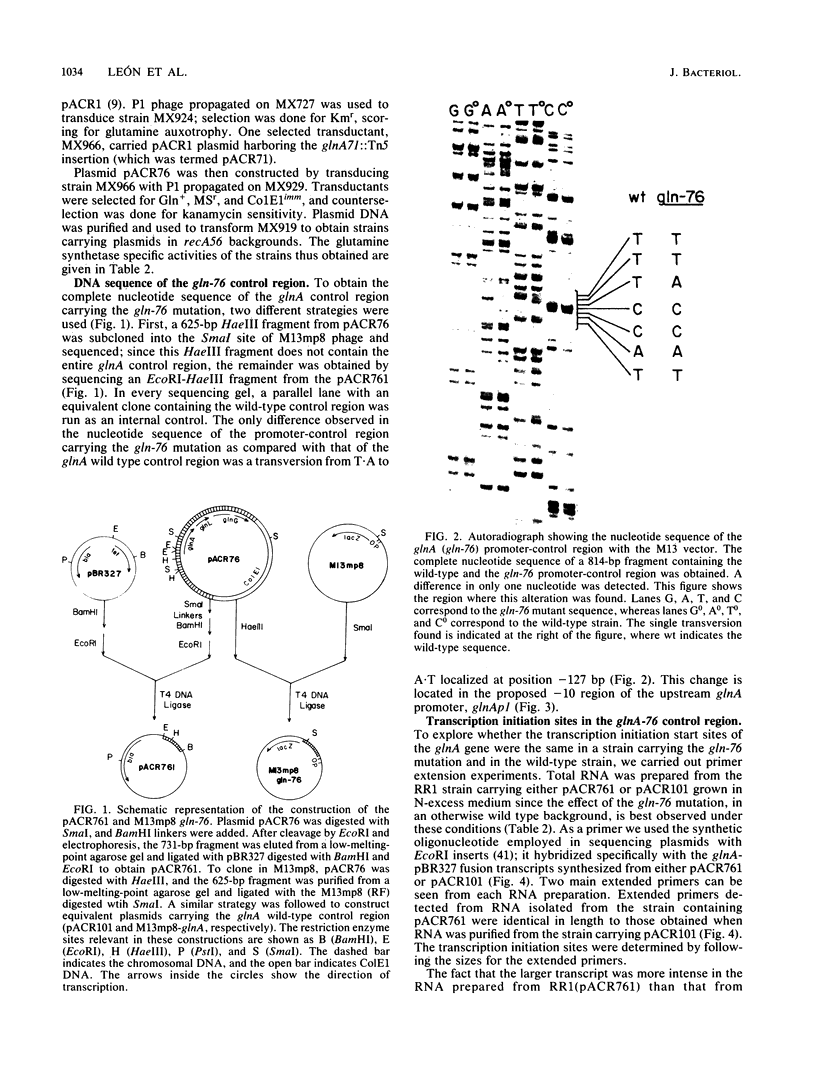
The spontaneous gln-76 mutation of Escherichia coli (Osorio et al., Mol. Gen. Genet. 194:114-123, 1984) was previously shown to be responsible for the cis-dominant constitutive expression of the glnA gene in the absence of a glnG-glnF activator system. Nucleotide sequence analysis has now revealed that gln-76 is a single transversion T.A to A.T, an up-promoter mutation affecting the -10 region of glnAp1, the upstream promoter of the glnALG control region. Both, wild-type and gln-76 DNA control regions were cloned into the promoter-probe plasmid pKO1. Galactokinase activity determinations of cells carrying the fused plasmids showed 10-fold more effective expression mediated by gln-76 than by the glnA wild-type control region. Primer extension experiments with RNA from strains carrying the gln-76 control region indicated that the transcription initiation sites were the same in both the gln-76 mutant and the wild type.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Morales A., Dixon R., Merrick M. Positive and negative control of the glnA ntrBC regulon in Klebsiella pneumoniae. EMBO J. 1984 Mar;3(3):501–507. doi: 10.1002/j.1460-2075.1984.tb01837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betlach M., Hershfield V., Chow L., Brown W., Goodman H., Boyer H. W. A restriction endonuclease analysis of the bacterial plasmid controlling the ecoRI restriction and modification of DNA. Fed Proc. 1976 Jul;35(9):2037–2043. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias A. A., Bastarrachea F. Nucleotide sequence of the glnA control region of Escherichia coli. Mol Gen Genet. 1983;190(1):171–175. doi: 10.1007/BF00330342. [DOI] [PubMed] [Google Scholar]
- Covarrubias A. A., Sánchez-Pescador R., Osorio A., Bolivar F., Bastarrachea F. ColE1 hybrid plasmids containing Escherichia coli genes involved in the biosynthesis of glutamate and glutamine. Plasmid. 1980 Mar;3(2):150–164. doi: 10.1016/0147-619x(80)90106-7. [DOI] [PubMed] [Google Scholar]
- Dixon R. Tandem promoters determine regulation of the Klebsiella pneumoniae glutamine synthetase (glnA) gene. Nucleic Acids Res. 1984 Oct 25;12(20):7811–7830. doi: 10.1093/nar/12.20.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G., Elford R. M., Holmes W. M. Fusion of the Escherichia coli tRNALeu1 promoter to the galK gene: analysis of sequences necessary for growth-rate-dependent regulation. Cell. 1982 Oct;30(3):855–864. doi: 10.1016/0092-8674(82)90290-2. [DOI] [PubMed] [Google Scholar]
- Garcia E., Bancroft S., Rhee S. G., Kustu S. The product of a newly identified gene, gInF, is required for synthesis of glutamine synthetase in Salmonella. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1662–1666. doi: 10.1073/pnas.74.4.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Roberts G., Tyler B. Polarity in the glnA operon: suppression of the reg- phenotype by rho mutations. J Bacteriol. 1982 Jun;150(3):1314–1321. doi: 10.1128/jb.150.3.1314-1321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Roberts G., Tyler B. Polarity in the glnA operon: suppression of the reg- phenotype by rho mutations. J Bacteriol. 1982 Jun;150(3):1314–1321. doi: 10.1128/jb.150.3.1314-1321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S. T., Bennett P. M. Effect of mutations in deoxyribonucleic acid repair pathways on the sensitivity of Escherichia coli K-12 strains to nitrofurantoin. J Bacteriol. 1976 Mar;125(3):1214–1216. doi: 10.1128/jb.125.3.1214-1216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Kustu S. Evidence that nitrogen regulatory gene ntrC of Salmonella typhimurium is transcribed from the glnA promoter as well as from a separate ntr promoter. Mol Gen Genet. 1984;193(1):135–142. doi: 10.1007/BF00327426. [DOI] [PubMed] [Google Scholar]
- Kustu S., Burton D., Garcia E., McCarter L., McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Tyler B. Fine-structure deletion map and complementation analysis of the glnA-glnL-glnG region in Escherichia coli. J Bacteriol. 1982 Jun;150(3):1302–1313. doi: 10.1128/jb.150.3.1302-1313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil T., Roberts G. P., MacNeil D., Tyler B. The products of glnL and glnG are bifunctional regulatory proteins. Mol Gen Genet. 1982;188(2):325–333. doi: 10.1007/BF00332696. [DOI] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- McCarter L., Krajewska-Grynkiewicz K., Trinh D., Wei G., Kustu S. Characterization of mutations that lie in the promoter-regulatory region for glnA, the structural gene encoding glutamine synthetase. Mol Gen Genet. 1984;197(1):150–160. doi: 10.1007/BF00327936. [DOI] [PubMed] [Google Scholar]
- McFarland N., McCarter L., Artz S., Kustu S. Nitrogen regulatory locus "glnR" of enteric bacteria is composed of cistrons ntrB and ntrC: identification of their protein products. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Merrick M. J. A new model for nitrogen control. Nature. 1982 Jun 3;297(5865):362–363. doi: 10.1038/297362a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Osorio A. V., Servín-González L., Rocha M., Covarrubias A. A., Bastarrachea F. cis-Dominant, glutamine synthetase constitutive mutations of Escherichia coli independent of activation by the glnG and glnF products. Mol Gen Genet. 1984;194(1-2):114–123. doi: 10.1007/BF00383506. [DOI] [PubMed] [Google Scholar]
- Pahel G., Tyler B. A new glnA-linked regulatory gene for glutamine synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4544–4548. doi: 10.1073/pnas.76.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1979–1983. doi: 10.1073/pnas.82.7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M., Vázquez M., Garciarrubio A., Covarrubias A. A. Nucleotide sequence of the glnA-glnL intercistronic region of Escherichia coli. Gene. 1985;37(1-3):91–99. doi: 10.1016/0378-1119(85)90261-6. [DOI] [PubMed] [Google Scholar]
- Rothstein D. M., Magasanik B. Isolation of Klebsiella aerogenes mutants cis-dominant for glutamine synthetase expression. J Bacteriol. 1980 Feb;141(2):671–679. doi: 10.1128/jb.141.2.671-679.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein D. M., Pahel G., Tyler B., Magasanik B. Regulation of expression from the glnA promoter of Escherichia coli in the absence of glutamine synthetase. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7372–7376. doi: 10.1073/pnas.77.12.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Ueno-Nishio S., Backman K. C., Magasanik B. Regulation at the glnL-operator-promoter of the complex glnALG operon of Escherichia coli. J Bacteriol. 1983 Mar;153(3):1247–1251. doi: 10.1128/jb.153.3.1247-1251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]